Abstract
Background-
Hypoxia during the first week of life can induce neuronal death in vulnerable brain regions usually associated with an impairment of cognitive function that can be detected later in life. The neurobiological changes mediated through neurotransmitters and other signaling molecules associated with neonatal hypoxia are an important aspect in establishing a proper neonatal care.
Methods-
The present study evaluated total GABA, GABAB receptor alterations, gene expression changes in GABAB receptor and glutamate decarboxylase in the cerebellum and brain stem of hypoxic neonatal rats and the resuscitation groups with glucose, oxygen and epinephrine. Radiolabelled GABA and baclofen were used for receptor studies of GABA and GABAB receptors respectively and Real Time PCR analysis using specific probes for GABAB receptor and GAD mRNA was done for gene expression studies.
Results-
The adaptive response of the body to hypoxic stress resulted in a reduction in total GABA and GABAB receptors along with decreased GABAB receptor and GAD gene expression in the cerebellum and brain stem. Hypoxic rats supplemented with glucose alone and with oxygen showed a reversal of the receptor alterations and changes in GAD. Resuscitation with oxygen alone and epinephrine was less effective in reversing the receptor alterations.
Conclusions-
Being a source of immediate energy, glucose can reduce the ATP-depletion-induced changes in GABA and oxygenation, which helps in encountering hypoxia. The present study suggests that reduction in the GABAB receptors functional regulation during hypoxia plays an important role in central nervous system damage. Resuscitation with glucose alone and glucose and oxygen to hypoxic neonatal rats helps in protecting the brain from severe hypoxic damage.
Keywords: GABAB, neonatal hypoxia, cerebellum and brain stem
Background
Hypoxia is one of the most common reasons for neonatal morbidity and mortality, causing reduced oxygen supply to the vital organs [1] and injury to the developing brain [2-5]. The response of central nervous system to hypoxia is vital in revealing mechanisms that participate in coordinated behavior of respiratory and vasomotor activities [6,7].
The ventilatory response to acute hypoxia (hypoxic ventilatory response; HVR) in humans and some other mammalian species is biphasic [8,9]. The initial rise in ventilation (early phase of the HVR) is followed by a marked decline after several minutes to values above the prehypoxic level. This decline in ventilation has been termed "ventilatory roll-off" or "hypoxic ventilatory decline" (HVD). Several neurotransmitters and neuromodulators, such as γ-aminobutyric acid (GABA), [10-13] serotonin [14], adenosine, [15,16] and platelet-derived growth factor [17,18] play important roles in HVD. The alterations in neurotransmitter signaling in the respiratory control centers in brain stem and stressed breathing facilitating regions in cerebellar deep nuclei highly influence the ventilatory response of the body.
At synaptic transmission level, experimental hypoxia or hypoxia/ischemia increases the release of aminoacid neurotransmitters [19-23], causing an imbalance in normal activity of glutamatergic and GABAergic neurones, resulting in acute cell excitotoxicity. Endogenous GABA acting on GABAA or GABAB receptors modulates ventilation during room air breathing as well that the ventilatory response to acute and sustained hypoxia [24]. Rhythm generation in mature respiratory networks is influenced strongly by synaptic inhibition. Zhang et al, 2002 [24] reported that GABAB-receptor-mediated postsynaptic modulation plays an important role in the respiratory network from P0 on. GABAB-receptor-mediated presynaptic modulation develops with a longer postnatal latency, and becomes predominant within the first postnatal week [25].
GABAB receptors may contribute essentially to the modulation of respiratory rhythm in adult mammals and may be involved in the control of respiratory neuronal discharge [26]. GABA, which is metabolized in GABA shunts, is produced through α-decarboxylation of glutamic acid catalyzed by glutamate decarboxylase (GAD; EC 4.1.1.15) under the presence of cofactor pyridoxal 5'-phoshate. GAD, the rate limiting enzyme of GABA synthesis and a key protein in the GABA pathway, is used as a marker for GABAergic activity.
Thus, understanding the diagnosis, pathogenesis, resuscitation and treatment of those infants suffering hypoxic brain injury is paramount to reducing disability, improving survival and enhancing quality of life. Upon delivery, 5--10% of all newborns require some degree of resuscitation and assistance to begin breathing [27-29]. The aim of resuscitation is to prevent neonatal death and adverse long-term neurodevelopment sequelae associated with neonatal hypoxic event [30] and rapidly reverse fetal hypoxemia, and acidosis [31]. Debate regarding the optimal concentration of oxygen at initiation of resuscitation continues in the international community. The present study focused on understanding the alterations in GABA content, total GABA and GABAB receptors and GAD expression in the cerebellum and brain stem of hypoxic neonatal rats and the effects of various resuscitations on these alterations. The effectiveness of various resuscitation methods like administration of 100% oxygen and intravenous fluids like 10% glucose and 0.10 g/Kg body wt epinephrine alone and in combinations in the management of hypoxia was analyzed to understand the neuroprotective role of glucose supplementation. Understanding the molecular mechanisms involved in the regulation of neurotransmitter receptors will lead to better therapies for neonatal hypoxia-ischemia.
Materials and methods
Animals
Neonatal Wistar rats were purchased from Amrita Institute of Medical Sciences, Kochi. Neonatal rats of four days old were weighed and used for experiments. All groups of neonatal rat were maintained with their mothers under optimal conditions - 12 hour light and 12 hour dark periods and were fed standard food and water ad libitum. All animal care and procedures were taken in accordance with the institutional, National Institute of Health guidelines and CPCSEA guidelines.
Induction of Acute Hypoxia in Neonatal Rats
Wistar neonatal rats of 4-days old (body weight, 6.06 ± 0.45 g) were used for the experiments and were grouped into seven as follows: (i) Control neonatal rats were given atmospheric air (20.9% oxygen) for 30 minutes (C); (ii) Hypoxia was induced by placing the neonatal rats in a hypoxic chamber provided with 2.6% oxygen for 30 minutes (Hx); (iii) Hypoxic neonatal rats were injected 10% dextrose (500 mg/Kg body wt) intra-peritoneally (i.p.) (Hx+G). (iv) Hypoxic neonatal rats were supplied with 100% oxygen for 30 minutes (Hx+O); (v) Hypoxic neonatal rats were injected 10% dextrose (500 mg/Kg body wt. i.p.) and treated with 100% oxygen for 30 minutes (Hx+G+O). (vi) Hypoxic neonatal rats were injected 10% dextrose (500 mg/Kg body wt), epinephrine (0.1 μg/Kg body wt. i.p.) and treated with 100% oxygen for 30 minutes (Hx+G+E+O) (vii) Hypoxic neonatal rats were injected with epinephrine (0.10 g/Kg body wt) i.p. (Hx + E). The experimental animals were maintained in the room temperature for one week.
Tissue preparation
Control and experimental neonatal rats were sacrificed by decapitation. The cerebellum and brain stem were dissected out quickly over ice according to the procedure of Glowinski and Iversen, 1966 [32] and was stored at -80°C for various experiments.
Quantification of GABA content Using [3H]Radioligands
GABA content in the cerebellum and brain stem of control and experimental rat groups was quantified by displacement method of Kurioka et al, 1981 [33] where the incubation mixture contained 30 nM [3H]GABA with and without GABA at a concentration range of 10-8 M to 10-4 M. The unknown concentrations were determined from the standard displacement curve using appropriate dilutions and calculated for μ moles/gm wt. of the tissue
GABA Receptor Binding Assay
[3H] GABA binding to the GABA receptor was assayed in Triton X-100 treated synaptic membranes [33]. Crude synaptic membranes were prepared using sodium-free 10 mM tris buffer, pH 7.4. Each assay tube contained a protein concentration of 0.1 - 0.2 mg. In saturation binding experiments, 5 nM to 40 nM concentrations of [3H]GABA was incubated with and without excess of unlabelled GABA (100 μM) and in competition binding experiments the incubation mixture contained 30 nM of [3H] GABA with and without GABA at a concentration range of 10-8M to 10-4M were used.
GABAB Receptor Binding Assay
[3H] baclofen binding to the GABAB receptor was assayed in Triton X-100 treated synaptic membranes [33]. Crude synaptic membranes were prepared using sodium-free 10 mM tris buffer, pH 7.4. Each assay tube contained a protein concentration of 0.1 - 0.2 mg. In saturation binding experiments, 5 nM to 40 nM concentrations of [3H]baclofen was incubated with and without excess of unlabelled baclofen (100 μM) were used.
Protein was measured by the method of Lowry et al, 1951 [34] using bovine serum albumin as standard.
Linear regression analysis of the receptor binding data for Scatchard plots
The data was analysed according to Scatchard, 1949 [35]. The specific binding was determined by subtracting non-specific binding from the total. The binding parameters, maximal binding (Bmax) and equilibrium dissociation constant (Kd), were derived by linear regression analysis by plotting the specific binding of the radioligand on X-axis and bound/free on Y-axis. The maximal binding is a measure of the total number of receptors present in the tissue and the equilibrium dissociation constant is the measure of the affinity of the receptors for the radioligand. The Kd is inversely related to receptor affinity.
Nonlinear regression analysis for displacement curve
Competitive binding data was analyzed using non-linear regression curve-fitting procedure (GraphPad PRISM™, San Diego, USA). The data of the competitive binding assays were represented graphically with the log of concentration of the competing drug on x-axis and percentage of the radioligand bound on the y-axis. The steepness of the binding curve can be quantified with a slope factor, often called a Hill slope. A one-site competitive binding curve that follows the law of mass action has a slope of 1.0 and a two site competitive binding curve has a slope less than 1.0. The concentration of competitor that competes for half the specific binding was defined as EC50, which is same as IC50. The affinity of the receptor for the competing drug is designated as Ki and is defined as the concentration of the competing ligand that binds to half the binding sites at equilibrium in the absence of radioligand or other competitors.
Gene expression studies in cerebellum and brain stem
RNA was isolated from the cerebellum and brain stem using Tri reagent. Total cDNA synthesis was performed using ABI PRISM cDNA Archive kit. Real-Time PCR assays were performed in 96-well plates in an ABI 7300 Real-Time PCR instrument (Applied Biosystems, Foster City, CA, USA). PCR analyses were conducted with gene-specific primers and fluorescently labeled Taq probe for GABA B (Rn 00578911) and GAD1 (Rn 00690304_g1) designed by Applied Biosystems. Endogenous control (β-actin) labeled with a reporter dye was used as internal control. All reagents were purchased from Applied Biosystems. The real-time data were analyzed with Sequence Detection Systems software version 1.7. All reactions were performed in duplicate.
The ΔΔCT method of relative quantification was used to determine the fold change in expression. This was done by first normalizing the resulting threshold cycle (CT) values of the target mRNAs to the CT values of the internal control β-actin in the same samples (ΔCT = CT Target - CT β-actin). It was further normalized with the control (ΔΔCT = ΔCT - CT Control). The fold change in expression was then obtained (2-ΔΔCT).
Statistical analysis
The equality of all the groups was tested by the analysis of variance (ANOVA) technique for different values of p. Further the pair wise comparisons of all the experimental groups were studied using Students-Newman-Keuls test at different significance levels. The testing was performed using GraphPad Instat (Ver. 2.04a, San Diego, USA) computer program.
Results
GABA Content in the cerebellum and brain stem of control and experimental neonatal rats
The GABA content was decreased significantly (p < 0.001) in the cerebellum and brain stem of hypoxic neonatal rats compared to control. The decreased content was reversed to near normal in glucose supplemented groups - Hx + G and Hx + G + O (Table 1).
Table 1.
GABA Content (μmoles/g wet wt.) in cerebellum and brain stem of Control and Experimental Groups of Neonatal Rats
| Experimental groups | GABA Content (μmoles/g wet wt.) | |
|---|---|---|
| Cerebellum | Brain stem | |
| Control | 6.45 ± 1.2 | 8.45 ± 1.8 |
| Hx | 2.02 ± 1.0a | 4.06 ± 1.4a |
| Hx + G | 6.25 ± 1.4 b | 9.85 ± 2.2 b |
| Hx + G + O | 6.60 ± 1.4 b | 8.66 ± 1.4 b |
| Hx + O | 3.55 ± 1.8 b | 6.01 ± 1.5 b |
| Hx + E | 3.05 ± 1.2 a | 4.55 ± 1.6 a |
| Hx + G + E + O | 3.12 ± 1.1 a | 5.02 ± 1.4 a |
Values are Mean ± S.E.M of 4-6 separate experiments. Each group consist 6-8 rats.
a p < 0.001 when compared to Control
b p < 0.001, c p < 0.01 when compared to hypoxic group
Hypoxic rats- Hx, Hypoxic rats glucose treated - Hx+G, Hypoxic rats oxygen treated - Hx+O, Hypoxic rats glucose and oxygen treated - Hx+G+O, Hypoxic rats epinephrine treated - Hx + E, Hypoxic rats glucose, epinephrine and oxygen treated - Hx+G+E+O
Total GABA receptors in the cerebellum and brain stem of control and experimental neonatal rats
Receptor studies for total GABA showed a significant decrease in receptor number compared to control in the cerebellum and brain stem (p < 0.01, p < 0.001 respectively) of hypoxic neonatal rats. In glucose supplemented groups, Hx + G and Hx + G + O, the receptor number was reversed to near control (p < 0.001) in both the brain regions. Epinephrine supplemented groups, Hx + E and Hx + G + E + O, showed no significant reversal in the altered receptor number to control level. In Hx + O, the Bmax was significantly decreased (p < 0.001) compared to control (Table 2).
Table 2.
Total GABA receptor binding parameters in the cerebellum and brain stem of control and experimental neonatal rats.
| Experimental groups | Cerebellum | Brain stem | ||
|---|---|---|---|---|
| Bmax (fmoles/mg protein) | Kd (nM) | Bmax (fmoles/mg protein) | Kd (nM) | |
| Control | 71.50 ± 2.41 | 11.11 ± 0.95 | 153.36 ± 3.7 | 4.77 ± 0.44 |
| Hx | 50.01 ± 1.80 a | 14.82 ± 0.82 a | 116.68 ± 2.8 a | 3.77 ± 0.22 a |
| Hx + G | 62.18 ± 1.50 b | 9.85 ± 0.36 b | 173.36 ± 2.5 b | 6.78 ± 0.35 a, b |
| Hx + G + O | 66.33 ± 2.00 b | 12.54 ± 0.42 | 160.84 ± 3.4 b | 5.01 ± 0.26 a, b |
| Hx + O | 55.34 ± 2.50 a | 15.72 ± 0.54 a | 136.68 ± 2.3 a, b | 4.73 ± 0.29 b |
| Hx + E | 44.02 ± 3.20 a | 10.46 ± 0.10 b | 122.08 ± 2.6 a | 3.30 ± 0.14 a |
| Hx + G + E + O | 45.50 ± 2.50 a | 7.46 ± 0.11a, b | 125.84 ± 4.5 a | 4.10 ± 0.22 b |
Values are Mean ± S.E.M of 4-6 separate experiments. Each group consist 6-8 neonatal rats.
a p < 0.001 when compared with control
b p < 0.001 when compared with hypoxic group.
Hypoxic rats- Hx, Hypoxic rats glucose treated - Hx+G, Hypoxic rats oxygen treated - Hx+O, Hypoxic rats glucose and oxygen treated - Hx+G+O, Hypoxic rats epinephrine treated - Hx + E, Hypoxic rats glucose, epinephrine and oxygen treated - Hx+G+E+O
Non linear regression analysis of total GABA receptors in the cerebellum and brain stem
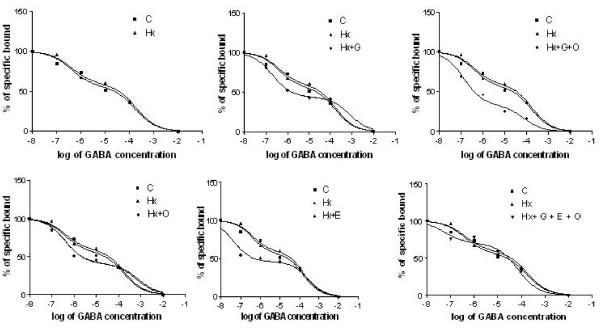
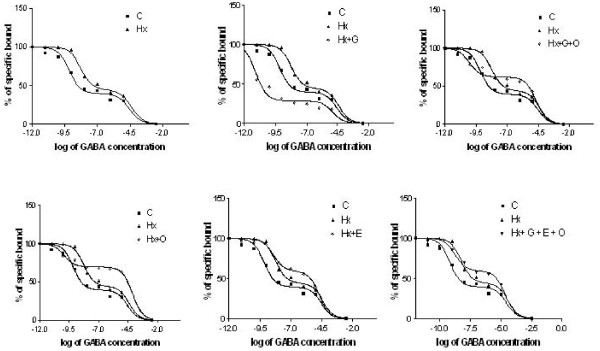
The binding data were confirmed by competition binding assay with [3H] GABA against different concentrations of GABA. GABA affinity in the cerebellum and brain stem of control and hypoxic neonatal rats fitted to a two site model with Hill slope value away from unity. GABA affinity of Hx + O, Hx + G, Hx + G + O, Hx + E and Hx + G + E + O also fitted to a two site model with Hill slope value away from unity. The Ki(H) increased in hypoxic neonatal rats along with an increase in the log (EC50)-1 indicating a shift in high affinity towards low affinity. Ki(L) also showed an increase in hypoxic neonatal rats with an increase in log (EC50)-2 denoting a shift in the low affinity site towards much lower affinity (Figure 1 &2).
Figure 1.
Displacement of [3H] GABA against GABA in cerebellum of control and experimental neonatal rats. Competition studies were carried out with 30 nM [3H] GABA in each tube with the unlabelled GABA concentrations varying from 10-8 to10-4 M. Values are representation of 4-6 separate experiments. Data from the curves as determined from nonlinear regression analysis using computer program PRISM fitted to a two-site model. The affinity for the first and second site for the competing drug is designated as Ki-1 (for high affinity) and Ki-2 (for low affinity). EC50 is the concentration of competitor that competes for half the specific binding. The equation built-in to the program is defined in terms of the log (EC50). If the concentrations of unlabelled compound are equally spaced on a log scale, the uncertainty of the log (EC50) will be symmetrical, but uncertainty of the EC50 will not be symmetrical
Figure 2.
Displacement of [3H] GABA against GABA in brain stem of control and experimental neonatal rats. Competition studies were carried out with 30 nM [3H] baclofen in each tube with the unlabelled baclofen concentrations varying from 10-12 to10-4 M. Values are representation of 4-6 separate experiments. Data from the curves as determined from nonlinear regression analysis using computer program PRISM fitted to a two-site model. The affinity for the first and second site for the competing drug is designated as Ki-1 (for high affinity) and Ki-2 (for low affinity). EC50 is the concentration of competitor that competes for half the specific binding. The equation built-in to the program is defined in terms of the log (EC50). If the concentrations of unlabelled compound are equally spaced on a log scale, the uncertainty of the log (EC50) will be symmetrical, but uncertainty of the EC50 will not be symmetrical.
GABAB receptors in the cerebellum and brain stem of control and experimental neonatal rats
GABAB receptors was significantly decreased (p < 0.001) with a significant increase in its affinity (p < 0.001, p < 0.05) in the cerebellum and brain stem of hypoxic neonatal rats compared to control. Hx + G and Hx + G + O showed a significant reversal of Bmax (p < 0.001) and Kd (p < 0.01) to near control in the cerebellum and a significant reversal of Bmax (p < 0.01, p < 0.001 respectively) to near control in the brain stem. In epinephrine and 100% oxygen supplemented groups, no reversal was observed (Table 3).
Table 3.
GABAB receptor binding parameters in the cerebellum and brain stem of control and experimental neonatal rats.
| Experimental groups | Cerebellum | Brain stem | ||
|---|---|---|---|---|
| Bmax (fmoles/mg protein) | Kd (nM) | Bmax (fmoles/mg protein) | Kd (nM) | |
| Control | 71.50 ± 2.41 | 11.11 ± 0.95 | 74.27 ± 1.20 | 13.31 ± 1.00 |
| Hx | 50.01 ± 1.80 a | 14.82 ± 0.82 a | 51.84 ± 1.50 a | 14.44 ± 0.99 b |
| Hx + G | 62.18 ± 1.50 b | 9.85 ± 0.36 b | 69.41 ± 1.40 b | 20.47 ± 0.99 a |
| Hx + G + O | 66.33 ± 2.00 b | 12.54 ± 0.42 | 70.47 ± 1.10 c | 26.10 ± 1.20 a |
| Hx + O | 55.34 ± 2.50 a | 15.72 ± 0.54 a | 49.10 ± 1.10 a | 16.36 ± 1.50 a |
| Hx + E | 44.02 ± 3.20 a | 10.46 ± 0.10 b | 43.59 ± 1.5 a | 14.53 ± 0.99 b |
| Hx + G + E + O | 45.50 ± 2.50 a | 7.46 ± 0.11a, b | 53.95 ± 1.5 a | 13.90 ± 0.99 b |
Values are Mean ± S.E.M of 4-6 separate experiments. Each group consist 6-8 neonatal rats.
a p < 0.001, b p < 0.05 when compared with control
c p < 0.001 when compared with hypoxic group.
Hypoxic rats- Hx, Hypoxic rats glucose treated - Hx+G, Hypoxic rats oxygen treated - Hx+O, Hypoxic rats glucose and oxygen treated - Hx+G+O, Hypoxic rats epinephrine treated - Hx + E, Hypoxic rats glucose, epinephrine and oxygen treated - Hx+G+E+O
Gene expression of GABAB receptor mRNA in the cerebellum and brain stem
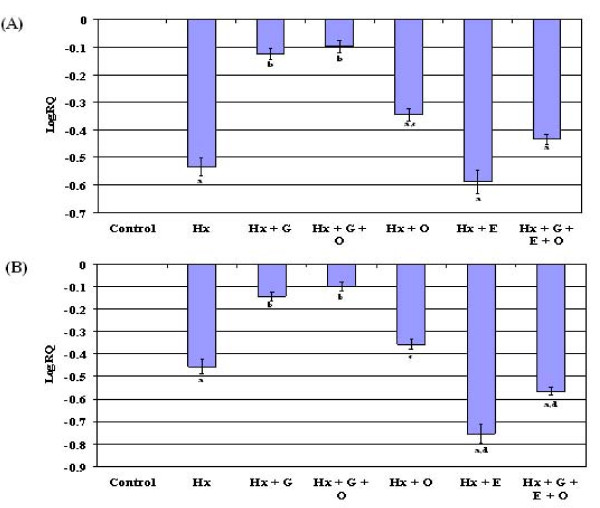
GABAB receptor mRNA was significantly down regulated (p < 0.001) in the cerebellum and brain stem of hypoxic neonatal rats compared to control. In the cerebellum, Hx + G, Hx + G + O and Hx + O showed a significant reversal of GABAB receptor expression (p < 0.001, p < 0.001 and p < 0.05 respectively) to near control where as epinephrine supplemented groups, Hx + E and Hx + G + E + O, showed no significant reversal of altered expression. In the brain stem, glucose supplemented groups, Hx + G, Hx + G + O, showed a significant reversal of the gene expression (p < 0.001) to near control, whereas Hx + O, Hx + E and Hx + G + E + O showed a down regulated GABAB receptor expression (p < 0.01, p < 0.001, p < 0.001 respectively) with out a significant reversal to near control (Figure 3).
Figure 3.
Real time PCR amplification of GABAB receptor subunit in mRNA form the cerebellum (A) and brain stem (B) of control and experimental neonatal rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. The relative ratios of mRNA levels were calculated using the ∆∆CT method normalized with β-actin. CT value as the internal control and Control CT value as the caliberator. PCR analyses were conducted in the cerebellum (A) and brain stem (B) with gene-specific primers and fluorescently labeled Taq probe GABAB (Rn 00578911)
Gene expression of GAD mRNA in the cerebellum and brain stem
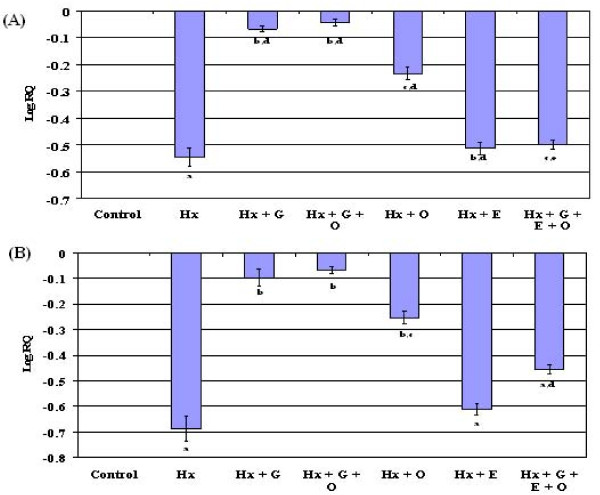
The expression of glutamate decarboxylase in cerebellum and brain stem also showed a significant down regulation (p < 0.001) in the hypoxic group compared to control. The cerebellar and brain stem GAD expression was significantly reversed to near control in Hx + G, Hx + G + O and Hx + O whereas in Hx + E and Hx + G + E + O, there was no significant reversal to near control (Figure 4).
Figure 4.
Real time PCR amplification of GAD mRNA form the cerebellum (A) and brain stem (B) of control and experimental neonatal rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. The relative ratios of mRNA levels were calculated using the ∆∆CT method normalized with β-actin. CT value as the internal control and Control CT value as the caliberator. PCR analyses were conducted in the cerebellum (A) and brain stem (B) with gene-specific primers and fluorescently labeled Taq probe GAD1 (Rn 00690304_g1).
Discussion
Hypoxia--ischemia (HI) occurring before or shortly after birth is a major cause of life-threatening injury and lifelong disability [36]. HI results in multi-organ failure and structural/functional damage especially devastating to the cardiovascular, renal, gastrointestinal and central nervous systems [37,38]. HI brain injury is very complex and has different neuropathological manifestations depending on the maturity of the newborn. Many of the structural changes that occur during the initial postnatal period in rodents are consistent with those seen during the late prenatal period in human brain development. Thus, exposure of rat to hypoxia on postnatal day 4 includes many of the neurodevelopmental events that may be affected by hypoxia in preterm human infants. In the present study, we investigated the functional regulation of GABAB receptors and GAD in hypoxic neonatal rats and the role of glucose, oxygen and epinephrine in altering the receptor status.
Numerous studies by different groups have confirmed that both inhibitory and excitatory amino acids are involved in the acute hypoxic ventilatory response [39-42]. Increases in GABA as a consequence of brain hypoxia can lead to depression of ventilation, which becomes more apparent in the absence of peripheral chemoreceptors. Blockade of GABA by biccuculine can significantly reduce this depressive effect of GABA on ventilation during hypoxia in chemodenervated animal or the newborn [43-45].
The present study reports a significant decrease in total GABA and GABAB receptor number with a down regulated receptor expression and glutamate decarboxylase expression in the cerebellum and brain stem regions of hypoxic neonatal rats. The decreased expression of GAD in turn results in the inhibition of GABA synthesizing pathway, which can be correlated to the decreased GABA receptors. The decreased GABA receptor is a response of the body to encounter hypoxic ventilatory decline. The reduction in GABAB receptor may help in overcoming the ventilatory decline during hypoxia but at the cost of severe central nervous system dysfunction. Louzoun-Kaplan et al, 2008 [46] reported that prenatal hypoxia at gestation day 17 in mice caused an immediate decrease in fetal cerebral cortex levels of glutamate decarboxylase. Decreased levels of key proteins in the GABA pathway in the cerebral cortex may lead to high susceptibility to seizures and epilepsy in newborns after prenatal or perinatal hypoxia. In the elevated plus maze, the agonist of GABA-B receptor was reported to improve consolidation of passive avoidance in rats undergoing hypoxia [47]. GABAB receptor-mediated activation of TASK-1 or a related channel provides a presynaptic autoregulatory feedback mechanism that modulates fast synaptic transmission in the rat carotid body [48]. The signaling cascade that triggers the altered transcription of GABA-B receptor and GAD under hypoxic stress can be related to the activation of apoptotic pathways by triggering Bax expression and deactivating CREB expression coupled with the activation of HIF. The accumulation of HIF-1α in ischemic or hypoxic tissues promote adaptive mechanisms for cell survival [49] and was found to be an important mediator of hypoxia-induced tolerance to ischemia [50]. Although HIF-1α is essential for adaptation to low oxygen levels, it has also been shown in vitro to mediate hypoxia-induced growth arrest and apoptosis [51]. The increased Hif 1 mRNA expression under hypoxia facilitates angiogenesis, vasodialation and erythropoiesis. But in severe hypoxic cases, HIF-1α is accumulated and leads to cell death by activating different target genes [52]. The role of HIF-1α in mediating pro death and pro survival responses, is dependent on the duration [53] and types of pathological stimuli [54] as well as the cell type that it induces [55].
We observed that glucose supplementation to hypoxic neonates alone and along with 100% oxygen showed a reversal in the altered GABAB receptor parameters and GAD expression in the cerebellum and brain stem. Glucose supplementation provides an instant source of energy to the brain cells thereby preventing ATP depletion mediated cell death. Hattori and Wasterlain, 2004 [56] observed a reduction in the blood glucose levels and substantially increased cerebral glucose utilization [57] as a result of hypoxic stress in experimental rats. Mónica Lemus et al, 2008 [58] reported that GABAB receptor agonist (baclofen) or antagonists (phaclofen and CGP55845A) locally injected into nucleus tractus solitarius modified arterial glucose levels and brain glucose retention.
The standard approach to resuscitation neonatal hypoxia is to use 100% O2. Further, resuscitation with 100% is recommended as a beneficial short-term therapy that is generally thought to be non-toxic [31,59]. Although the use of 100% O2 appears intuitive to maximize the gradient required to drive O2 into hypoxic cells [30], a building body of evidence derived from animal models, has demonstrated that although resuscitation with 100% O2 improves restoration of cerebral and cortical perfusion, it may occur at the price of greater biochemical oxidative stress [31]. Resuscitation with 100% O2 significantly increased glutamate and glycine in the dorsal cortex contralateral to the ligated common carotid artery, compared to piglets resuscitated with 21% O2. These data suggest that persistent changes in neurochemistry occur 4 days after hypoxic ischemia and further studies are warranted to elucidate the consequences of this on neonatal brain development [60]. We observed that 100% oxygen resuscitation for neonatal hypoxia is not as effective as the combination of glucose and oxygen or administration of glucose alone. In cerebellum and brain stem of 100% oxygen resuscitated groups, GABAB receptors showed a significant decrease compared to control. One hundred percentage of oxygen generated abnormally high levels of reactive oxygen species (ROS) which causes dysfunction of defensive antioxidant system of cells by altering enzyme activity [61,62] and act as a factor for neurodegeneration [63]. Hypoxemic piglets resuscitated with 100% O2 also showed increased cerebral injury, cortical damage and early neurologic disorders [64-66]. Previous studies on acetylcholinesterase [67], GABAA and serotonin receptors [68] reported the neuroprotective role of glucose and combination of glucose and oxygen resuscitation and the damaging effects of oxygen supplementation alone. The reduction in GABAB receptor number in the cerebellar and brain stem regions during oxygen supplementation is suggested to be due to tissue damage caused by the formation of free radicals or reactive oxygen species and the changes in amino acids resulting in neuronal cell death. During oxygen resuscitation, the accumulation of ROS activates the over stimulation of HIF 1 alpha which can in turn results in the activation of apoptotic pathways by altering the expression of transcription factors like CREB and NF-Kappa-B.
Epinephrine is routinely used in the resuscitation for persistent severe neonatal hypoxia. The present study points out the adverse effects of epinephrine supplementation, alone and even in combination with glucose and oxygen, by studying the changes in GABAB receptor, expression of GABAB receptor and GAD in the brain stem and cerebellum. The GABAB receptor was significantly decreased in epinephrine treated groups. A reflex action of epinephrine firing occurs during hypoxia. Supplementation of epinephrine to already excited system results in its hyper activity and it affects the balance of various neurotransmitters like dopamine [69] and glutamate. Epinephrine induces a hypoxia-neovascularization cascade and plays a primary role in vascular proliferation within soft tissues [70]. It is reported that repetitive hypoxic stress induced by labour is a powerful stimulus for catecholamine release in fetus and is accompanied by typical alterations of fetal heart rate. The high influx of this excitatory neurotransmitter affects the balance of other neurotransmitters thereby disrupting the cascade of signal transduction.
There has been much interest in the acute neurological changes associated with neonatal hypoxia, along with the mechanisms of subsequent central nervous system dysfunction in the adult [71-74]. Hypoxia during the first week of life can induce neuronal death in vulnerable brain regions usually associated with an impairment of cognitive function that can be detected later in life [75]. Postnatal hypoxia resulting from lung immaturity and respiratory disturbances in infants is an important pathophysiological mechanism underlying the devastating neurological complications. This points the importance of a proper resuscitation program to overcome neonatal hypoxia for a better intellect in the later stages of life.
Conclusions
Our studies point out the neuroprotective role of glucose in the management of neonatal hypoxic stress. The down regulated GABAB receptor in cerebellum and brain stem led to hypoxia induced ventilatory decline and activation of apoptotic pathways. These receptor alterations are reversed back to near control by the timely resuscitation with glucose, alone and in combination with oxygen. The deleterious effect of oxygen alone and epinephrine resuscitation in neuronal response through alterations in neurotransmitters was also observed. Thus it is suggested that glucose administration immediately after hypoxia with oxygenated air as a resuscitation programme will be of tremendous advantage especially in neonatal care. Deeper understanding of mechanisms, through which hypoxia regulates the neurotransmitters, could point towards the development of new therapeutic approaches to reduce or suppress the pathological effects of hypoxia.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TRA carried out the receptor assays, gene expression and drafted the manuscript. SJ participated participated in the design of the study and performed the statistical analysis. CSP conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Contributor Information
Thoppil R Anju, Email: anjutr@gmail.com.
Sadanandan Jayanarayanan, Email: anju_thoppil@yahoo.co.in.
Cheramadatikudiyil S Paulose, Email: biomncb@cusat.ac.in.
Acknowledgements
This work was supported by the research grants from DBT, DST, ICMR, Govt. of India and KSCSTE, Govt. of Kerala to Dr. C. S. Paulose. Anju T R thanks Council of Scientific and Industrial Research for Senior Research Fellowship.
References
- Low JA, Froese AB, Galbraith RS, Smith JT, Sauerbrei EE, Derrick EJ. The association between preterm newborn hypotension and hypoxemia and outcome during the first year. Acta Paediatrica. 1993;82:433–437. doi: 10.1111/j.1651-2227.1993.tb12717.x. [DOI] [PubMed] [Google Scholar]
- Delivoria-Papadopoulos M, Mishra POM. Mechanisms of perinatal cerebral injury in fetus and newborn. Annals of the New York Academy of Sciences. 2000;900:159–168. doi: 10.1111/j.1749-6632.2000.tb06226.x. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxicreoxygenation injury. American Journal of Physiology. 2002;282:227–241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Fernandez AP, Serrano J, Peinado MA, Martinez A. The role of free radicals in cerebral hypoxia and ischemia. Free Radical Biology and Medicine. 2005;39:26–50. doi: 10.1016/j.freeradbiomed.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Xu W, Chi L, Row BW. et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Acker T, Acker H. oxygen sensing need in CNS function: Physiological and pathological implications. Journal of Experimental Biology. 2004;207:3171–3188. doi: 10.1242/jeb.01075. [DOI] [PubMed] [Google Scholar]
- Solomon IC. Excitation of phrenic and sympathetic output during acute hypoxia: Contribution of medullary oxygen detectors. Respiration Physiology. 2000;121:101–117. doi: 10.1016/S0034-5687(00)00122-5. [DOI] [PubMed] [Google Scholar]
- Neubauer JA, Melton JE, Edelman NH. Modulation of respiration during brain hypoxia. J Appl Physiol. 1990;68:441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Weil JV, Zwillich CW. Assessment of ventilatory response to hypoxia. Chest. 1976;70(1):124–128. doi: 10.1378/chest.70.1.124. [DOI] [PubMed] [Google Scholar]
- Kneussl MP, Pappagianopoulos P, Hoop B, Kazemi H. Reversible depressiozn of ventilation and cardiovascular function by ventriculocisternal perfusion with γ-aminobutyric acid in dogs. Am Rev Respir Dis. 1986;133:1024–1028. doi: 10.1164/arrd.1986.133.6.1024. [DOI] [PubMed] [Google Scholar]
- Taveira da Silva AM, Hartley B, Hamosh P, Quest JA, Gillis RA. Respiratory depressant effects of GABA α- and β-receptor agonists in the cat. J Appl Physiol. 1987;62:2264–2272. doi: 10.1063/1.339481. [DOI] [PubMed] [Google Scholar]
- Kazemi H, Hoop B. Glutamic acid and gamma-aminobutyric acid neurotransmitters in central control of breathing. J Appl Physiol. 1991;70:1–7. doi: 10.1063/1.350309. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Neylon M, Marshall JM. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnazir B, Marshall JM, Kumar P. Postnatal development of the pattern of respiratory and cardiovascular response to systemic hypoxia in the piglet: the roles of adenosine. J Physiol. 1996;492:573–585. doi: 10.1113/jphysiol.1996.sp021330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Simakajornboon N, Czapla MA, Xue YD, Gozal E, Vlasic V, Lasky JA, Liu JY. Brainstem activation of platelet-derived growth factor-β receptor modulates the late phase of the hypoxic ventilatory response. J Neurochem. 2000;74:310–319. doi: 10.1046/j.1471-4159.2000.0740310.x. [DOI] [PubMed] [Google Scholar]
- Simakajornboon N, Kuptanon T. Maturational changes in neuromodulation of central pathways underlying hypoxic ventilatory response. Respir Physiol Neurobiol. 2005;149:273–286. doi: 10.1016/j.resp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Cataltepe O, Towfighi J, Vannucci RC. Cerebrospinal fluid concentrations of glutamate and GABA during perinatal cerebral hypoxia-ischemia and seizures. Brain Res. 1996;709:326–330. doi: 10.1016/0006-8993(95)01437-3. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Andersson P, Kjellmer I, Thiringer K, Thordstein M. Extracellular overflow of glutamate, aspartate, GABA and taurine in the cortex and basal ganglia of fetal lambs during hypoxia-ischemia. Neurosci Lett. 1987;78:311–317. doi: 10.1016/0304-3940(87)90379-X. [DOI] [PubMed] [Google Scholar]
- Rego AC, Santos MS, Oliveira CR. Oxidative stress, hypoxia, and ischemia-like conditions increase the release of endogenous amino acids by distinct mechanisms in cultured retinal cells. J Neurochem. 1996;66:2506–2516. doi: 10.1046/j.1471-4159.1996.66062506.x. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Enhanced GABA release in cell-damaging conditions in the adult and developing mouse hippocampus. Int J Devl Neurosci. 1997;15(2):163–174. doi: 10.1016/S0736-5748(97)80001-9. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Release of endogenous glutamate, aspartate, GABA, and taurine from hippocampal slices from adult and developing mice under cell-damaging conditions. Neuochem Res. 1998;23:563–570. doi: 10.1023/A:1022494921018. [DOI] [PubMed] [Google Scholar]
- Zhang W, Barnbrock A, Gajic S, Pfeiffer A, Ritter B. Differential ontogeny of GABAB-receptor-mediated pre- and postsynaptic modulation of GABA and glycine transmission in respiratory rhythm-generating network in mouse. The Journal of Physiology. 2002;540:435–446. doi: 10.1113/jphysiol.2001.013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Tetsuka M, Endo M. GABA(B) receptors in the nucleus tractus solitarii modulate the carotid chemoreceptor reflex in rats. Neurosci Lett. 1999;260:21–24. doi: 10.1016/S0304-3940(98)00942-2. [DOI] [PubMed] [Google Scholar]
- Yang AL, Lo MJ, Ting H, Chen JS, Huang CY, Lee SD. GABAA and GABAB receptors differentially modulate volume and frequency in ventilatory compensation in obese Zucker rats. J Appl Physiol. 2007;102:350–357. doi: 10.1152/japplphysiol.01463.2005. [DOI] [PubMed] [Google Scholar]
- Schubert S, Brandl U, Brodhun M, Ulrich C, Spaltmann J, Fiedler N, Bauer R. Neuroprotective effects hypoxia--ischemia in newborn piglets. Brain Res. 2005;1058:129–136. doi: 10.1016/j.brainres.2005.07.061. [DOI] [PubMed] [Google Scholar]
- Davis PG, Tan A, O'Donnell CPF, Schulze A. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet. 2004;364:1329–33. doi: 10.1016/S0140-6736(04)17189-4. [DOI] [PubMed] [Google Scholar]
- Tan A, Schulze A, O'Donnell CPF, Davis PG. Air versus oxygen for resuscitation of infants at birth. The cochrane database of systemic reviews 2006. 2005;2 doi: 10.1002/14651858.CD002273.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corff KE, McCann DL. Room air resuscitation versus oxygen resuscitation in the delivery room. J Perinat Neonat Nurs. 2005;19:379–90. doi: 10.1097/00005237-200510000-00013. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Walsh MC, Carlo WA. Reevaluating neonatal resuscitation with 100% oxygen. Am J Respir Crit Care Med. 2005;172:1360. doi: 10.1164/rccm.2509002. [DOI] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain: The disposition of [3H] Norepinephrine, [3H] DOPA in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Kurioka S, Toshiaki K, Makoto M. Effects of sodium and bicarbonate ions on gamma amino butyric acid receptor binding in synaptic membranes of rat brain. J Neurochem. 1981;37:418–421. doi: 10.1111/j.1471-4159.1981.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall J. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. doi: 10.1111/j.1749-6632.1949.tb27297.x. [DOI] [Google Scholar]
- du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol. 2002;15:151–7. doi: 10.1097/00019052-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Shah P, Riphagen S, Beyene J, Perlman JM. Multiorgan dysfunction in infants with post-asphyxial hypoxic--ischemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89:F152–5. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento M, Sastre J, Asensi MA, Vina J. Room-air resusciatation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med. 2005;172:1393–8. doi: 10.1164/rccm.200412-1740OC. [DOI] [PubMed] [Google Scholar]
- Mitra J, Prabhakar NR, Overholt JL, Cherniack NS. Respiratory effects of N-methyl-D-aspartate on the ventrolateral medullary surface. J Appl Physiol. 1989;67:1814–1819. doi: 10.1152/jappl.1989.67.5.1814. [DOI] [PubMed] [Google Scholar]
- Lin J, Suguihara C, Huang J, Hehre D, Devia C, Bancalari E. Effect of N-methyl-D-aspartate-receptor blockage on hypoxic ventilatory response in unanesthetized piglets. J Appl Physiol. 1996;80:1759–1763. doi: 10.1152/jappl.1996.80.5.1759. [DOI] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Torres JE, Gozal YM, Nuckton TJ, Hornby PJ. Nitric oxide modulates ventilatory responses to hypoxia in the developing rat. Am J Respir Crit Care Med. 1997;155:1755–1762. doi: 10.1164/ajrccm.155.5.9154888. [DOI] [PubMed] [Google Scholar]
- Gozal D, Graff GR, Torres JE, Khicha SG, Nayak GS, Simakajornboon N, Gozal E. Cardiorespiratory responses to systemic administration of a protein kinase C inhibitor in conscious rats. J Appl Physiol. 1998;84:641–648. doi: 10.1063/1.368090. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Lipski J. The role of inhibitory amino acids in control of respiratory motor output in an arterially perfused rat. Respir Physiol. 1992;89:47–63. doi: 10.1016/0034-5687(92)90070-D. [DOI] [PubMed] [Google Scholar]
- Huang J, Suguihara C, Hehre D, Lin J, Bancalari E. Effects of GABA receptor blockage on the respiratory response to hypoxia in sedated newborn piglets. J Appl Physiol. 1994;77:1006–1010. doi: 10.1152/jappl.1994.77.2.1006. [DOI] [PubMed] [Google Scholar]
- Soto-Arape I, Burton MD, Kazemi H. Central amino acid neurotransmitters and the hypoxic ventilatory response. Am J Respir Crit Care Med. 1995;151:1113–1120. doi: 10.1164/ajrccm/151.4.1113. [DOI] [PubMed] [Google Scholar]
- Louzoun-Kaplan V, Zuckerman M, Regino Perez-Polo J, Golan HM. Prenatal hypoxia down regulates the GABA pathway in newborn mice cerebral cortex; partial protection by MgSO4. International Journal of Developmental Neuroscience. 2008;26:77–85. doi: 10.1016/j.ijdevneu.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Car H, Oksztel R, Nadlewska A, Wi K. Baclofen prevents hypoxia-induced consolidation impairment for passive avoidance in rats. Pharmacological Research. 2001;44:329–335. doi: 10.1006/phrs.2001.0868. [DOI] [PubMed] [Google Scholar]
- Fearon IM, Zhang M, Vollmer C, Nurse CA. GABA mediates autoreceptor feedback inhibition in the rat carotid body via presynaptic GABAB receptors and TASK-1. The Journal of Physiology. 2003;553:83–94. doi: 10.1113/jphysiol.2003.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48:285–296. doi: 10.1002/1531-8249(200009)48:3<285::AID-ANA2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003;23:359–369. doi: 10.1128/MCB.23.1.359-369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- Halterman MW, Federoff HJ. HIF-1alpha and p53 promote hypoxia-induced delayed neuronal death in models of CNS ischemia. Exp Neurol. 1999;159:65–72. doi: 10.1006/exnr.1999.7160. [DOI] [PubMed] [Google Scholar]
- Aminova LR, Chavez JC, Lee J, Ryu H, Kung A, Lamanna JC, Ratan RR. Prosurvival and prodeath effects of hypoxia-inducible factor-1alpha stabilization in a murine hippocampal cell line. J Biol Chem. 2005;280:3996–4003. doi: 10.1074/jbc.M409223200. [DOI] [PubMed] [Google Scholar]
- Vangeison G, Carr D, Federoff HJ, Rempe DA. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci. 2008;28:1988–1993. doi: 10.1523/JNEUROSCI.5323-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori H, Wasterlain CG. Posthypoxic glucose supplement reduces hypoxicischemic brain damage in the neonatal rat. Ann Neurol. 2004;28:122–128. doi: 10.1002/ana.410280203. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3749–3154. doi: 10.1242/jeb.01209. [DOI] [PubMed] [Google Scholar]
- Lemus Mónica, Montero S, Cadenas JL, Lara JJ, Tejeda-Chávez HR, Álvarez-Buylla R, de Álvarez-Buylla Elena Roces. GabaB receptors activation in the NTS blocks the glycemic responses induced by carotid body receptor stimulation. Autonomic Neuroscience. 2008;141:73–82. doi: 10.1016/j.autneu.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Kuisma M, Boyd J, Voipio V, Alaspaa A, Roine RO, Rosenberg P. Comparison of 30 and the 100% inspired oxygen concentrations during early post-resuscitation period: a randomised controlled pilot study. Resuscitation. 2006;69:199–206. doi: 10.1016/j.resuscitation.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Lauren LJ, Po-Yin C, Obaid L, Emara M, Johnson ST, Bigam DL, Todd KG. Persistent neurochemical changes in neonatal piglets after hypoxia-ischemia and resuscitation with 100%, 21% or 18% oxygen. Resuscitation. 2008;77:111–120. doi: 10.1016/j.resuscitation.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay U, Das D, Ranajit K, Banerjee V. Reactive oxygen species: Oxidative damage and pathogenesis. Current Science. 1999;77:658–666. [Google Scholar]
- Anju TR, Athira B, Paulose CS. Superoxide dismutase functional regulation in neonatal hypoxia: Effect of glucose, oxygen and epinephrine. Indian J Biochem Biophys. 2009;46:166–171. [PubMed] [Google Scholar]
- Matharan TS, Laemmel E, Duranteau J, Vicaut E. After hypoxia and glucose depletion causes reactive oxygen species production by mitochondria in HUVEC. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2004;287:R1037–R1043. doi: 10.1152/ajpregu.00048.2004. [DOI] [PubMed] [Google Scholar]
- Temesvari P, Karg E, Bódi I, Németh I, Pintér S, Lazics K. Impaired early neurologic outcome in newborn piglets reoxygenated with 100% oxygen compared with room air after pneumothorax-induced asphyxia. Pediatric Research. 2001;49:812–819. doi: 10.1203/00006450-200106000-00017. [DOI] [PubMed] [Google Scholar]
- Munkeby BH, Borke WB, Bjornland K, Sikkeland LL, Borge GL, Halvorsen B, Saugstad OD. Resuscitation with 100% O2 increases cerebral injury in hypoxemic piglets. Pediatric Research. 2004;56(5):783–790. doi: 10.1203/01.PDR.0000141988.89820.E3. [DOI] [PubMed] [Google Scholar]
- Shimabuku R, Ota A, Pereyra S, Veliz B, Paz E, Nakachi G. Hyperoxia with 100% oxygen following hypoxia-ischemia increases brain damage in newborn rats. Biology of Neonate. 2005;88:168–171. doi: 10.1159/000086206. [DOI] [PubMed] [Google Scholar]
- Chathu F, Krishnakumar A, Paulose CS. Acetylcholine esterase activity and behavioral response in hypoxia induced neonatal rats: Effect of glucose, oxygen and epinephrine supplementation. Brain and cognition. 2008;68:59–66. doi: 10.1016/j.bandc.2008.02.124. [DOI] [PubMed] [Google Scholar]
- Anju TR, Jobin M, Jayanarayanan S, Paulose CS. Cerebellar 5-HT2A receptor function under hypoxia in neonatal rats: Role of glucose, oxygen, and epinephrine resuscitation. Respir Physiol Neurobiol. 2010;172(3):147–153. doi: 10.1016/j.resp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Binoy J, Nandhu MS, Paulose CS. Dopamine D (1) and D(2) receptor functional down regulation in the cerebellum of hypoxic neonatal rats: Neuroprotective role of glucose and oxygen, epinephrine resuscitation. Pharmacological Research. 2009. [DOI] [PubMed]
- Karacaoglu E, Bayram I, Celiköz B, Zienowicz RJ. Does sustained epinephrine release trigger a hypoxia-neovascularization cascade? Plast Reconstr Surg. 2007;119:858–64. doi: 10.1097/01.prs.0000252004.78314.56. [DOI] [PubMed] [Google Scholar]
- Soulier V, Peyronnet J, Pequignot JM, Cottet-Emard JM, Lagercrantz H, Dalmaz Y. Long-term impairment in the neurochemical activity of the sympathoadrenal system after neonatal hypoxia in the rat. Pediatr Res. 1997;42:30–38. doi: 10.1203/00006450-199707000-00006. [DOI] [PubMed] [Google Scholar]
- Peterson BS. Brain imaging studies of the anatomical and functional consequences of preterm birth for human brain development. Ann NY Acad Sci. 2003;1008:219–237. doi: 10.1196/annals.1301.023. [DOI] [PubMed] [Google Scholar]
- Lindahl E, Michelsson K, Helenius M, Parre M. Neonatal risk factors and later neurodevelopmental disturbances. Dev Med Child Neurol. 1988;30:571–589. doi: 10.1111/j.1469-8749.1988.tb04795.x. [DOI] [PubMed] [Google Scholar]
- Berg AT. Childhood neurological morbidity and its association with gestational age, intrauterine growth retardation and perinatal stress. Paediatr Perinat Epidemiol. 1988;2:229–238. doi: 10.1111/j.1365-3016.1988.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Casolini P, Zuena AR, Cinque C. Sub-neurotoxic neonatal anoxia induces subtle behavioural changes and specific abnormalities in brain group-I metabotropic glutamate receptors in rats. J Neurochem. 2005;95:137–145. doi: 10.1111/j.1471-4159.2005.03349.x. [DOI] [PubMed] [Google Scholar]