Van Trump et al. (2010) recently demonstrated that the aminoglycoside antibiotic gentamicin, previously thought to be selectively toxic to hair cells of the canal neuromasts (CN) of the fish lateral line system, is additionally toxic to hair cells of the superficial neuromasts (SN). The authors used the fluorescent vital dyes DASPEI (2-(4-(dimethylamino)styryl)-N-ethylpyridinium iodide) and FM1-43 ((n-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl) pyridinium dibromide) to visualize by fluorescence microscopy the survival of hair cells in the CN and SN of zebrafish (Danio rerio) and Mexican blind cave fish (Astyanax fasciatus) treated in a solution of 0.001% gentamicin sulfate for 24h. Extensive hair cell death was observed in populations of CN and SN of gentamicin-treated animals of both species compared to control animals. On the basis of this result, the authors concluded, (p. 49), “(1) That hair cells in the SNs of the lateral line can no longer be regarded as functionally resistant to gentamicin toxicity, (2) that this drug should therefore no longer be used as a pharmacological tool for selective blocking of CN, but not SN hair cells, and (3) that the conclusions of some previous studies need to be reevaluated.” Using an alternative approach to themselves reevaluate a 15-year-old assumption in the literature drawn from SEM imaging (e.g., Song et al., 1995), Van Trump et al. (2010) convincingly demonstrated that gentamicin in fact damages a high proportion of both SN and CN, similar in effect to another commonly used aminoglycoside, streptomycin (e.g., Kaus, 1987; Blaxter and Fuiman, 1989; Montgomery et al., 1997; Montgomery et al., 2003). Toward further caution in the interpretation of lateral line behavioral studies that have used aminoglycoside antibiotics to chemically ablate SN and/or CN without rigorous verification of treatment efficacy, we are inspired to share our own recent observations on limitations in the efficacy of both gentamicin and streptomycin in damaging the lateral line systems of three fish species.
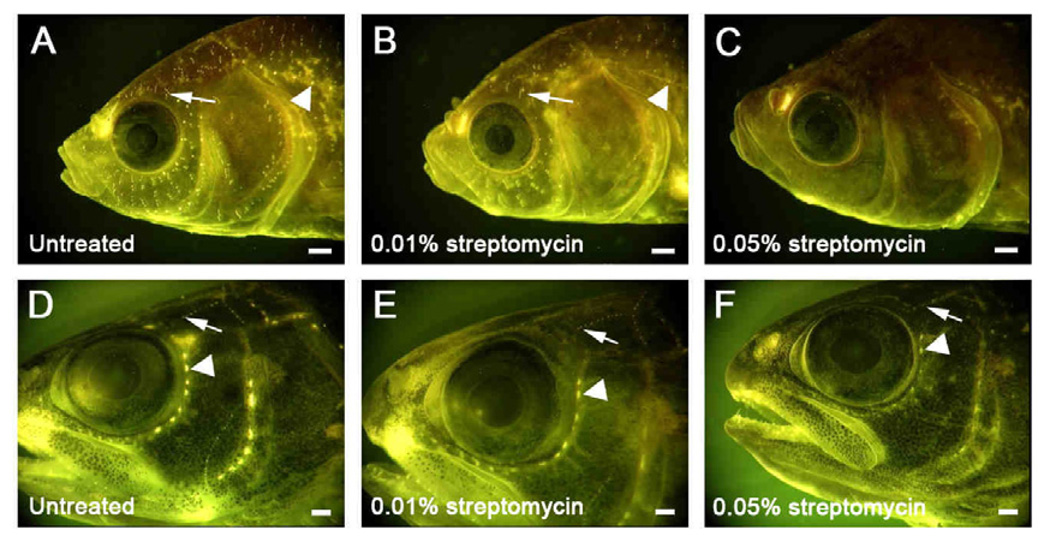
The images presented here (Figs. 1–3) are drawn primarily from two projects. Images comprising Fig. 1 were obtained over the course of a series of experiments designed to examine the role of the lateral line system in swimming behaviors of goldfish (Carassius auratus) and juvenile steelhead trout (Oncorhynchus mykiss). During these experiments, prior to behavioral testing, fish were held for 24h in 10 L buckets of aerated water containing one of several concentrations of streptomycin sulfate, expected on the basis of previously published reports to chemically ablate both CN and SN (Kaus, 1987; Montgomery et al., 1997; Montgomery et al., 2003). Following treatment, fish were tested in a behavioral assay, labeled with DASPEI, anesthetized, and examined under a fluorescent dissecting microscope to assess the extent of streptomycin-induced hair cell death. Labeling patterns in streptomycin-treated fish were compared to labeling patterns in untreated fish. Figs. 1A–C display goldfish treated with 0% (untreated), 0.01% and 0.05% streptomycin, respectively; Figs. 1D–F display juvenile steelhead treated at the same concentrations. The 0.05% streptomycin treatment (cf. Montgomery et al., 1997) appeared to ablate both the CN and SN of the goldfish (Fig. 1C), but left a number of partially intact neuromasts (primarily CN) in the steelhead (Fig. 1F). The lower 0.01% concentration left a number of partially or wholly intact CN and SN in animals of both species, as indicated by persistent DASPEI labeling in these animals (Figs. 1B and 1E). While the mechanism of DASPEI uptake is unknown, possible entry routes include the transduction channel and apical endocytosis, both of which are transduction-dependent process such that DASPEI will only be taken up by active hair cells (Van Trump et al. 2010; D. Raible, personal communication). Although DASPEI intensity is an indirect method for hair cell assessment, studies in zebrafish have demonstrated that DASPEI intensity is proportional to direct counts of labeled hair cells (Harris et al. 2003; Coffin et al. 2009). The observed changes in DASPEI labeling at increasing streptomycin concentrations (Fig. 1) are thus consistent with increased but incomplete hair cell loss in O. mykiss at 0.05% and in both O. mykiss and C. auratus at 0.01%.
Fig. 1.
DASPEI-labeled SN (arrows) and CN (arrowheads) on the heads of goldfish (Carassius auratus) (A–C) and steelhead (Oncorhynchus mykiss) (D–E). Fish were treated in 10 L buckets of aerated water for 24 h with streptomycin sulfate at concentrations of (A,D) 0% (untreated) (B,E) 0.01%, or (C,F) 0.05%. Higher streptomycin concentrations resulted in increased hair cell death, evidenced by a reduction in the number of labeled neuromasts, although a number of partially intact neuromasts remained in the steelhead at the highest concentration tested (F). All scale bars = 1.0 mm.
Fig. 3.
Confocal brightest-point projections of midshipman SN (A–E) and CN (F–J) labeled with Alexa 488 phalloidin, which labels the actin-rich hair bundles at the apical end of each hair cell. (A) Intact SN from an untreated fish, (B) SN treated with 0.002% gentamicin are largely intact, as are (C) SN treated with 0.05% streptomycin. (D) SN partially or (E) completely damaged by liquid nitrogen (LN). (F) Intact CN from an untreated fish), (G) intact CN treated with 0.002% gentamicin, (H) intact CN treated with 0.05% streptomycin, (I) intact CN from a fish treated with LN ablation, as an example of a neuromast skipped during the procedure, (J) LN-ablated CN. Scale bars in A, E, and G = 20 µm, bars in B, C, and J = 10 µm.
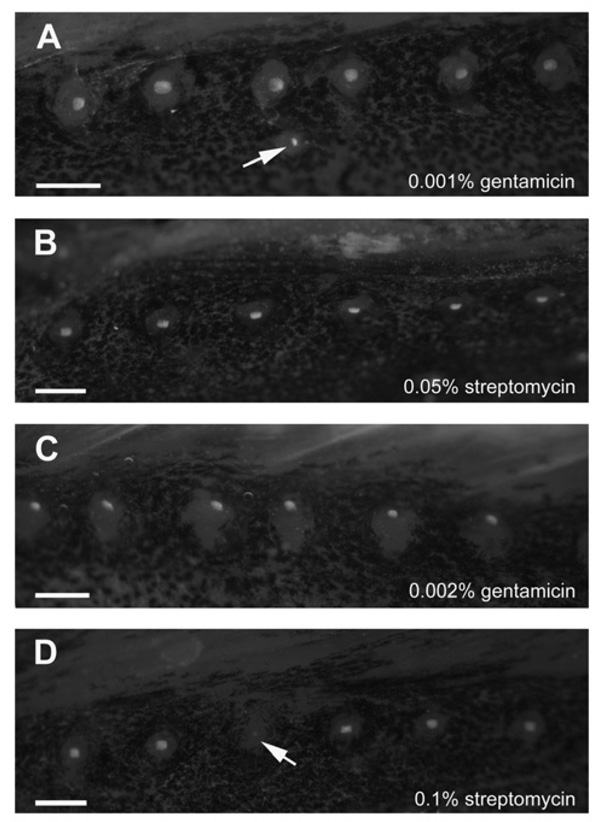
Images comprising Figs. 2 and 3 were obtained over the course of a project designed to evaluate the role of the lateral line system in sound source localization by a marine species, the plainfin midshipman (Porichthys notatus). While an influential theoretical paper published several decades ago posited that the lateral line must be critical for sound source localization by fish (van Bergeijk, 1964), more recent theories (e.g., the “phase model”) have posited that auditory mechanisms of the inner ear may be sufficient for source localization (see Sand and Bleckmann, 2008; Rogers and Zeddies, 2008). Thus, in order to test the hypothesis that fish without a functioning lateral line system would retain the ability to localize a biologically relevant signal emanating from a distant underwater speaker, test animals were treated with gentamicin or streptomycin in an attempt to chemically ablate both CN and SN. Fish were treated in seawater (salinity approximately 23–28 parts per thousand) containing 0.001% gentamicin sulfate for 24h (after Van Trump et al., 2010) or 0.05% streptomycin sulfate for 3h (after Montgomery et al., 1997). As in the goldfish and steelhead experiments, treated and untreated midshipman were then labeled with DASPEI and examined under a fluorescent dissecting microscope to assess the extent of aminoglycoside-induced hair cell death. Fig. 2 displays DASPEI-labeled images of the anterior dorsal trunk lateral line from gentamicin- (Fig. 2A) and streptomycin-treated (Fig. 2B) animals. Extensive neuromast survival was evident in both cases. Although this result was consistent with reduced uptake of aminoglycosides in seawater noted in past investigations (Blaxter and Fuiman, 1989; see below), it was also consistent with the limited efficacy of streptomycin noted above in O. mykiss at the same 0.05% concentration (Fig. 1C) and in both O. mykiss and C. auratus at a lower 0.01% concentration (Figs. 1B and 1E). We thus subsequently probed the effect of higher aminoglycoside concentrations, treating additional fish in seawater containing doubled concentrations of gentamicin (0.002%) for 24h or streptomycin (0.1%) for 3h. As before, extensive neuromast survival was evident (Figs. 2C and 2D), with 20 or fewer missing or damaged neuromasts (out of several hundred neuromasts total) in each fish examined, regardless of aminoglycoside type or concentration (mean=11.6, s.d.=5.5, n=8 fish). Since comorbid nonsensory effects have been associated with exposure to high concentrations of aminoglycosides (Kaus, 1987; see Janssen, 2000; Coffin, personal observation), we elected to abandon aminoglycosides altogether as a means of lateral line ablation, eventually selecting a physical method of ablation – direct application of a liquid nitrogen-chilled probe to SN and surgically exposed CN – to enable the completion of our sound source localization experiments.
Fig. 2.
DASPEI-labeled neuromasts from the dorsal lateral line of plainfin midshipman (Porichthyus notatus), just ventral to the dorsal fin. Fish were treated with (A) 0.001% gentamicin sulfate for 24 h, (B) 0.05% streptomycin sulfate for 3 h, (C) 0.002% gentamicin sulfate for 24 h, or (D) 0.1% streptomycin sulfate for 3 h. Neuromasts were largely intact in all animals examined, although some damage was noted (indicated by arrows in A and D). Images were taken with a Leica MZFLIII stereomicroscope and a Leica DFC350FX CCD-cooled camera. All scale bars = 0.5 mm.
Liquid nitrogen ablation of SN has been discussed previously (e.g., Montgomery et al., 2003); extension of the technique to CN in midshipman was straightforward. Briefly, a liquid nitrogen-cooled copper probe (diameter ~1 mm) was applied for ~2 s to each of the well-defined stitches of SN on the head, trunk, and caudal fin of anesthetized midshipman (see Greene, 1899). Cranial lateral line canals were then opened with microdissection scissors and exposed CN were treated with a liquid nitrogen-cooled surgical steel probe (diameter ~0.5 mm). After CN treatment the canals were closed with superglue and the fish was resuscitated by flushing water over the gills until normal righting behavior resumed. For comparison of the pharmacological and physical methods, Fig. 3 displays images obtained by confocal microscopy of phalloidin-labeled SN (Figs. 3A–E) and CN (Figs. 3F–J) hair bundles. Little to no hair bundle damage was observed in any SN or CN exposed to either streptomycin or gentamicin, as demonstrated by SN or CN treated with 0.002% gentamicin (Figs. 3B and 3G, respectively) or 0.05% streptomycin (Figs. 3C and 3H, respectively). Physical ablation using liquid nitrogen, in contrast, successfully damaged both SN (Fig. 3E) and CN (Fig. 3J), although neuromasts were occasionally incompletely damaged or spared if the cooled probe was not properly applied (Figs. 3D and 3I).
While the ototoxicity of aminoglycoside antibiotics in the vertebrate inner ear (Li et al., 1995; Kil et al., 1997; Forge and Li, 2000) and in the neuromasts of the lateral line system of some fish species (e.g., zebrafish: Harris et al. 2003; Ton and Parng, 2005; Coffin et al., 2009; Owens et al., 2009; Van Trump et al., 2010) is well established, rigorous validation of aminoglycoside-induced damage to lateral line hair cells in many model species is lacking. Using a fluorescent vital dye to assess neuromast survival in streptomycin- or gentamicin-treated fish of two freshwater species (juvenile O. mykiss, C. auratus) and a marine species (P. notatus), we have provided evidence that many lateral line hair cells may survive aminoglycoside exposure. While streptomycin treatment at the higher 0.05% concentration (but not the lower 0.01% concentration) appeared to ablate a majority of hair cells in juvenile steelhead and especially goldfish, the hair cells of the midshipman lateral line appeared extraordinarily resistant to aminoglycoside toxicity. Although previous investigators have noted that hair cell uptake of aminoglycosides may be reduced in seawater by high levels of Ca2+ (Blaxter and Fuiman, 1989; see also Coffin et al. 2009), streptomycin has been used in behavioral studies of lateral line function in at least two other marine species (Clupea harengus, Blaxter and Fuiman, 1989; Pagothenia borchgrevinki, Montgomery et al., 1997). While changes in behavior following streptomycin treatment reported in those studies were attributed to a loss of lateral line function, Janssen (2000) has suggested that behavioral changes observed following pharmacological treatment may be partially attributable to comorbid nonsensory effects; our data indicate that this possibility is worth further consideration.
Although caution is certainly warranted in generalizing our results and those of Van Trump et al. (2010) to other species and treatment paradigms, Van Trump et al.’s (2010) investigation offered a valuable caveat on the importance of rigorous verification of treatment efficacy, which we would underscore: While pharmacological manipulation of the lateral line seems straightforward methodologically, comparative biologists studying lateral line-mediated behavior must understand precisely how – and to what extent - treatment has altered sensory anatomy before any functional inferences should be drawn. In species with thousands of distributed neuromasts (e.g., C. auratus) and/or extensive canal systems (e.g., O. mykiss), pharmacological treatment with aminoglycosides or other ototoxic agents (e.g., cobalt chloride; but see Janssen, 2000) may prove the only feasible treatment option. In species with localized and accessible SN and CN (e.g., P. notatus), however, physical ablation, which avoids the potential comorbid nonsensory effects of aminoglycosides and can provide for a more thorough ablation of hair cells, may prove a more effective alternative.
Acknowledgements
We thank Drs. David Furlow, David Raible, Ed Rubel, and Chris Stecker for their support and Jon Reardon for technical assistance, and the UC Davis Center for Aquatic Biology and Aquaculture and Bodega Marine Laboratory for housing and maintaining the fish. We also thank two anonymous reviewers for comments that greatly improved the manuscript. This research was funded in part by NSF grant IOS-0642214 (J.A.S.), the California Energy Commission’s Public Interest Energy Research Program (T.D.M.), and by NIH Grant Nos. F32DC009931 (A.B.C.), T32DC000033, F31DC010543 (A.D.B.), and P30DC004661.
Abbreviations
- CN
canal neuromast
- DASPEI
2-(4-(dimethylamino)styryl)-N-ethylpyridinium iodide
- FM1-43
n-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl) pyridinium dibromide
- SN
superficial neuromast
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew D. Brown, Department of Speech and Hearing Sciences, University of Washington, Seattle, WA 98105, andrewdb@u.washington.edu
Timothy D. Mussen, Department of Wildlife, Fish and Conservation Biology, University of California, Davis, Davis, CA 95616, tdmussen@ucdavis.edu
Joseph A. Sisneros, Departments of Psychology and Biology, University of Washington, Seattle, WA 98105, sisneros@u.washington.edu
Allison B. Coffin, Virginia Merrill Bloedel Hearing Research Center, Department of Otolaryngology-Head and Neck Surgery, University of Washington, Seattle, WA 98105, coffina@u.washington.edu.
References
- Blaxter JA, Fuiman LA. Function of the free neuromasts of marine teleost larvae. In: Coombs S, Gorner P, Munz H, editors. The Mechansensory Lateral line. Springer: Berlin Heidelberg New York; 1989. pp. 481–499. [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear. Res. 2009;253:42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear. Res. 2000;139:97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Greene CW. The phosphorescent organs of the toadfish, Porichthyus notatus. J. Morphol. 1899;15:667–696. [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J. Assoc. Res. Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaus S. The effect of aminoglycoside antibiotics on the lateral line organ of Aplocheilus lineatus (Cyprinodontidae) Acta Otolaryngol. 1987;103:291–298. doi: 10.3109/00016488709107796. [DOI] [PubMed] [Google Scholar]
- Kil J, Warchol ME, Corwin JT. Cell death, cell proliferation, and estimates of hair cell life spans in the vestibular organs of chicks. Hear. Res. 1997;114:117–126. doi: 10.1016/s0378-5955(97)00166-4. [DOI] [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J. Comp. Neurol. 1995;355:405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, Baker CF, Carton AG. The lateral line can mediate rheotaxis in fish. Nature. 1997;38:960–963. [Google Scholar]
- Montgomery JC, MacDonald F, Baker CF, Carton AG. Hydrodymanic contributions to multimodal guidance of prey capture behavior in fish. Brain Behav. Evol. 2002;59:190–198. doi: 10.1159/000064906. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, McDonald F, Baker CF, Carton AG, Ling N. Sensory integration in the hydrodynamic world of rainbow trout. Proc. R. Soc. Lond. 2003;270:195–197. doi: 10.1098/rsbl.2003.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Coffin AB, Hong LS, Bennett KO, Rubel EW, Raible DW. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear. Res. 2009;253:32–41. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PH, Zeddies DG. Multipole mechanisms for directional hearing in fish. In: Webb JF, Fay RR, Popper AN, editors; Fay RR, Popper AN, editors. Fish Bioacoustics. Springer Handbook of Auditory Research. Vol. 32. New York: Springer; 2008. (series eds) [Google Scholar]
- Song J, Yan HY, Popper AN. Damage and recovery of hair cells in the fish canal (but not superficial) neuromasts after gentamicin exposure. Hear. Res. 1995;91:63–71. doi: 10.1016/0378-5955(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear. Res. 2005;208:79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Van Bergeijk WA. Directional and nondirectional hearing in fish. In: Tavolga WN, editor. Marine Bio-acoustics. Oxford: Pergamon Press; 1964. pp. 281–299. [Google Scholar]
- Sand O, Bleckmann H. Auditory and lateral line stimuli. In: Webb JF, Fay RR, Popper AN, editors; Fay RR, Popper AN, editors. Fish Bioacoustics. Springer Handbook of Auditory Research. Vol. 32. New York: Springer; 2008. (series eds) [Google Scholar]