Abstract
Background
For the clinical applicability of regulatory T cells (Tregs) in transplantation, it is critical to determine if donor antigen specificity is required for their immunosuppressive function. We developed an allospecific CD4+ T cell receptor transgenic (TCR-tg) mouse as a source for large numbers of Tregs with defined allospecificity and tested whether they are more effective than polyclonal Tregs at suppressing allograft rejection.
Materials and Methods
CD4+CD25+CD62Lhi T cells were sorted from the spleen and peripheral lymph nodes of wild-type (WT-Tregs) and TCR-tg (Allo-Tregs) mice, and expanded using IL-2 and anti-CD3/anti-CD28 conjugated magnetic beads. Tregs were tested for their ability to suppress the proliferation and cytokine production of alloreactive CD4+CD25- T cells in mixed leukocyte assays. Syngeneic WT hosts were adoptively transferred 5×106 Tregs and transplanted with allogeneic hearts.
Results
Using anti-CD3/anti-CD28 conjugated beads, Tregs were expanded in vitro 100-fold and maintained their suppressor phenotype and function. Allo-Tregs were 6-8 times more potent on a cell-for-cell basis than WT-Tregs in suppressing allospecific proliferation in vitro. Allo-Tregs were unable to suppress in the absence of allo-antigen. Adoptive transfer of expanded Allo-Tregs into WT recipients prolonged the graft survival in a F1 heart transplant model compared to WT-Treg or no treatment [20.0±4.4 d (n=6) vs. 10.4±1.2 (n=8) and 9.7±1.6 d (n=6)].
Conclusions
Unlike polyclonal Tregs, allospecific Tregs are able to prolong allograft survival. However, large numbers of Allo-Tregs were unable to induce tolerance, suggesting that Treg therapy in immunocompetent recipients will require conditioning and/or additional immunomodulation for the induction of tolerance.
Keywords: immunology, transplantation, alloimmunity, rejection, transgenic T cells, regulatory T cells
Introduction
Regulatory T cells (Tregs) are negative regulators of the innate and adaptive immune response that are critical to the development and maintenance of immune tolerance [1-3]. While several subtypes of Tregs have now been described [4], the best-characterized are thymic-derived CD4+ Tregs that express high levels of CD25 (the IL-2 receptor alpha chain) and the transcription factor, FoxP3 [5-7]. These Tregs have been shown to be critical in the prevention of several types of autoimmune diseases in mice [8, 9] and in humans [10, 11]. Within this population, those that express high levels of CD62L (L-selectin) may be more potent suppressors [12, 13].
The demonstration that Tregs are involved in the development and maintenance of allospecific tolerance in animal models [14, 15] has lead to the concept that Tregs may be useful therapeutically in transplantation. A number of studies have documented that Tregs can prevent rejection of allografts when co-transferred with T effector cells into lymphopenic hosts [16-23]. While these studies have afforded important mechanistic information and support for using Treg therapy in graft-versus-host disease, it remains unclear whether the adoptive transfer of allospecific Tregs can provide long-term graft survival or tolerance in immunocompetent hosts.
The immune response to allografts is fundamentally different from other immune responses in that it constitutes both the direct (recipient T cells directly recognize donor MHC on donor APCs) and the indirect (recipient T cells recognize donor-derived peptides bound to MHC on recipient APCs) pathways of antigen recognition [24]. Due to the direct pathway's overlapping recognition of conformational determinants of foreign-peptide and self-MHC with donor-peptides and donor-MHC, the precursor frequency of the alloimmune response is orders of magnitude greater than the frequency of antigen-specific responses [25]. In addition, the alloimmune response is distinct from autoimmunity in that the direct and indirect donor-reactive T cells have not been selected against donor antigen during development. Therefore, in contrast to the role of Treg therapy to control autoimmune diseases, which may involve a relatively small repertoire, the large repertoire of allospecific T cells may be a significant barrier to the use of Treg therapy in transplantation.
The use of allospecific Tregs, as compared to polyclonal Tregs, can potentially increase the efficacy of Treg therapy in mouse transplant models [17, 20, 26]. Here we report the use of a recently developed CD4+ T cell receptor transgenic (TCR-tg) mouse called “4C” that has direct alloreactivity to the MHC class II molecule, I-Ad [27]. We demonstrate that Tregs derived from 4C TCR-tg mice (Allo-Tregs) can be expanded in vitro and retain the phenotype and suppressive function equivalent to freshly isolated Tregs. In comparison to similarly expanded polyclonal wild-type Tregs (WT-Tregs), the alloantigen specific Allo-Tregs are more potent at suppressing alloreactive T cells in vitro when stimulated by allogenic antigen presenting cells and can prolong cardiac allograft survival in MHC mismatched (haploidentical) and otherwise immunologically unmanipulated mice. However, even when administered in large numbers, Allo-Tregs were insufficient, as a monotherapy, to produce long-term graft survival or immune tolerance.
Materials and Methods
Mice
C57BL/6 and BALB/c (National Cancer Institute, Frederick, MD), OT-II TCR-tg (Taconic, Oxnard, CA), and 4C TCR-tg mice previously developed in our laboratory [27] were housed and bred at the University of California San Francisco Animal Barrier Facility. All animal protocols were approved by our IACUC.
Antibodies and reagents
PE-anti-CD4 (GK1.5), APC-anti-CD25 (PC61.5), and PE-anti-FoxP3 (FJK-16s) were from eBioscience (San Diego, CA). FITC-anti-CD62L (MEL-14), biotin-anti-Ly5.1 (A20), biotin-anti-Vβ13 (MR12-3), NALE format anti-CD3 (145-2C11), and APC-conjugated streptavidin were from BD-Pharmingen (San Diego, CA). IL-2 ELISA kits were from R&D, Minneapolis, MN.
Cell sorting and flow cytometry
CD4+ T cells were purified from the peripheral lymph nodes and spleens of C57BL/6 and 4C TCR-tg mice using anti-CD4 magnetic beads (Dynal Biotech) and detached with Detachabead (Invitrogen, Carlsbad, CA). Cells were then stained with anti-CD4-PE, anti-CD25-APC, and anti-CD62L-FITC and sorted on a Mo-Flo™ cytometer (DakoCytomation, Copenhagen, Denmark). Cells sorted to be CD4+CD62LhiCD25+ were classified as Tregs and cells sorted to be CD4+CD62LhiCD25- were classified as conventional T cells (Tconv). Post-sort purity was >98% for both. 4C TCR-tg Rag+/+ mice were used for Treg purification since we found this cell population to be absent in 4C TCR-tg Rag-/- mice, similar to what has been described with the AMB and other TCR-tg mice [20].
In vitro expansion of Tregs
Treg and Tconv expansion was carried out as described previously [9]. Mo Flo-purified T cells were stimulated with anti-CD3 and anti-CD28 coupled to 4.5-μM paramagnetic beads (provided by Xcyte Therapeutics, Inc.) supplemented with 2,000 IU/ml recombinant human IL-2 (Chiron, Emeryville, CA) in complete medium consisting of 10% fetal bovine serum (Biosource International, Camarillo, CA), nonessential amino acids, 0.5 mM sodium pyruvate, 5 mM HEPES, 1 mM GlutaMax (Invitrogen, Carlsbad, CA) and 55 μM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) in DMEM medium. Tregs were plated in 24-well tissue-culture plates at a density of 0.5 - 0.8×106 per well with 1×106 beads in 1.5 ml culture medium. Cultures were split 1:4 after 72 hrs and then split every 1 - 2 days to maintain the cultures at 0.5 – 1.0×106 /ml. Prior to use, the beads were removed from the Tregs using a magnet. Cultures were routinely checked for surface expression of CD4, CD25, CD62L, and the transgenic TCR marker, Vβ13, prior to use in experiments.
In vitro suppression assays
Expanded or fresh Tregs were co-cultured with 50,000 4C Tconv cells at varying Treg:Tconv ratios with 100,000 BALB/c antigen presenting cells (APCs). BALB/c APC consisted of freshly isolated BALB/c splenocytes that were T cell depleted using anti-Thy1.2 magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany) and activated by pre-incubating with 1 μg/ml LPS (Sigma-Aldrich, St. Louis, MO) for 6 hrs, rinsed extensively, and irradiated with 2000 rad. In some experiments, Tregs and Tconv were co-cultured with irradiated self-APCs and stimulated with 1 μg/ml anti-CD3 antibody; and in some experiments, CD4+ T cells from OT-II TCR-tg mice were used as responders and the co-cultures were stimulated with 0.1 μg/ml ovalbumin (Ova) peptide (323-339). Cultures were incubated at 37°C for 72 hrs and pulsed with 1 μCi/well [3H]thymidine for the final 16 hrs of incubation.
Transplantation
Vascularized heterotopic cardiac allografts were performed as previously described [28]. Rejection was defined by the cessation of a palpable heartbeat. Mice were administered 0.5×106, 5×106, or 30×106 Tregs by intravenous injection one day prior to transplantation.
Immunohistology
Cryostat sections (5 mM) were dried, acetone fixed, and incubated for 1 hr in blocking buffer (0.1 M Tris, 0.05 M NaPhos, 0.3% Tween 20, 3% goat serum) at room temperature (RT). Intrinsic peroxidase activity was blocked by incubation in 0.3% H2O2 in PBS for 5' at RT and tissue biotin was blocked with the Avidin-Biotin Blocking Kit from Vector Labs (Burlingame, CA). Slides were incubated with biotin-conjugated anti-Ly5.1 antibody overnight at 4°C in 0.1% BSA in PBS and subsequently with streptavidin-HRP complex (Vector Labs) for 30 min. Slides were then developed with DAB substrate (Vector Labs) and counterstained with hematoxylin (Sigma-Aldrich).
Statistical analysis
Data are expressed as mean±SD. Significance of differences between 2 groups was determined by unpaired 2-tailed Student's t test. Statistical analysis of Kaplan-Meier curves was performed using MedCalc Software (Mariakerke, Belgium).
Results
Isolation and expansion of polyclonal and TCR-tg Tregs
We have recently developed a CD4+ TCR-tg with direct alloreactivity called “4C” [27]. The 4C TCR-tg was developed on the B6 (H-2b) background and has direct alloreactivity against the MHC class II molecule, I-Ad. Potentially, alloantigen-specific CD4+ TCR-tg mice could serve as a source of allospecific Tregs that would facilitate the characterization of the potential therapeutic effect of allospecific Tregs in prolonging allograft survival.
The frequency of CD4+CD25+ T cells was compared between wild-type B6 mice and the 4C TCR-tg in the B6 background. Approximately 3% of total CD4+ T cells were found to co-express CD25 (Fig. 1A) in both wild-type and 4C TCR-tg mice. Of the CD4+CD25+ T cells, approximately two-thirds were CD62Lhi. Previous studies have shown that CD62L marks a Treg subset with optimal suppressive properties [12, 13]. For this purpose, and to exclude activated conventional CD4+ T cells (which demonstrate CD62Llow expression), cell sorting was performed gating on the CD4+, CD25+ and CD62Lhi surface phenotype (Fig. 1B). Sorted cells were then expanded by co-culture with anti-CD3/ anti-CD28 conjugated magnetic beads and 2000 IU/ml of recombinant human IL-2 (Fig. 1C) as previously described [9]. Sorted cells could be expanded approximately 100-fold by 12-15 days in culture. Phenotypic analysis of the expanded cells, WT Tregs and 4C Tregs, demonstrated that the cells remained CD4+, CD25+, and CD62Lhi and >90% demonstrated expression of the Treg-specific transcription factor, FoxP3 (Fig. 1D). Of the Tregs expanded from the 4C TCR-tg mouse, >99% were found to maintain expression of the transgenic TCR β chain, Vβ13 (Fig. 1D).
Fig. 1. 4C TCR-tg has physiological numbers of CD4+CD25+ Tregs that can be expanded in vitro.
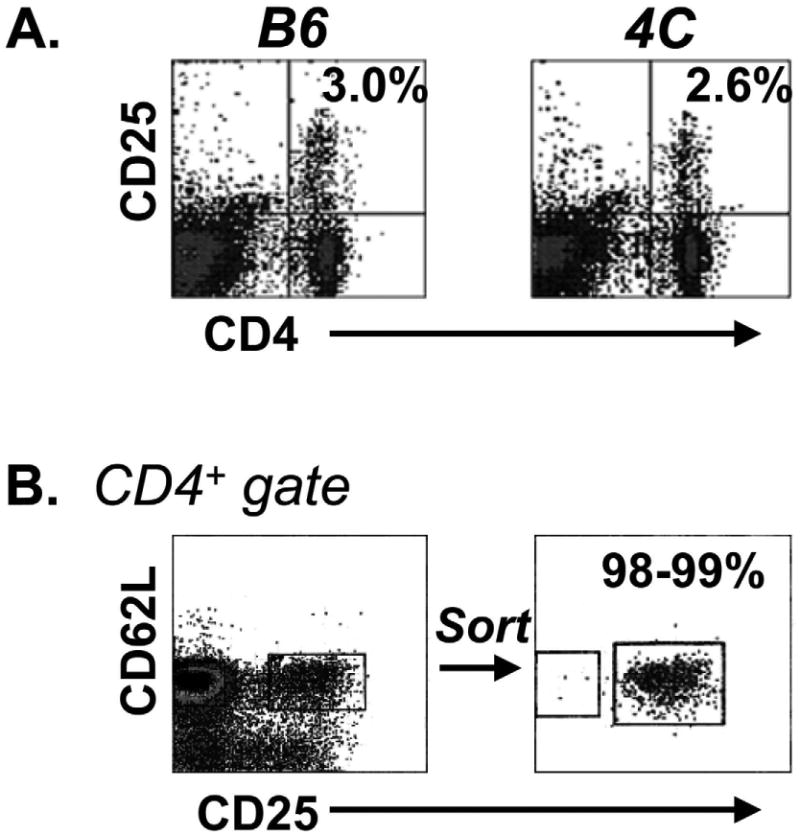
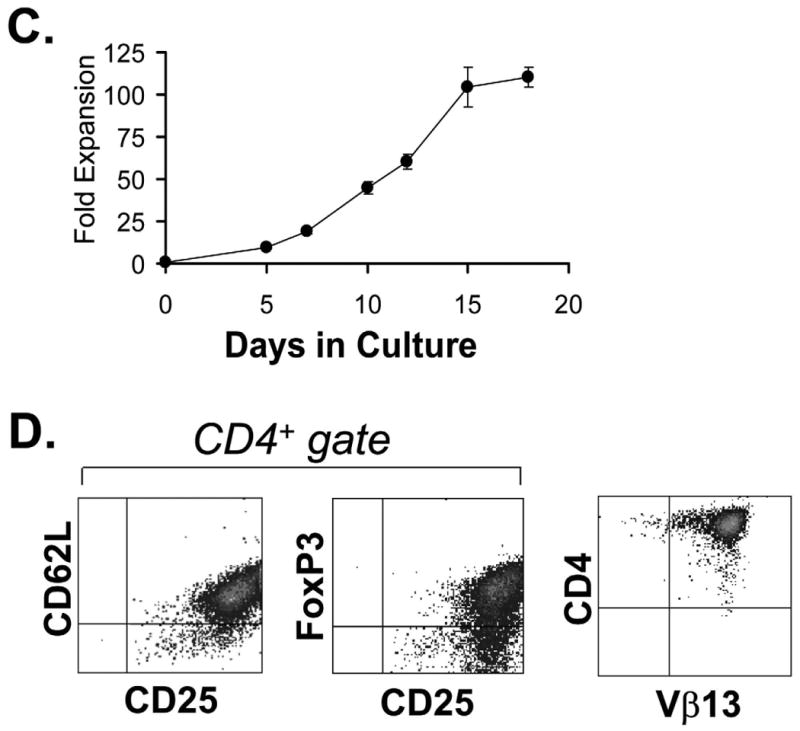
(A) Wild type (B6) and 4C TCR-tg mice have similar frequencies of splenic CD4+CD25+ T cells. (B) Tregs (B6 or 4C TCR-tg) were isolated based on a CD4+CD25+CD62Lhi surface phenotype by MoFlo cell sorting. (C) 4C TCR-tg were expanded over 100-fold in vitro by co-culture with anti-CD3/anti-CD28-conjugated magnetic beads and 2000 IU/ml rhIL-2. (D) Post-expansion, 4C TCR-tg Tregs (Allo-Tregs) were found to remain CD62Lhi and maintain expression of CD25, FoxP3 expression and the transgenic TCR marker, Vβ13.
Function and specificity of expanded Tregs
Next we examined the function of the expanded Tregs. When compared to freshly isolated 4C TCR-tg Treg (Allo-Treg), in vitro expanded Allo-Tregs demonstrated similar capabilities to suppress the proliferation and IL-2 production by BALB/c APC-stimulated 4C Tconv cells (Fig. 2). Expanded Allo-Tregs were compared to expanded polyclonal WT Tregs in their ability to suppress the CD4+ alloresponse to BALB/c APC in vitro. When stimulated by BALB/c APCs, the Allo-Tregs were approximately 6- to 8-fold more potent at suppressing a CD4+ alloreactive T cell response than similarly expanded polyclonal WT Tregs (Fig. 3A). However, when anti-CD3ε (a pan-T cell stimulator) was used to activate T cells, the polyclonal WT Tregs and Allo-Tregs displayed comparable suppressive activity (Fig. 3B). Therefore, Allo-Tregs are more potent at suppressing BALB/c alloreactive T cells than polyclonal Tregs, but show the same suppressive capability when similar TCR ligation is applied.
Fig. 2. Cultured Allo-Tregs retain similar in vitro suppressive abilities compared to freshly isolated Allo-Tregs.
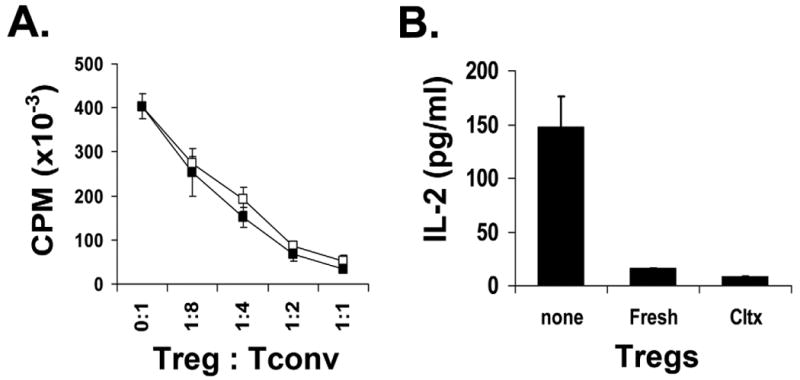
(A) Freshly isolated (open squares) and expanded (solid squares) Allo-Tregs were co-cultured at different ratios with 5×104 4C Tconv responders and 105 LPS-stimulated, T-cell depleted, and irradiated BALB/c splenocytes in suppression assays. (B) Supernatants from the suppression assay were tested by ELISA for IL-2 concentration. Data from fresh and cultured Tregs at the 1:1 Treg:Tconv ratio are shown. Suppression assays were performed in triplicate and data are shown as mean+/-SD. The results shown are representative of 3 independent experiments.
Fig. 3. Allospecific Tregs suppress allospecific T cells in vitro better than polyclonal Tregs.
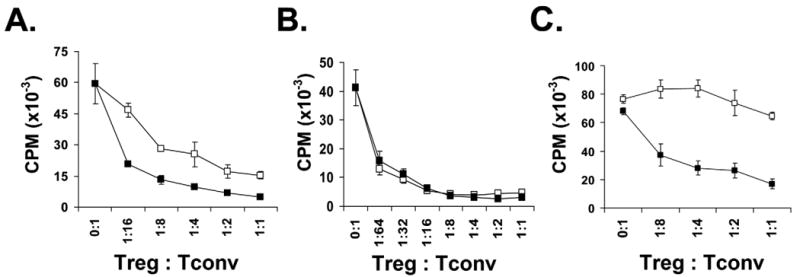
(A) Allo-Tregs (filled squares) or polyclonal WT Tregs (open squares) were co-cultured at different ratios with 5×104 4C Tconv responders and 105 BALB/c APCs in suppression assays. (B) In similar suppression assays, Allo-Tregs (filled squares) and WT Tregs (open squares) were stimulated with anti-CD3 antibody (1 μg/ml) and syngeneic B6 APC and demonstrated equivalent suppressive capabilities. (C) Allo-Tregs are unable to suppress the I-Ab-restricted OT-II TCR-tg T cell response to ovalbumin peptide presented by B6 APCs (open squares), but are able to suppress the same OT-II TCR-tg T cell response when BALB/c APCs are added to the co-culture (filled squares). Suppression assays were performed in triplicate and data are shown as mean+/-SD. The results shown are representative of 2 independent experiments.
To further examine the necessity of antigen-recognition for Treg function, we tested the ability of the Allo-Tregs to suppress an antigen-specific response to ovalbumin with or without stimulation of the 4C TCR. In this assay, Allo-Tregs were co-cultured with ovalbumin specific CD4+ T cells (OT-II, I-Ab restricted) that were stimulated with ovalbumin peptide and irradiated B6 splenocytes with or without the addition of irradiated BALB/c splenocytes. We found that the Allo-Tregs only exhibited suppressive function when BALB/c splenocytes were present in the culture (Fig. 3C), demonstrating that expanded Allo-Tregs need to be activated to exert their suppressive function. Furthermore, the in vitro suppression did not require the responders and Tregs to be activated by the same antigen presenting cells.
Alloantigen-specific Tregs promote allograft survival in wild-type recipients
To test the ability of Allo-Tregs to suppress the alloresponse in vivo, expanded Allo-Tregs or polyclonal WT Tregs were tested for their ability to prolong allograft survival in a heterotopic vascularized heart transplant model. Expanded Tregs were adoptively transferred into otherwise unmanipulated, sex-matched, wild type B6 mice one day prior to heterotopic cardiac allograft transplantation from F1 (BALB/c × B6) cardiac donors. Recipients that had either received 5×106 Allo-Tregs or no treatment were harvested 5 days after transplantation and examined histologically. Mice that had received Allo-Tregs demonstrated decreased myocardial leukocyte infiltrates compared to hearts transplanted into recipients that had not received Allo-Tregs (Fig. 4A).
Fig. 4. Allo-Tregs localize to the cardiac allograft and decrease graft leukocytic infiltrate.
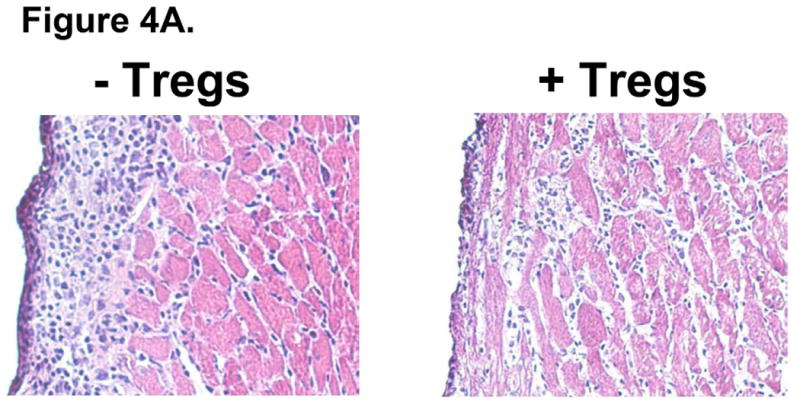
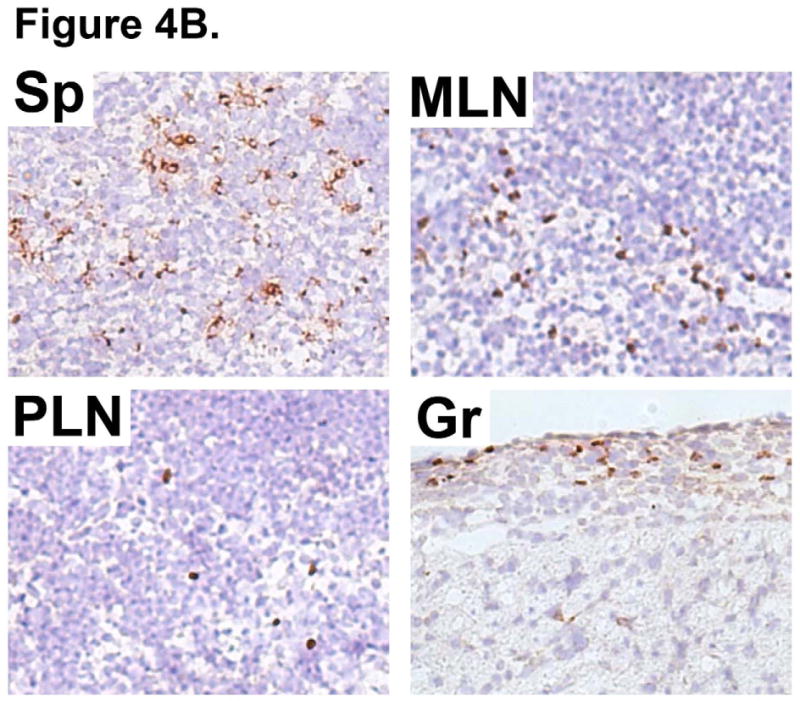
WT B6 mice were intravenously injected with 5×106 Tregs derived from 4C TCR-tg mice (Allo-Tregs) one day prior to heterotopic cardiac transplantation with F1 (BALB/c × B6). (A) Cardiac allografts from recipients treated with Allo-Tregs (+Tregs) have decreased subepicardial leukocytic infiltrates compared to untreated recipients (-Tregs); 200× magnification. Results are representative of 5 mice. (B) Immunohistological staining of Ly5.1+ Allo-Tregs (Brown staining) in Ly5.2+ B6 hosts of Ly5.2+ F1 (BALB/c × B6) cardiac grafts five days post-transplantation. Representative sections from spleen (Sp), mesenteric lymph node (MLN), peripheral lymph node (PLN), and cardiac graft (Gr); 200× magnification. Results are representative of 5 mice.
Allo-Tregs expressing the Ly5.1 congenic marker were used to determine the localization of infused Allo-Tregs after cardiac transplantation. On post-transplant day 5, the recipient spleen, mesenteric lymph nodes, and peripheral lymph nodes were examined by immunohistochemistry. As shown in Fig. 4B, the highest density of transferred Tregs was found in the recipient spleen (Sp) and mesenteric lymph nodes (MLN). The Allo-Tregs were also present in the peripheral lymph nodes (PLN) and amongst subepicardial lymphocytic infiltrates in the heart grafts (Gr). Thus, Allo-Tregs were found to migrate to lymphoid organs as well as to the allograft.
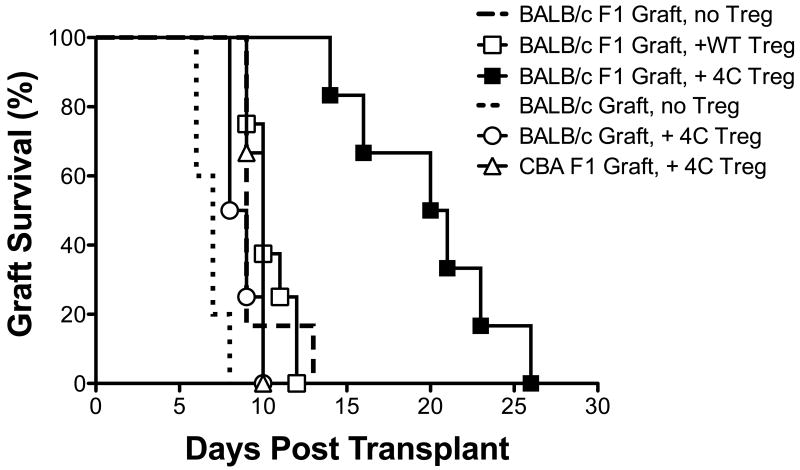
Allo-Tregs were able to prolong graft survival despite an intact host immune system while same number of polyclonal WT Tregs provided no graft prolongation. As shown in Fig. 5, the intravenous transfer of 5×106 expanded Allo-Tregs was able to significantly prolong the survival of F1 cardiac grafts when compared to no treatment (MST = 20.0 ± 4.4 days [n = 6] versus 9.7 ± 1.6 days [n = 6]; p < 0.001). However, the same number of similarly expanded polyclonal B6 Tregs had no effect on graft survival (MST = 10.4 ± 1.2 days [n = 8]; p = 0.60). The effect of the Allo-Tregs on allograft survival was donor specific, as the Allo-Tregs were unable to prolong the survival of a third-party F1 (CBA × B6) graft (MST = 9.67 ± 0.58 days [n = 3]; p = 0.69). When the number of Allo-Tregs was decreased 10-fold (5×105), no prolongation was observed (MST = 9.44 ± 0.55 days [n = 5]), and increasing the number of infused Allo-Tregs to 30×106 did not further prolong graft survival compared to the 5×106 infusion (data not shown). When fully HLA-mismatched BALB/c (not F1) grafts were transplanted the administration of Allo-Tregs resulted in a small prolongation of survival (6.80 ± 0.84 days [n=5] without Allo-Tregs versus 8.75 ± 0.96 days [n = 4] with 5×106 Allo-Tregs; p = 0.02) (Fig. 5).
Fig. 5. Allo-Tregs suppress alloreactivity in vivo better than polyclonal Tregs in a donor specific manner.
WT B6 mice were intravenously injected with 5×106 Allo-Tregs (4C) or polyclonal WT Tregs (B6) one day prior to heterotopic cardiac transplantation with F1 (BALB/c × B6), BALB/c, or 3rd party F1 (CBA × B6) donors and graft survival was compared by Kaplan-Meier analysis. Rejection was defined by the cessation of a palpable graft pulse.
Discussion
In this study, we demonstrate that Tregs with direct allospecificity are more potent than polyclonal Tregs at suppressing alloimmunity both in vitro and in vivo. Allospecific Tregs were able to significantly prolong graft survival as a single immunosuppressive agent in mice that were otherwise not manipulated or immunologically impaired. Similar to other studies, we found polyclonal Tregs or 3rd party-Tregs to be ineffective [22, 26, 29], confirming the importance of antigen specificity in Treg therapy. Importantly, we find that despite a large number of transferred allospecific Tregs, only limited immunological protection was afforded.
Because of their role as natural immune suppressors, Tregs are an attractive therapeutic agent for preventing allograft rejection. To date, many studies have demonstrated the efficacy of Tregs in prolonging or preventing graft survival in rodent models that are immunologically impaired, such as Rag-/- recipients [23], nu/nu recipients [20, 22], T cell-depleted recipients [21], and irradiated recipients [17]. While these studies have generated a tremendous interest in the potential use of Tregs for induction of transplantation tolerance [30-32], the ability of Tregs to suppress the alloresponse in otherwise unmanipulated and MHC incompatible hosts is not well characterized [26, 33].
CD4+ TCR-tg mice can serve as a source of antigen specific CD4+CD25+ Tregs for use in rodent models to investigate the therapeutic efficacy of antigen-specific Tregs. Expanded Tregs from the BDC2.5 TCR-tg, a TCR-tg derived from an islet-specific autoimmune NOD CD4+ T cell, have been shown to be effective in preventing autoimmune diabetes when transferred to pre-diabetic NOD mice; whereas similarly expanded non-specific NOD Tregs were less effective [9]. In addition, Tregs from the ABM TCR-tg, a CD4+ TCR-tg specific for the MHC class II molecule I-Abm12, can prolong skin and heart allografts in lymphopenic mice that are syngeneic with the exception of this molecule [20].
While it has been traditionally considered that T cells with direct alloreactivity are the primary mediators of acute rejection and those with indirect alloreactivity mediate chronic rejection [34], a substantial role is now also appreciated for the indirect pathway in acute rejection [35-37]. Recent findings have similarly demonstrated that Tregs with indirect allospecificity are indeed potent suppressors of the alloresponse [17, 21, 38, 39] and that co-administration of Tregs with direct or indirect alloreactivity improves their efficacy [17]. An interesting future study would be to compare the efficacy of TCR-tg Tregs with direct specificity to those with indirect specificity and to test for synergism between these subsets.
Treg administered to immunocompetent recipients will likely require adjuvant immunosuppression and/or lymphodepletion to achieve long-term graft survival [26]. Recent evidence has found that anti-thymocyte globulin (ATG) [40] and Rapamycin [18] increase Treg generation through the conversion of allospecific effector T cells into Tregs. Pu et al. were able to increase survival of liver transplant recipients in a rat model through the co-administration of Tacrolimus and Tregs [29]. Similarly, Rapamycin has been used successfully to increase Treg effectiveness in murine transplant experiments [21, 26, 41] and to increases human Treg suppressive capabilities in vitro [42].
It is now known that activation of innate immune pathways through TLR signaling pathways leads to dendritic cell maturation and the inhibition of Treg function [43-46]. Surgical trauma, inflammation, and ischemia-reperfusion injury that occurs during procurement, storage, and implantation of transplant organs can lead to the release of endogenous molecules that act as innate immune stimulants [30, 47]. Therefore, delayed or multiple administrations of Tregs postoperatively may improve their efficacy [21, 26].
These results show that, on a cell-per-cell basis, allospecific Tregs are more potent at suppressing alloreactive T cells than are wild-type, polyclonal Tregs. However, as a monotherapy, the observed allograft protection in a haploidentical mouse heart transplant model is only modest. Supplementation with immunomodulatory agents, addition of Tregs with indirect antigen specificity, and/or alterations in Treg expansion technique, dosage, timing, and dosing frequency may be required to improve their efficacy.
Acknowledgments
This work was supported by a grant from the American College of Surgeons (T.V.B), an ASTS-Roche Award (T.V.B.), an ASTS Faculty Development Award (S.M.K.), the National Institutes of Health NIDDK K08 DK61970 (S.M.K.) and R37 AI46643 (J.A.B), the JDRF Center 4-2004-372 (J.A.B.), ROTRF (Q.T.), the UCSF Transplant Innovative Research Fund, and Nicholas family fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–16. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 5.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 8.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatila TA, Blaeser F, Ho N, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106(12):R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 12.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105(5):2220–6. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 13.Fu S, Yopp AC, Mao X, et al. CD4+ CD25+ CD62+ T-regulatory cell subset has optimal suppressive and proliferative potential. Am J Transplant. 2004;4(1):65–78. doi: 10.1046/j.1600-6143.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 14.Wood KJ, Luo S, Akl A. Regulatory T cells: potential in organ transplantation. Transplantation. 2004;77(1 Suppl):S6–8. doi: 10.1097/01.TP.0000106477.70852.29. [DOI] [PubMed] [Google Scholar]
- 15.Jiang S, Lechler RI. Regulatory T cells in the control of transplantation tolerance and autoimmunity. Am J Transplant. 2003;3(5):516–24. doi: 10.1034/j.1600-6143.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 16.Bushell A, Jones E, Gallimore A, Wood K. The generation of CD25+ CD4+ regulatory T cells that prevent allograft rejection does not compromise immunity to a viral pathogen. J Immunol. 2005;174(6):3290–7. doi: 10.4049/jimmunol.174.6.3290. [DOI] [PubMed] [Google Scholar]
- 17.Joffre O, Santolaria T, Calise D, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7(7):1722–32. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MKt, Moore DJ, Jarrett BP, et al. Promotion of allograft survival by CD4+CD25+ regulatory T cells: evidence for in vivo inhibition of effector cell proliferation. J Immunol. 2004;172(11):6539–44. doi: 10.4049/jimmunol.172.11.6539. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Fueyo A, Sandner S, Habicht A, et al. Specificity of CD4+CD25+ regulatory T cell function in alloimmunity. J Immunol. 2006;176(1):329–34. doi: 10.4049/jimmunol.176.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang JY, Tanriver Y, Jiang S, et al. Indefinite mouse heart allograft survival in recipient treated with CD4(+)CD25(+) regulatory T cells with indirect allospecificity and short term immunosuppression. Transpl Immunol. 2009;21(4):203–9. doi: 10.1016/j.trim.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Velasquez-Lopera MM, Eaton VL, Lerret NM, et al. Induction of transplantation tolerance by allogeneic donor-derived CD4(+)CD25(+)Foxp3(+) regulatory T cells. Transpl Immunol. 2008;19(2):127–35. doi: 10.1016/j.trim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Xia G, He J, Zhang Z, Leventhal JR. Targeting acute allograft rejection by immunotherapy with ex vivo-expanded natural CD4+ CD25+ regulatory T cells. Transplantation. 2006;82(12):1749–55. doi: 10.1097/01.tp.0000250731.44913.ee. [DOI] [PubMed] [Google Scholar]
- 24.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3(5):525–33. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 25.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166(2):973–81. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 26.Raimondi G, Sumpter TL, Matta BM, et al. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J Immunol. 2010;184(2):624–36. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan TV, Hoang V, Garrod KR, et al. A new T-cell receptor transgenic model of the CD4+ direct pathway: level of priming determines acute versus chronic rejection. Transplantation. 2008;85(2):247–55. doi: 10.1097/TP.0b013e31815e883e. [DOI] [PubMed] [Google Scholar]
- 28.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–50. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Pu LY, Wang XH, Zhang F, et al. Adoptive transfusion of ex vivo donor alloantigen-stimulated CD4(+)CD25(+) regulatory T cells ameliorates rejection of DA-to-Lewis rat liver transplantation. Surgery. 2007;142(1):67–73. doi: 10.1016/j.surg.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Alegre ML, Goldstein DR, Chong AS. Toll-like receptor signaling in transplantation. Curr Opin Organ Transplant. 2008;13(4):358–65. doi: 10.1097/MOT.0b013e3283061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7(6):1457–63. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 32.Yong Z, Chang L, Mei YX, Yi L. Role and mechanisms of CD4+CD25+ regulatory T cells in the induction and maintenance of transplantation tolerance. Transpl Immunol. 2007;17(2):120–9. doi: 10.1016/j.trim.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 34.Briscoe DM, Sayegh MH. A rendezvous before rejection: where do T cells meet transplant antigens? Nat Med. 2002;8(3):220–2. doi: 10.1038/nm0302-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brennan TV, Jaigirdar A, Hoang V, et al. Preferential priming of alloreactive T cells with indirect reactivity. Am J Transplant. 2009;9(4):709–18. doi: 10.1111/j.1600-6143.2009.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honjo K, Xu X, Bucy RP. CD4+ T-cell receptor transgenic T cells alone can reject vascularized heart transplants through the indirect pathway of alloantigen recognition. Transplantation. 2004;77(3):452–5. doi: 10.1097/01.TP.0000112937.12491.42. [DOI] [PubMed] [Google Scholar]
- 37.Auchincloss H, Jr, Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc Natl Acad Sci U S A. 1993;90(8):3373–7. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105(12):4871–7. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 39.Tsang JY, Tanriver Y, Jiang S, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118(11):3619–28. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17(10):2844–53. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 41.Pilat N, Baranyi U, Klaus C, et al. Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am J Transplant. 2010;10(4):751–62. doi: 10.1111/j.1600-6143.2010.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawamoto K, Pahuja A, Hering BJ, Bansal-Pakala P. Transforming growth factor beta 1 (TGF-beta1) and rapamycin synergize to effectively suppress human T cell responses via upregulation of FoxP3(+) Tregs. Transpl Immunol. 2010 doi: 10.1016/j.trim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 44.Sutmuller RP, den Brok MH, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116(2):485–94. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309(5739):1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103(18):7048–53. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23(11):509–12. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]