Abstract
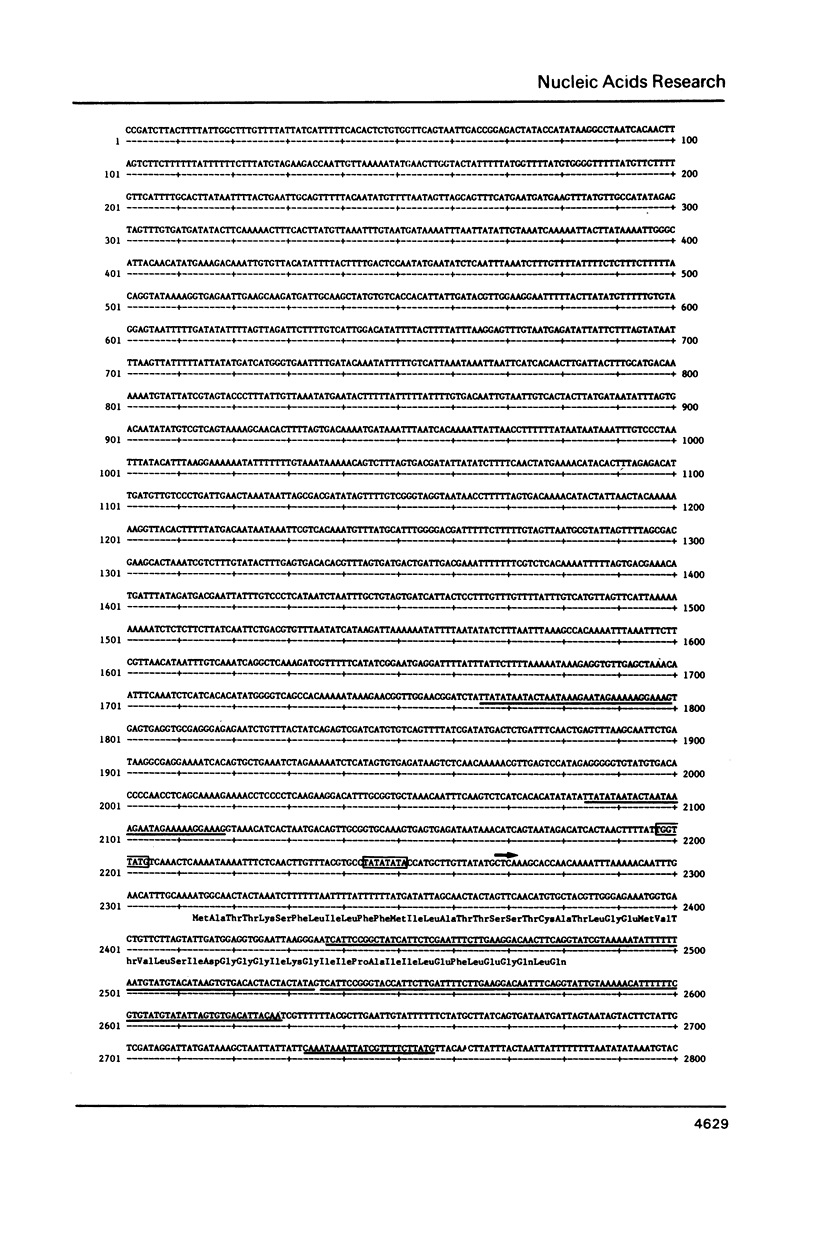
We have isolated recombinant lambda clones containing intact major tuber protein (patatin) genes and flanking sequences from the commercial tetraploid variety Maris Piper. The gene is composed of seven exons and six introns, spread over 4 kb of DNA. Nuclease mapping defined the 5' end of the mRNA approximately 45 bp upstream of the initiation codon. The 5' end of the gene is preceeded by a canonical TATA box sequence. The three known patatin genes encode proteins of nearly identical Mr but very different isoelectric points. The sequence of the gene does not indicate a role for patatin as one of the globulin class of plant storage proteins.
Full text
PDF






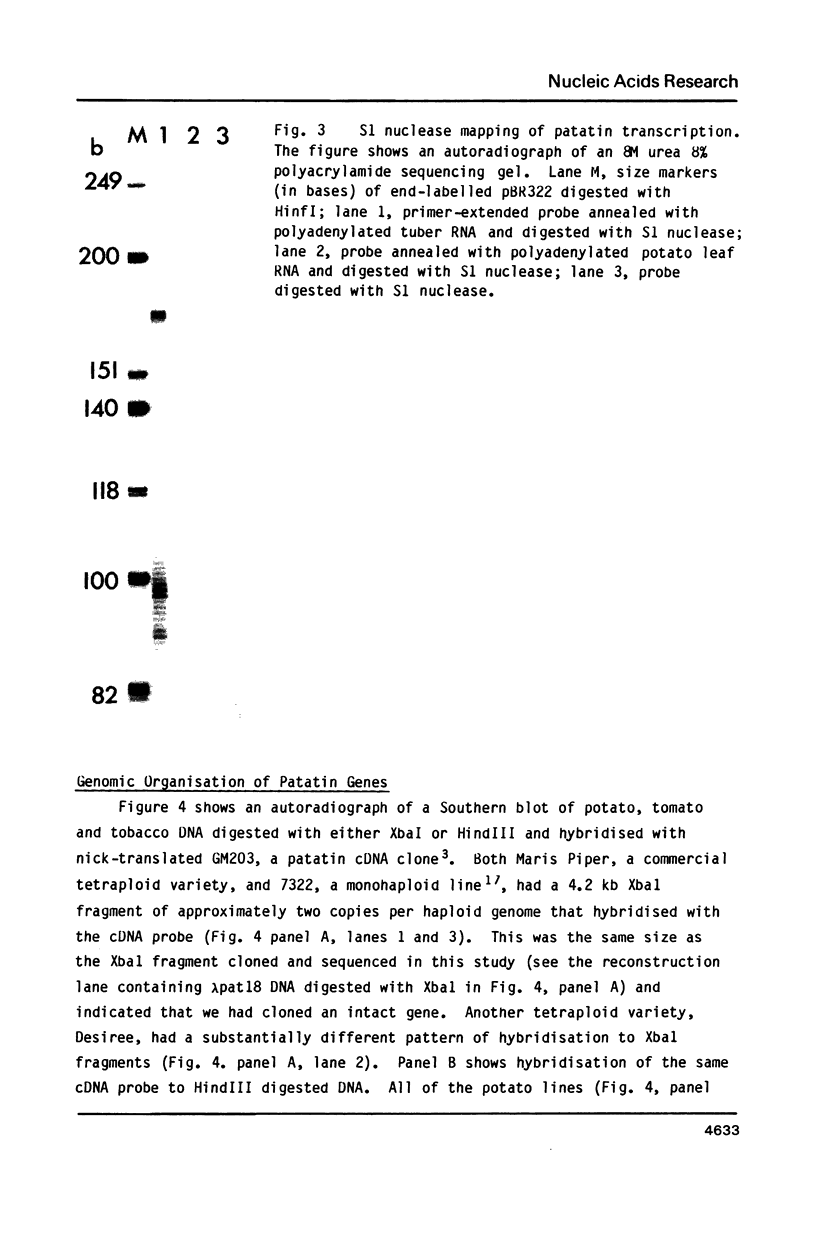
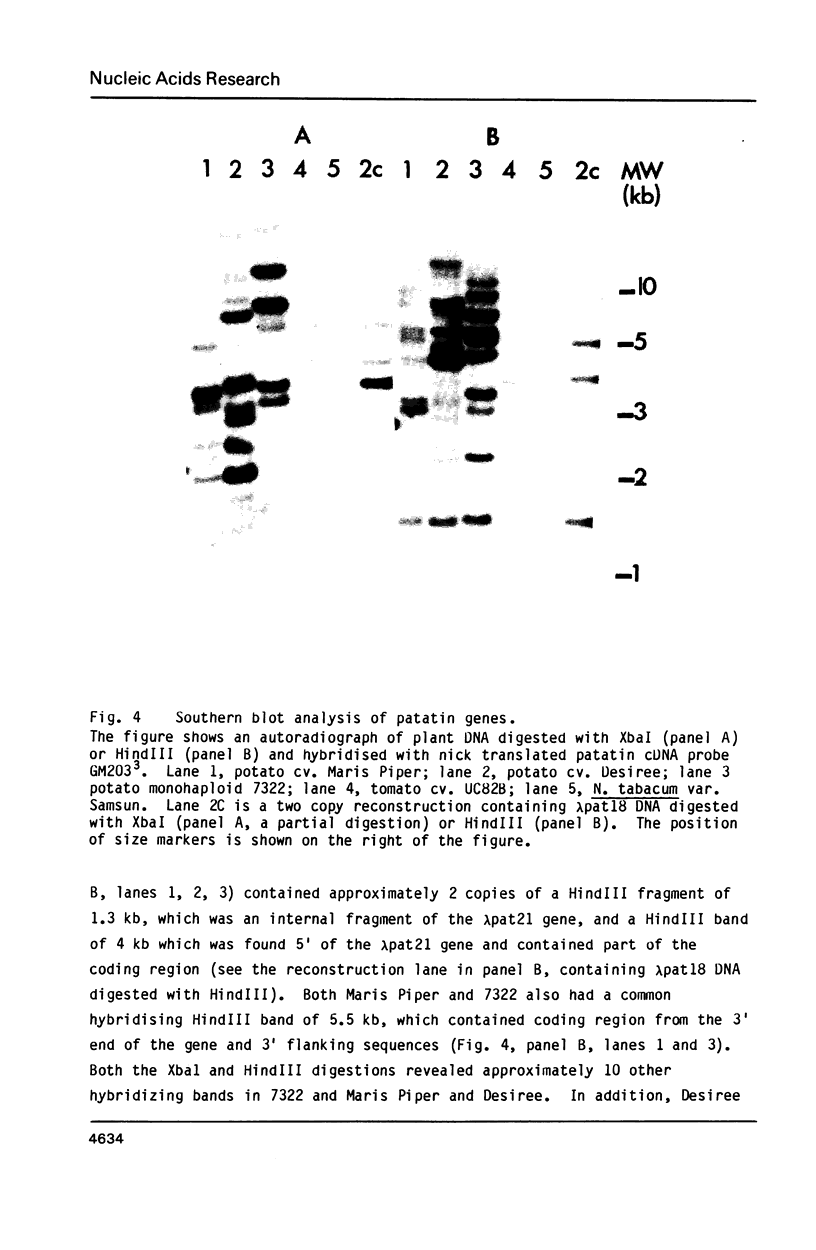




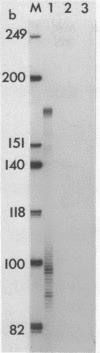
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Galliard T., Matthew J. A. Lipids of potato tubers. II. Lipid-degrading enzymes in different varieties of potato tuber. J Sci Food Agric. 1973 May;24(5):623–627. doi: 10.1002/jsfa.2740240516. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett G. W., Delauney A. J., Gatehouse J. A., Gilroy J., Croy R. R., Boulter D. The vicilin gene family of pea (Pisum sativum L.): a complete cDNA coding sequence for preprovicilin. Nucleic Acids Res. 1983 Apr 25;11(8):2367–2380. doi: 10.1093/nar/11.8.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Pikaard C. S., Hannapel D. J., Park W. D. Isolation and sequence analysis of cDNAs for the major potato tuber protein, patatin. Nucleic Acids Res. 1984 Nov 12;12(21):7987–8000. doi: 10.1093/nar/12.21.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. Molecular analysis of a cell lineage. Nature. 1983 Apr 21;302(5910):670–676. doi: 10.1038/302670a0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Paiva E., Lister R. M., Park W. D. Induction and accumulation of major tuber proteins of potato in stems and petioles. Plant Physiol. 1983 Jan;71(1):161–168. doi: 10.1104/pp.71.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W. D., Blackwood C., Mignery G. A., Hermodson M. A., Lister R. M. Analysis of the Heterogeneity of the 40,000 Molecular Weight Tuber Glycoprotein of Potatoes by Immunological Methods and by NH(2)-Terminal Sequence Analysis. Plant Physiol. 1983 Jan;71(1):156–160. doi: 10.1104/pp.71.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. The end of the message. Nature. 1982 Aug 5;298(5874):516–517. doi: 10.1038/298516a0. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Comparative biochemistry of plant glycoproteins. Biochem Soc Trans. 1979 Aug;7(4):783–799. doi: 10.1042/bst0070783. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Sun S. M., Hall T. C. Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1897–1901. doi: 10.1073/pnas.80.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]