Abstract
Background
Evidence suggests that there is widespread decline in male reproductive health and that antiandrogenic pollutants may play a significant role. There is also a clear disparity between pesticide exposure and data on endocrine disruption, with most of the published literature focused on pesticides that are no longer registered for use in developed countries.
Objective
We used estimated human exposure data to select pesticides to test for antiandrogenic activity, focusing on highest use pesticides.
Methods
We used European databases to select 134 candidate pesticides based on highest exposure, followed by a filtering step according to known or predicted receptor-mediated antiandrogenic potency, based on a previously published quantitative structure–activity relationship (QSAR) model. In total, 37 pesticides were tested for in vitro androgen receptor (AR) antagonism. Of these, 14 were previously reported to be AR antagonists (“active”), 4 were predicted AR antagonists using the QSAR, 6 were predicted to not be AR antagonists (“inactive”), and 13 had unknown activity, which were “out of domain” and therefore could not be classified with the QSAR (“unknown”).
Results
All 14 pesticides with previous evidence of AR antagonism were confirmed as antiandrogenic in our assay, and 9 previously untested pesticides were identified as antiandrogenic (dimethomorph, fenhexamid, quinoxyfen, cyprodinil, λ-cyhalothrin, pyrimethanil, fludioxonil, azinphos-methyl, pirimiphos-methyl). In addition, we classified 7 compounds as androgenic.
Conclusions
Due to estimated antiandrogenic potency, current use, estimated exposure, and lack of previous data, we strongly recommend that dimethomorph, fludioxonil, fenhexamid, imazalil, ortho-phenylphenol, and pirimiphos-methyl be tested for antiandrogenic effects in vivo. The lack of human biomonitoring data for environmentally relevant pesticides presents a barrier to current risk assessment of pesticides on humans.
Keywords: antiandrogen, AR-Lux, biomonitoring, endocrine disruption, fungicide
Evidence suggests that prenatal and early-life exposure to pesticides may be causative factors in a variety of human disorders. For example, a meta-analysis by Wigle et al. (2009) showed that maternally exposed offspring have increased risk of childhood leukemia [odds ratio = 2.64; 95% confidence interval (CI), 1.4–5].
There are also indications that reproductive abnormalities, expressed as cryptorchidism, hypospadias, and decreased penile length, may be linked to pesticide exposure, most strikingly in maternally exposed boys (Andersen et al. 2008; Damgaard et al. 2006; Rocheleau et al. 2009). This is significant because male fertility is thought to be declining in many countries (Andersson et al. 2008), and perinatal hypospadias/cryptorchidism are risk factors for reduced sperm quality and testicular cancer in adulthood (Skakkebaek et al. 2001). Banned persistent organochlorines [p,p′-DDT (1,1,1-trichloro-2-[o-chlorophenyl]-2,2-[p-chlorophenyl]ethane), p,p′-DDE (p,p′-1,1-bis-(4-chlorophenyl)-2,2-dichloroethene), β-hexachlorocyclohexane, hexachlorobenzene, α-endosulfan, cis-heptachloroepoxide, oxychlordane, dieldrin] were detected in all samples of breast milk in a case–control study of mothers in Denmark and Finland. Also, levels were significantly higher in samples from mothers of sons with cryptorchidism than in samples from matched controls (1997–2001; Damgaard et al. 2006). Female Danish greenhouse workers exposed to current-use pesticides were more likely to give birth to a son with cryptorchidism than were a random sample of mothers from the Copenhagen area (6.2% and 1.9%). Furthermore, sons of mothers who directly handled treated plants or were engaged in spraying pesticides had significantly smaller penises than did sons of mothers who had noncontact roles in the greenhouse industry (Andersen et al. 2008). Last, in a recent meta-analysis of studies from the United States and Europe, Rocheleau et al. (2009) reported that maternal occupational exposure to pesticides was associated with a 36% increased risk of hypospadias relative to the risk in mothers without exposure (risk ratio = 1.36; 95% CI, 1.04–1.77). The risk of developing cryptorchidism (Pierik et al. 2004) and hypospadias (Brouwers et al. 2007) was also associated with paternal exposures to pesticides, mainly in greenhouses for the production of vegetables and flowers.
The term “testicular dysgenesis syndrome” (TDS) has been proposed to explain the interrelated nature of these abnormalities (Skakkebaek et al. 2001). It is conceivable that estrogenic and/or antiandrogenic contaminants play a role in TDS. Experimental studies with rats have shown that maternal exposure to flutamide (a pharmaceutical antiandrogen) affects androgen-dependent developmental outcomes such as anogenital distance and nipple retention (McIntyre et al. 2001). However, ethinylestradiol has not been shown to affect these end points (Howdeshell et al. 2008). Furthermore, hormone receptor screening in vitro suggests a preponderance of antiandrogenic activity compared with estrogenic activity in nonorganochlorine (current-use) pesticides. For example, Kojima et al. (2004) screened 161 pesticides and reported that 52 were antiandrogenic, whereas only 29 were estrogenic, and Orton et al. (2009) reported that 6 of 12 pesticides screened were antiandrogenic and none were estrogenic. There is a good correlation between androgen receptor (AR) antagonist properties and in vivo antiandrogenic effects, and there is also good evidence that androgen-sensitive end points are demasculinized in male rats when exposed in utero to a wide range of pesticides. Antiandrogenic effects both in vitro and via maternal exposure in vivo have been reported in response to the herbicide linuron (Gray et al. 1999; Lambright et al. 2000); the fungicides prochloraz (Vinggaard et al. 2005), procymidone (Ostby et al. 1999), tebuconazole (Taxvig et al. 2007), and vinclozolin (Anway et al. 2006; Uzumcu et al. 2004); the organochlorine insecticides DDE (Gray et al. 1999) and endosulfan (Sinha et al. 2001); the organophosphate dimethoate (Verma and Mohanty 2009); and the pyrethroid insecticide deltamethrin (Andrade et al. 2002). However, with the exception of linuron, dimethoate, deltamethrin, and tebuconazole, the pesticides listed above have not been authorized for use in Europe during the past 5 years, which should result in lower occupational, residential, and dietary exposures. Endocrine-relevant data on current use pesticides is minimal—and in some cases completely absent—with most of the published literature focused on pesticides that are no longer registered for use.
Therefore, the aim of this study was to test the antiandrogenic activity of currently used pesticides, with a view to informing future studies to determine their likely role in causing TDS. We selected compounds for testing based on evidence of human exposure (dietary intake data for Europe) and predicted AR antagonism according to the quantitative structure–activity relationship (QSAR) model developed by Vinggaard et al. (2008). Compounds predicted to be AR antagonists and compounds with high exposure scores were analyzed for AR antagonist properties using the MDA-kb2 assay (Ermler et al. 2010; Wilson et al. 2002). In addition, we used the yeast antiandrogen screen (YAS) to further test a subset of pesticides that were newly identified as AR antagonists or that had MDA-kb2 assay results that were discordant with QSAR predictions.
Materials and Methods
Test compound selection
Pesticides were selected using a combination of exposure scores and data about receptor-mediated antiandrogenic activity [see Supplemental Material, Figure 1 (doi:10.1289/ehp.1002895)]. First, we identified 134 pesticides with data suggesting relevant human exposures, including 58 pesticides identified at the highest concentrations and most frequently in European foods (European Commission 2008); 30 additional pesticides with relatively high daily dietary intakes (> 0.0004 μg/kg/day) identified by the FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) Joint Meeting on Pesticide Residues (JMPR) (FAO/WHO 2011); 44 additional pesticides identified in > 0.4% of fruits and vegetables during routine testing [European Food Safety Authority (EFSA) 2009]; and o,p′- and p,p′-DDE, which we included because of known adipose tissue levels (Fernández et al. 2004). Each pesticide was assigned four scores, with each ranging from 1 to 10: a) maximum food residue level (European Commission 2008); b) estimated daily dietary intake (FAO/WHO 2011); c) frequency of detection in fruits and vegetables (EFSA 2009), with a score of 5 assigned when data were not available; and d) a score according to the number of times pesticides were listed as one of the top 10 pesticides identified in fruits and cereals in Europe (a frequency score), with a score of 0 assigned if they were never listed (European Commission 2008). The four scores were summed to generate a “total exposure score,” with a maximum possible score of 40 (see Supplemental Material, Table 1).
The second stage of compound selection for testing was an assessment of in vitro evidence of AR interaction in the available literature (Andersen et al. 2002; Bauer et al. 2002; Kojima et al. 2004; Okubo et al. 2004; Orton et al. 2009; Vinggaard et al. 2008). Compounds previously shown not to be AR antagonists in vitro (n = 43) were removed from the list, which reduced the number of candidate pesticides from 134 to 91. Compounds previously reported to be AR antagonists (n = 27) were removed if the ratio of their total exposure score to their published IC20 [concentration that inhibits the androgenicity of DHT by 20%; total exposure score/published IC20 = “environmental relevance ratio” (ERR)] was < 3 (ERR was recalculated using our experimental data after the selection process). This left 14 previously reported AR antagonists for testing by the MDA-kb2 assay. For pesticides without published data (n = 64), AR antagonist activity was predicted using the QSAR developed by Vinggaard et al. (2008). These pesticides were tested using the MDA-kb2 assays if they were predicted to have AR antagonist activity (n = 4) or if they had high exposure scores (> 8) regardless of their QSAR status, including 6 pesticides that were predicted not to have AR antagonist activity and 13 pesticides that could not be predicted because they were out of the domain of the QSAR model. In total, 37 compounds were selected for testing in the MDA-kb2 assay. Finally, 8 pesticides that were newly described as highly active antiandrogens in the MDA-kb2 assay and 4 pesticides for which the QSAR prediction differed from the experimental result (including 1 out of the model domain) were subjected to further testing using the YAS (n = 14). For a summary of the selection process, see Supplemental Material, Figure 1 (doi:10.1289/ehp.1002895).
Chemicals
Dihydrotestosterone (DHT; > 97% purity) was purchased from Steraloids Ltd. (Croydon, Surrey, UK); novaluron, dimethomorph, p,p′-DDE, methiocarb, and indoxacarb were purchased from Greyhound Chromatography and Allied Chemicals (all > 98.7% pure; Birkenhead, Merseyside, UK); and all other pesticides (all > 97% pure) were purchased from Sigma Aldrich (Poole, Dorset, UK). Ethanol (> 99.7% purity) was obtained from VWR International Ltd. (Leicestershire, UK). All test compounds were dissolved in ethanol to make stock solutions to be used in the assays.
MDA-kb2 assay
MDA-kb2 cells are human breast cancer cells stably transfected with a firefly luciferase reporter gene that is driven by an androgen-response element–containing promoter (Wilson et al. 2002). Details of the modified assay were published previously (Ermler et al. 2010). Briefly, cells were seeded at a concentration of 1 × 105 cells/mL in phenol red–free Leibowitz-15 medium (Invitrogen Ltd., Paisley, UK) containing 10% (charcoal-stripped) fetal calf serum (Invitrogen Ltd.) in white luminometer plates and allowed to attach for 24 hr. Cells were then exposed to eight serial dilutions of selected pesticides with or without DHT (0.25 nM). After 24 hr, luciferase activity was determined with SteadyGlo assay reagent (Promega UK Ltd., Southampton, Hampshire, UK) and measured in a plate reader (FLUOstar Optima, BMG Labtech GmbH, Offenburg, Germany). The following controls were run on each plate: media, ethanol, DHT coexposure (0.25 nM), DHT serial dilutions (0.002–10 nM), and flutamide (0.013–8 μM) or procymidone (0.005–3.2 μM) serial dilutions. All concentrations were tested in duplicate over two plates, and each pesticide was measured at least twice in separate experiments. For comparative purposes, luminescence was normalized to DHT alone at coexposure concentration (maximum response, 100%) and solvent-only (ethanol) controls (minimum response, 0%). Initially, flutamide was used as the internal quality control for antiandrogenicity; however, because of overlap of toxic effects on the cells with antiandrogenic activity, it was replaced by procymidone, which is more potent [IC50 (50% concentration that inhibits): flutamide, 1.56 μM; procymidone, 0.53 μM] but nontoxic to MDA-kb2 cells in the concentration range associated with receptor antagonism. Pesticides were initially tested over a concentration range of 0.64 nM–50 μM (5× dilutions) as a range-finding exercise. Subsequently, the concentration ranges were modified to reflect the potency and toxicity of each individual compound. Because cytotoxic effects could not be distinguished from antiandrogenic effects in the coexposed treatments, any readings of the pesticide statistically significantly below the mean ethanol control level (0%) were considered toxic to MDA-kb2 cells, and the corresponding coexposure data were not classified as antiandrogenic. Sixty percent of the pesticides were repeat tested using the same product but with new stock solutions and by a different experimenter.
YAS
The methods for the YAS have been described previously (Sohoni and Sumpter 1998). Briefly, stimulation of the transfected AR causes a color change in the media, which is measured by absorbance at 540 nm (Labsystems Multiskan Multisoft, Vienna, VA, USA). Plates were also measured at 620 nm to measure cell growth (turbidity) to check for any cytotoxic effects that may have occurred. Pesticides were coincubated with DHT (6.4 nM). Controls run in each experiment were ethanol, DHT serial dilutions (0.0026–100 nM), and flutamide serial dilutions (0.19–100 μM). The pesticide concentration range varied according to potency observed in MDA-kb2 assay but was between 0.016 and 750 μM for all test compounds. Incubation time was 53 hr at 28°C. Where turbidity readings were significantly depressed, toxicity was indicated and the effect could not be considered antiandrogenic; therefore, these dilutions were removed from analysis. Pesticide serial dilutions were tested in duplicate over two plates and were tested in two separate experiments.
Statistics
To analyze antiandrogenic action, raw luminescence readings were normalized on a plate-by-plate basis to the means of the positive DHT controls (n = 8) and the solvent controls (n = 8) (Ermler et al. 2010). We pooled all data from the same test compound and conducted statistical concentration–response regression analyses using the best-fit approach (Scholze et al. 2001). Specifically, a variety of nonlinear regression models were fitted independently to the same data set, and the best-fitting model was selected using a statistical goodness-of-fit criterion. Concentration–response data from different researchers were first analyzed one by one using regression models, and differences in regression analyses due to data from different researchers were judged as statistically significant when the 95% CIs of the regression curves did not overlap. Such statistical differences between researchers were not observed, and thus data were pooled for final analysis. Luminescence readings from pesticides tested in the absence of DHT were divided by the mean of the solvent controls from the same plate and analyzed for negative and positive trends (suggestive of cytotoxic or androgenic action, respectively) by nonparametric contrast tests (Neuhaeuser et al. 2000). Data considered to be statistically significant at p < 0.05 were analyzed using the best-fit approach as described above. All statistical analysis was performed using SAS statistical software (SAS Institute Inc., Cary, NC, USA). From the best-fitting model, we derived inhibitory concentrations for antiandrogenicity and effect concentrations for cytotoxicity.
Results
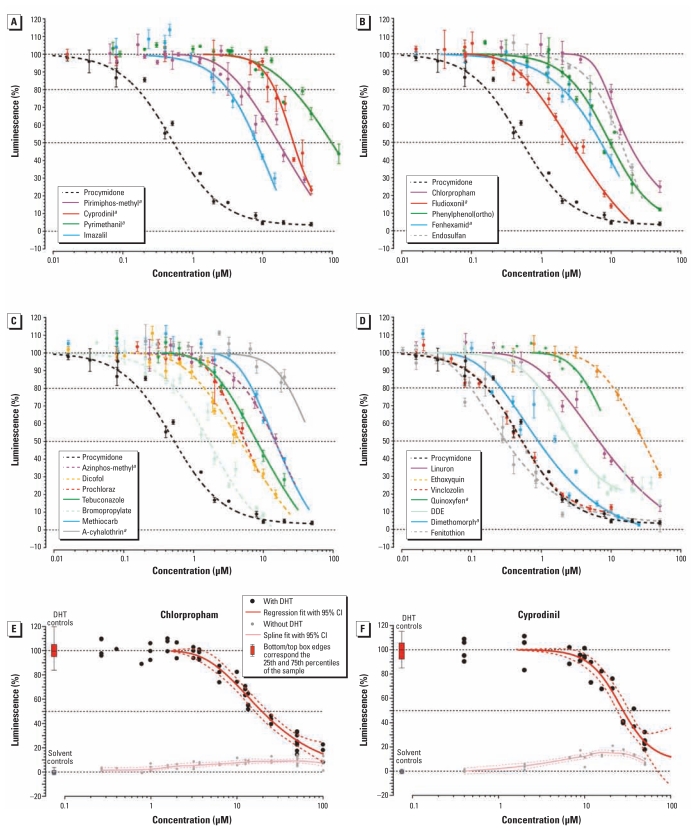
We derived the within-plate variation from readings of the positive DHT controls as a coefficient of variation (CV), with 95% of all CVs falling between 2.1% and 12.9% (mean, 6.5%). Of the 37 tested compounds, 24 pesticides were antiandrogenic in the MDA-kb2 assay, 9 of which are newly described (Table 1, Figure 1). The most potent in vitro AR antagonist was fenitrothion (IC20 = 0.098 μM), and the least potent was pyrimethanil (IC20 = 27.2 μM). All 14 compounds previously reported in the literature as antiandrogenic were confirmed using our test system. Two of 4 previously untested pesticides that were predicted to be AR antagonists in the QSAR were positive in the MDA-kb2 assay, and 3 of 13 pesticides that could not be predicted using the QSAR (i.e., they were out of the model domain) were also antiandrogenic. Five of 6 pesticides predicted to be inactive based on the QSAR were AR antagonists in the MDA-kb2 assay, but 3 were out of the QSAR prediction range because they were antiandrogenic at a concentration higher than the exclusion criterion of the QSAR (limit of detection, IC25 ≤ 10 μM; IC20: cyprodinil, 15.1 μM; pyrimethanil, 27.2 μM; cyhalothrin, 23.1 μM).
Table 1.
Receptor-mediated antiandrogenic activity and cytotoxicity in the MDA-kb2 and YAS assays.
| Compound | Expirationa | Scoreb | QSAR prediction | Antiandrogen IC20 (μM)
|
Cytotoxic EC20 (μM)
|
Androgenc EC20 (μM) MDA-kb2 | ERR | ||
|---|---|---|---|---|---|---|---|---|---|
| MDA-kb2 | YAS | MDA-kb2 | YAS | ||||||
| Fungicides
| |||||||||
| Cyprodinild | Apr 2017 | 33 | Inactive | 15.1 | 1.34 | > 50 | 27.8 | 1.91 | 2.2 |
| Procymidonee | Jun 2008 | 33 | AA | 0.163 | 0.956 | > 50 | > 160 | Neg | 202.5 |
| Imazalil | Dec 2011 | 32 | AA | 3.23 | — | 19.0 | — | Neg | 9.9 |
| Pyrimethanild | May 2017 | 28 | Inactive | 27.2 | 9.15 | > 125 | 167 | 27.8 | 1.0 |
| Fludioxonild | Oct 2018 | 25 | OD | 0.801 | 0.730 | 28.5 | > 160 | Neg | 31.2 |
| Azoxystrobin | Dec 2011 | 24 | Inactive | Neg | — | 2.9 | — | Neg | NA |
| Fenhexamidd | May 2011 | 24 | Active | 2.02 | — | 21.6 | — | Neg | 11.9 |
| Tolylfluanid | Sep 2016 | 24 | OD | Neg | 0.234 | 8.09 | 1.14 | Neg | NA |
| O-Phenylphenol | Dec 2019 | 21 | AA | 3.43 | — | > 50 | — | Neg | 6.1 |
| Prochloraze | Dec 2010 | 18 | AA | 2.39 | — | 12.5 | — | Neg | 7.5 |
| Pyraclostrobin | May 2014 | 17 | OD | Neg | — | 0.089 | — | Neg | NA |
| Mandipropamid | Jul 2011 | 18 | OD | Neg | — | 8.92 | — | Neg | NA |
| Tebuconazole | Aug 2019 | 16 | AA | 2.89 | — | 38.9 | — | Neg | 5.5 |
| Difenoconazole | Dec 2018 | 13 | Active | Neg | Neg | 2.91 | 0.109 | Neg | NA |
| Vinclozoline | Jan 2007 | 13 | AA | 0.163 | — | > 50 | — | 2.9 | 79.8 |
| Dimethomorphd | Sep 2017 | 12 | Active | 0.263 | 38.5 | > 25 | > 50 | Neg | 45.6 |
| Quinoxyfend | Aug 2014 | 12 | Inactive | 4.79 | 1.21 | 10.1 | > 75 | Neg | 2.5 |
| Spiroxamine | Dec 2011 | 9 | OD | Neg | — | 9.29 | — | Neg | NA |
| Ethoxyquine | Mar 2008 | 8 | AA | 10.7 | 11.1 | > 50 | > 200 | Neg | 0.75 |
| Insecticides
| |||||||||
| Pirimiphos-methyld | Sep 2017 | 30 | OD | 5.49 | 3.08 | > 50 | > 200 | Neg | 5.5 |
| Endosulfane | Jun 2006 | 19 | AA | 6.05 | — | 33.8 | — | Neg | 3.1 |
| Methiocarb | Sep 2017 | 17 | AA | 6.82 | — | > 46 | — | Neg | 2.5 |
| Spirotetramat | Pending | 17 | OD | Neg | — | > 50 | — | Neg | NA |
| Azinphos-methyld,e | Jan 2007 | 16 | OD | 5.38 | 2.25 | 33.9 | > 150 | Neg | 2.9 |
| Bifenthrine | May 2010 | 16 | Active | Neg | 99.8 | 22.2 | > 200 | Neg | NA |
| Indoxacarb | Mar 2016 | 16 | OD | Neg | — | 11.3 | — | Neg | NA |
| Spinosad | Jan 2017 | 16 | OD | Neg | — | 13.1 | — | Neg | NA |
| λ-Cyhalothrind | Dec 2011 | 15 | Inactive | 23.1 | 95.4 | 51.4 | > 200 | Neg | 0.65 |
| Dicofole | Mar 2009 | 15 | AA | 1.43 | — | 29.0 | — | Neg | 10.5 |
| Bromopropylatee | Jul 2007 | 13 | AA | 0.540 | — | 27.2 | — | Neg | 24.1 |
| Propargitee | Dec 2010 | 13 | OD | Neg | — | 0.487 | — | Neg | NA |
| Fenitrothione | Nov 2007 | 11 | AA | 0.098 | — | > 50 | — | 4.9 | 112.2 |
| Novaluron | Jul 2011 | 9 | OD | Neg | — | > 50 | — | Neg | NA |
| Profenofose | Jul 2003 | 8 | OD | Neg | — | 8.61 | — | Neg | NA |
| p,p′-DDEe | 1986 | —f | AA | 0.948 | — | > 50 | — | 3.6 | NA |
| Herbicides
| |||||||||
| Chlorpropham | Jan 2015 | 22 | Inactive | 7.66 | 10.2 | > 50 | > 40 | 2.67 | 2.9 |
| Linuron | Dec 2013 | 12 | AA | 1.74 | — | > 50 | — | 3.48 | 6.9 |
Abbreviations: AA, antiandrogenic, refers to known antiandrogens (not assessed by QSAR); EC20, concentration that produces a 20% effect; IC20, concentration that inhibits the androgenicity of DHT by 20%; NA, not applicable; Neg, no response was observed; OD, out of domain (QSAR was not able to predict activity for this compound).
Expiration date is taken from Annex 1 of Council Directive 91/414/EEC concerning the “placing of plant protection products on the market” (European Union 1991).
For details of exposure score, see text and Supplemental Material, Tables 1 and 2 (doi:10.1289/ehp.1002895).
Androgenic in the absence of DHT.
A newly described antiandrogenic compound.
The expiration date is in the past, so the compound can no longer be used in Europe.
Not included in ranked exposure.
Figure 1.
Results of the MDA-kb2 assay showing regression curves for antiandrogenic pesticides (A–D) and stimulatory activity for chlorpropham (E) and cyprodinil (F). Values for luminescence were normalized to those of controls. In A–D, compounds are grouped by exposure scores (see Table 1), from highest (A) to lowest (D), with procymidone shown in each as a point of reference. Regression lines end at the toxic threshold. Dashed lines indicate pesticides with lapsed registration, and solid lines indicate pesticides with current registration; data shown are mean ± SE. Data for chloropham (E) and cyprodinil (F) demonstrate overlap of AR antagonism (black data points and curves) with receptor agonism (gray curves).
aNewly described antiandrogens.
All 14 pesticides tested using the YAS were antiandrogenic, including two that lacked activity in the MDA-kb2 assay [tolylfluanid (out of domain of QSAR) and bifenthrin (predicted active in QSAR)] (Table 1).
Twenty-two of the 37 pesticides analyzed in the MDA-kb2 assay were cytotoxic. The concentrations required to elicit cytotoxicity were between 2.1 times (quinoxyfen) and 50 times (bromopropylate) higher than the concentrations associated with antiandrogenicity [based on the ratio of EC20 (concentration that produces a 20% effect) for cytotoxicity and IC20 for antiandrogenicity]. Seven of the chemicals analyzed in the MDA-kb2 assay showed AR agonist activity when tested in the absence of DHT coexposure, including two (cyprodinil and chlorpropham) with androgenic activity occurring at lower concentrations than antiandrogenic activity (Table 1, Figure 1). Four of 14 pesticides were cytotoxic in the YAS assay (cyprodinil, pyrimethanil, tolylfluanid, and difenoconazole), whereas we observed no AR agonism in this assay (Table 1).
Discussion
Our results indicate that systematic testing for antiandrogenic activity of currently used pesticides is urgently required. For example, 20 of the 50 pesticides with the highest exposure scores were antiandrogenic in at least one assay, including 8 that have not been identified as antiandrogens previously [see Supplemental Material, Figure 2 (doi:10.1289/ehp.1002895)]. In previous in vitro screenings of current-use pesticides, proportions of antiandrogenic pesticides were broadly similar [32% (52 of 161), Kojima et al. 2004; 50% (6 of 12), Orton et al. 2009; 62% (38 of 61), Vinggaard et al. 2008], further supporting the possibility that a large fraction of untested pesticides may be antiandrogenic. In contrast, estrogenic activity appears to be less common in current-use pesticides [18% (29 of 161), Kojima et al. 2004; 0% (0 of 100), Nishihara et al. 2000; 0% (0 of 12), Orton et al. 2009]. Some discrepancy between our data and published data exists; for example, pirimiphos-methyl was previously reported to have no antiandrogenic activity (Kojima et al. 2004), and chlorpropham has been reported to have no activity (Kojima et al. 2004) and to be antiandrogenic (Orton et al. 2009). These differences are most likely due to differences among the assay systems used. We also observed differences between findings based on the MDA-kb2 assay and the YAS assay. However, IC20 values based on the two assays never deviated by more than one order of magnitude, with the exception of two pesticides (tolylfluanid, bifenthrin) that were cytotoxic in the MDA-kb2 assay, and dimethomorph, for which we observed a large divergence in AR antagonist activity (IC20: MDA-kb2, 0.263 μM; YAS, 38.5 μM).
We did not design our study to evaluate the QSAR by Vinggaard et al. (2008), and the number of chemicals falling within the applicability domain of the model was low; however, we note that several pesticides with antiandrogenic activity in vitro were not predicted by the QSAR, in part because some of the compounds were less potent than the prediction domain of the QSAR, which classifies chemicals with an IC25 > 10 μM as devoid of antiandrogenicity. The large percentage of pesticides for which the QSAR was not able to provide predictions (45 of 64) suggests that extending the applicability domain would increase the usefulness of the model.
The ranking according to our exposure scoring system was similar to the listed “adjusted theoretical maximum dietary intake” of pesticides (58% concordance among the top 40 compounds) previously reported by Menard et al. (2008), which is based on actual French consumption data and maximum residue levels. Consequently, the ERR was similar, using either our exposure scores or the adjusted theoretical dietary intake published by Menard et al. (2008) [see Supplemental Material, Table 2 (doi:10.1289/ehp.1002895)]. Both our exposure data and those used by Menard et al. (2008) were sourced from before 2008 (except JMPR reports from 2008 and 2009) and therefore may not be fully representative of current exposures. Indeed, from 2005 through 2010, the authorizations for use granted by European Union authorities expired for 12 of the tested pesticides, including several in vitro AR antagonists (procymidone, prochloraz, vinclozolin, ethoxyquin, endosulfan, azinphos-methyl, bromopropylate, dicofol, and fenitrothion) and 3 without evidence of antiandrogenic activity (bifenthrin, propargite, and profenofos). Thus, exposure to some of the tested compounds should decrease, whereas exposure to replacement products may increase. For example, a pesticide formulation called Switch, which contains cyprodinil and fludioxonil (both of which were antiandrogenic in our test system), was recommended as a replacement for the vinclozolin formulation Ronilan (Shah et al. 2002).
To our knowledge, except for two reports to date (Heudorf and Angerer 2001; Saieva et al. 2004), there is a complete absence of published human biomonitoring data for pesticides in Europe, and therefore, it is impossible to predict how the levels eliciting an effect in vitro may correspond to human internal concentrations. Similarly, although the National Health and Nutrition Examination Survey (NHANES) in the United States incorporates human biomonitoring of pesticides, exposure concentrations in human target tissues are very poorly understood, because of the almost complete lack of toxicokinetic data, short half-lives of current use pesticides, unspecific urinary metabolites, and unknown metabolic pathways (see Barr 2008). Pesticides with relatively large ERRs, including dimethomorph (expiration of European Union authorization, September 2017), fludioxonil (October 2018), fenhexamid (May 2011), imazalil (December 2011), linuron (December 2013), ortho-phenylphenol (December 2019), tebuconazole (August 2019), and pirimiphos-methyl (September 2017), may be important antiandrogenic pollutants at present and in the future (Table 1). Linuron and tebuconazole are known in vivo antiandrogens (Lambright et al. 2000; Taxvig et al. 2007); however, data on the other pesticides are much more limited. This is especially true of dimethomorph, fludioxonil, and fenhexamid, for which we were unable to identify previous publications regarding endocrine disruption. These compounds are newly formulated fungicides (dimethomorph, 2007; fludioxonil, 2008; fenhexamid, 2001), which are stable on food commodities (> 70% of the parent compound) and remain unchanged on the commodity when reaching the consumer (EFSA 2007, 2010a, 2010b). Dimethomorph and fenhexamid belong to the fungicide group of sterol biosynthesis inhibitors (Leroux 2004), as do the in vivo antiandrogenic conazoles (e.g., Taxvig et al. 2007) and imidazoles (Vinggaard et al. 2005). A study of the sterol biosynthesis inhibitors imazalil, propiconazole, triadimefon, triadimenol, and prochloraz indicated that all inhibited aromatase in human placental microsomes (Vinggaard et al. 2000), but to our knowledge, effects of dimethomorph and fenhexamid on steroidogenesis in mammalian cells have not been assessed. Imazalil and the in vivo antiandrogen prochloraz (Vinggaard et al. 2005) are both classified as imidazole fungicides, and in vitro potency estimates for the two compounds were similar (IC20: imazalil, 3.23 μM; prochloraz, 2.39 μM), but the possible effects of imazalil in vivo have not been evaluated. Therefore, it is our view that dimethomorph, fludioxonil, fenhexamid, and imazalil should be tested in vivo as a matter of urgency. Another relevant pesticide may also be ortho-phenylphenol, which is used as a fungicide in agriculture and as a wood preservative, and also has a wide variety of industrial applications (e.g., preservation of glues, plastic additives in flame retardants, disinfectant in hospitals) (LANXESS Corp. 2010). In our exposure ranking system, it ranked 12th out of 37 test compounds (Table 1). Considering that ortho-phenylphenol was highly ranked by exposure and that nonagricultural sources were absent from our exposure scores, it is not surprising that it was detected in all human urine samples tested in two studies [mean concentration, 2.9 nM, n = 30 samples (Ye et al. 2005); 35.2 nM, n = 22 samples (Bartels et al. 1997)], 85% of breast milk samples [mean concentration, 10.6 nM, n = 20 samples (Ye et al. 2006)], and 30% of amniotic fluid samples [mean concentration, 0.76 nM, n = 20 samples (Bradman et al. 2003)] in the United States. ortho-Phenylphenol was previously identified as a receptor-mediated antiandrogen (Kojima et al. 2004), but no data are available on its possible effects in vivo. Pirimiphos-methyl is an organothiophosphate insecticide that is stable on stored grain (< 24 weeks, 70% unchanged parent compound; EFSA 2005). There are also indications that it may be antiandrogenic in vivo because maternal and postnatal exposure of rats to 12 mg/kg body weight/day caused testicular tubular atrophy (EFSA 2005). In addition, treatment of adult male rats for 90 days resulted in decreased sperm density and mobility (125 mg/kg body weight/day), testicular atrophy (lowest observed adverse effect level, 41.67 mg/kg body weight/day), and decreased fertility (125 mg/kg body weight/day) (Ngoula et al. 2007). There is insufficient evidence to assess the risk of tested pesticides to human health because of a lack of data. However, to our knowledge, all of the pesticides (with the possible exception of fenitrothion; Okahashi et al. 2005; Turner et al. 2002) identified as in vitro AR antagonists in our study have also been reported to have antiandrogenic effects in vivo in animal models (Anway et al. 2006; Gray et al. 1999; Lambright et al. 2000; McIntyre et al. 2002; Ostby et al. 1999; Sinha et al. 2001; Taxvig et al. 2007; Uzumcu et al. 2004; Vinggaard et al. 2005). We also identified 7 compounds that appeared to be androgenic because they stimulated activity in the absence of DHT. The mechanism of action for this response is not well characterized; however, it has been previously detected in this assay (Tamura et al. 2006; Wilson et al. 2002) and was proposed to be due to conformational change of the ligand-binding pocket in such a way that simultaneous androgenic and antiandrogenic activities were possible (Tamura et al. 2006). We are unable to confirm or reject these data; however, preliminary data from our laboratory suggests that the stimulatory response is neither via stimulation of the receptor, because we have not observed evidence of androgenic effects in the YAS for any compounds, nor due to cell proliferation, as evidenced by transient transfection of cells with a nonandrogenic responsive element. Cyprodinil and chlorpropham were more potent AR agonists (EC20 = 1.91 and 2.67, respectively) than antagonists (IC20 = 15.1 and 7.66, respectively) in the MDA-kb2 assay.
Conclusions
In addition to identifying new candidate antiandrogens, our findings highlight important data gaps that prevent accurate assessment of male reproductive health risks from pesticides. The most important of these are the absence of in vivo studies and human biomonitoring data for environmentally relevant pesticides. In addition, fungicides typically had high exposure scores and were thus well represented in the testing set, presumably because they are often applied just before or after harvest to food commodities. They are typically applied as mixtures in order to increase effectiveness and prevent development of resistant strains (Fungicide Resistance Action Committee 2010), and therefore, human exposure to mixtures of these in vitro antiandrogens may be considerable. The contribution of pesticides to declining male reproductive health requires further investigation, particularly to clarify the relationship between effective concentrations in vivo and exposure.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.1002895 via http://dx.doi.org/).
Thanks to the colleagues from the National Food Institute, Danish Technical University, Soborg, Denmark, for help with the quantitative structure–activity relationship–aided compound selection.
We gratefully acknowledge funding from the European Commission (grant 212502).
References
- Andersen HR, Schmidt IM, Grandjean P, Jensen TK, Budtz-J⊘rgensen E, Kjaerstad MB, et al. Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environ Health Perspect. 2008;116:566–572. doi: 10.1289/ehp.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Vinggaard AM, Rasmussen TH, Gjermandsen IM, Bonefeld-J⊘rgensen EC. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol Appl Pharmacol. 2002;179(1):1–12. doi: 10.1006/taap.2001.9347. [DOI] [PubMed] [Google Scholar]
- Andersson AM, J⊘rgensen N, Main KM, Toppari J, Meyts ERD, Leffers H, et al. Adverse trends in male reproductive health: we may have reached a crucial ‘tipping point’. Int J Androl. 2008;31(2):74–80. doi: 10.1111/j.1365-2605.2007.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade AJM, Araujo S, Santana GM, Ohi M, Dalsenter PR. Reproductive effects of deltamethrin on male offspring of rats exposed during pregnancy and lactation. Regul Toxicol Pharmacol. 2002;36(3):310–317. doi: 10.1006/rtph.2002.1586. [DOI] [PubMed] [Google Scholar]
- Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J Androl. 2006;27(6):868–879. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB. Biomonitoring of exposure to pesticides. J Chem Health Saf. 2008;15(6):20–29. [Google Scholar]
- Bartels MJ, Brzak KA, Bormett GA. Determination of ortho-phenylphenol in human urine by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 1997;703(1):97–104. doi: 10.1016/s0378-4347(97)00405-2. [DOI] [PubMed] [Google Scholar]
- Bauer ERS, Bitsch N, Brunn H, Sauerwein H, Meyer HHD. Development of an immuno-immobilized androgen receptor assay (IRA) and its application for the characterization of the receptor binding affinity of different pesticides. Chemosphere. 2002;46(7):1107–1115. doi: 10.1016/s0045-6535(01)00145-x. [DOI] [PubMed] [Google Scholar]
- Bradman A, Barr DB, Henn BGC, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect. 2003;111:1779–1782. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers MM, Feitz WFJ, Roelofs LAJ, Kiemeney LALM, de Gier RPE, Roeleveld N. Risk factors for hypospadias. Eur J Pediatr. 2007;166:671–678. doi: 10.1007/s00431-006-0304-z. [DOI] [PubMed] [Google Scholar]
- Damgaard IN, Skakkebaek NE, Toppari J, Virtanen HE, Shen HQ, Schramm KW, et al. Persistent pesticides in human breast milk and cryptorchidism. Environ Health Perspect. 2006;114:1133–1138. doi: 10.1289/ehp.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Initial Risk Assessment Provided by the Rapporteur Member State United Kingdom for the Existing Active Substance Pirimiphos-methyl of the Second Stage of the Review Programme Referred to in Article 8(2) of Council Directive 91/414/EEC. York, UK: EFSA; 2005. Draft Assessment Report. [Google Scholar]
- EFSA (European Food Safety Authority) Conclusion regarding the peer review of the pesticide risk assessment of the active substance fludioxonil. [[accessed 20 April 2011]];EFSA Sci Rep. 2007 110:1–85 . Available: http://www.efsa.europa.eu/en/efsajournal/doc/110r.pdf. [Google Scholar]
- EFSA (European Food Safety Authority) 2007 Annual report on pesticide residues according to Article 32 of Regulation (EC) No 396/2005. [[accessed 20 April 2011]];EFSA Sci Rep. 2009 305:1–106 . Available: http://www.efsa.europa.eu/en/efsajournal/doc/305r.pdf. [Google Scholar]
- EFSA (European Food Safety Authority) Modification of the existing MRLs for dimethomorph in various crops. [[accessed 20 April 2011]];EFSA J. 2010a 8(5):1622. Available: http://www.efsa.europa.eu/en/scdocs/doc/1622.pdf. [Google Scholar]
- EFSA (European Food Safety Authority) Modification of the existing MRLs for fenhexamid in various leafy vegetables. [[accessed 20 April 2011]];EFSA J. 2010b 8(1):1455. Available: http://www.efsa.europa.eu/en/efsajournal/doc/1455.pdf. [Google Scholar]
- Ermler S, Scholze M, Kortenkamp A. The sensitivity of the MDA-kb2 cell in vitro assay in detecting anti-androgenic chemicals—identification of sources of variability and estimation of statistical power. Toxicol in Vitro. 2010;24(6):1845–1853. doi: 10.1016/j.tiv.2010.05.007. [DOI] [PubMed] [Google Scholar]
- European Commission. Monitoring of Pesticide Residues in Products of Plant Origin in the European Union, Norway, Iceland and Liechtenstein 2006. Brussels:Commission of the European Communities. 2008. [[accessed 19 April 2011]]. Available: http://ec.europa.eu/food/fvo/specialreports/pesticide_residues/report_2006_en.pdf.
- European Union. Corrigendum to Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant products on the market (OJ No L 230 of 19. 8. 1991) 1991. [[accessed 3 May 2011]]. Available: http://eur-lex.europa.eu/Notice.do?val=185439:cs&lang=en&list=447073:cs,185439:cs,172911:cs,&pos=2&page=1&nbl=3&pgs=10&hwords=
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) AGP - JMPR Reports and Evaluations. 2011. [[accessed 19 April 2011]]. Available: http://www.fao.org/agriculture/crops/core-themes/theme/pests/pm/jmpr/jmpr-rep/en/
- Fernández MF, Rivas A, Olea-Serrano F, Cerrillo I, Molina-Molina JM, Araque P, et al. Assessment of total effective xenoestrogen burden in adipose tissue and identification of chemicals responsible for the combined estrogenic effect. Anal Bioanal Chem. 2004;379(1):163–170. doi: 10.1007/s00216-004-2558-5. [DOI] [PubMed] [Google Scholar]
- FRAC (Fungicide Resistance Action Committee) FRAC Recommendations for Fungicide Mixtures Designed to Delay Resistance Evolution. 2010. [[accessed 19 May 2010]]. Available: http://www.frac.info/frac/publication/anhang/Resistance%20and%20Mixtures%20Jan2010_ff.pdf.
- Gray LE, Jr, Wolf C, Lambright C, Mann P, Price M, Cooper RL, et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p′-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health. 1999;15(1–2):94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Angerer J. Metabolites of organophosphorous insecticides in urine specimens from inhabitants of a residential area. Environ Res. 2001;86(1):80–87. doi: 10.1006/enrs.2001.4237. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male Long Evans hooded rat. Toxicol Sci. 2008;102(2):371–382. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect. 2004;112:524–531. doi: 10.1289/ehp.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC, et al. Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol Sci. 2000;56(2):389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- LANXESS Corp. PREVENTOL® O extra flakes. 2010. [[accessed 20 April 2011]]. Available: http://www.protectedbypreventol.com/mpp/en/global/products/product_selection_tool/?Action=prod&ID=50.
- Leroux P. Chemical control of Botrytis and its resistance to chemical fungicides. In: Elad Y, Williamson B, Tudzynski P, Delen N, editors. Botrytis: Biology, Pathology and Control. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2004. pp. 195–222. [Google Scholar]
- McIntyre BS, Barlow NJ, Foster PMD. Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol Sci. 2001;62(2):236–249. doi: 10.1093/toxsci/62.2.236. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Foster PMD. Male rats exposed to linuron in utero exhibit permanent changes in anogenital distance, nipple retention, and epididymal malformations that result in subsequent testicular atrophy. Toxicol Sci. 2002;65(1):62–70. doi: 10.1093/toxsci/65.1.62. [DOI] [PubMed] [Google Scholar]
- Menard C, Heraud F, Nougadere A, Volatier JL, Leblanc JC. Relevance of integrating agricultural practices in pesticide dietary intake indicator. Food Chem Toxicol. 2008;46(10):3240–3253. doi: 10.1016/j.fct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Neuhaeuser M, Seidel D, Hothorn L, Urfer W. Robust trend test with application to toxicology. Environ Ecol Stat. 2000;7:43–56. [Google Scholar]
- Ngoula F, Watcho P, Dongmo MC, Kenfack A, Kamtchouing P, Tchoumboue J. Effects of pirimiphos-methyl (an organophosphate insecticide) on the fertility of adult male rats. Afr Health Sci. 2007;7(1):3–9. doi: 10.5555/afhs.2007.7.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara T, Nishikawa J, Kanayama T, Dakeyama F, Saito K, Imagawa M, et al. Estrogenic activities of 517 chemicals by yeast two-hybrid assay. J Health Sci. 2000;46(4):282–298. [Google Scholar]
- Okahashi N, Sano M, Miyata K, Tamano S, Higuchi H, Kamita Y, et al. Lack of evidence for endocrine disrupting effects in rats exposed to fenitrothion in utero and from weaning to maturation. Toxicology. 2005;206(1):17–31. doi: 10.1016/j.tox.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Okubo T, Yokoyama Y, Kano K, Soya Y, Kano I. Estimation of estrogenic and antiestrogenic activities of selected pesticides by MCF-7 cell proliferation assay. Arch Environ Contam Toxicol. 2004;46(4):445–453. doi: 10.1007/s00244-003-3017-6. [DOI] [PubMed] [Google Scholar]
- Orton F, Lutz I, Kloas W, Routledge EJ. Endocrine disrupting effects of herbicides and pentachlorophenol: in vitro and in vivo evidence. Environ Sci Technol. 2009;43(6):2144–2150. doi: 10.1021/es8028928. [DOI] [PubMed] [Google Scholar]
- Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE., Jr The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol Ind Health. 1999;15(1–2):80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RFA. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect. 2004;112:1570–1576. doi: 10.1289/ehp.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau CM, Romitti PA, Dennis LK. Pesticides and hypospadias: a meta-analysis. J Pediatr Urol. 2009;5(1):17–24. doi: 10.1016/j.jpurol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saieva C, Aprea C, Tumino R, Masala G, Salvini S, Frasca G, et al. Twenty-four-hour urinary excretion of ten pesticide metabolites in healthy adults in two different areas of Italy (Florence and Ragusa) Sci Total Environ. 2004;332(1–3):71–80. doi: 10.1016/j.scitotenv.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Scholze M, Boedeker W, Faust M, Backhaus T, Altenburger R, Grimme LH. A general best-fit method for concentration-response curves and the estimation of low-effect concentrations. Environ Toxicol Chem. 2001;20(2):448–457. [PubMed] [Google Scholar]
- Shah DA, Dillard HR, Cobb AC. Alternatives to Vinclozolin (Ronilan) for Controlling Gray and White Mold on Snap Bean Pods in New York. 2002. [[accessed 20 April 2011]]. Available: http://www.plantmanagementnetwork.org/pub/php/research/snapbean/
- Sinha N, Adhikari N, Saxena DK. Effect of endosulfan during fetal gonadal differentiation on spermatogenesis in rats. Environ Toxicol Pharmacol. 2001;10(1–2):29–32. doi: 10.1016/s1382-6689(01)00066-7. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: opinion. Hum Reprod. 2001;16(5):972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158(3):327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Tamura H, Ishimoto Y, Fujikawa T, Aoyama H, Yoshikawa H, Akamatsu M. Structural basis for androgen receptor agonists and antagonists: interaction of SPEED 98-listed chemicals and related compounds with the androgen receptor based on an in vitro reporter gene assay and 3D-QSAR. Bioorg Med Chem. 2006;14:7160–7174. doi: 10.1016/j.bmc.2006.06.064. [DOI] [PubMed] [Google Scholar]
- Taxvig C, Hass U, Axelstad M, Dalgaard M, Boberg J, Andeasen HR, et al. Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol Sci. 2007;100:464–473. doi: 10.1093/toxsci/kfm227. [DOI] [PubMed] [Google Scholar]
- Turner KJ, Barlow NJ, Struve MF, Wallace DG, Gaido KW, Dorman DC, et al. Effects of in utero exposure to the organophosphate insecticide fenitrothion on androgen-dependent reproductive development in the Crl:CD(SD)BR rat. Toxicol Sci. 2002;68(1):174–183. doi: 10.1093/toxsci/68.1.174. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Suzuki H, Skinner MK. Effect of the anti-androgenic endocrine disruptor vinclozolin on embryonic testis cord formation and postnatal testis development and function. Reprod Toxicol. 2004;18(6):765–774. doi: 10.1016/j.reprotox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Verma R, Mohanty B. Early-life exposure to dimethoate-induced reproductive toxicity: evaluation of effects on pituitary-testicular axis of mice. Toxicol Sci. 2009;112(2):450–458. doi: 10.1093/toxsci/kfp204. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Christiansen S, Laier P, Poulsen ME, Breinholt V, Jarfelt K, et al. Perinatal exposure to the fungicide prochloraz feminizes the male rat offspring. Toxicol Sci. 2005;85(2):886–897. doi: 10.1093/toxsci/kfi150. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Hnida C, Breinholt V, Larsen JC. Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol in Vitro. 2000;14(3):227–234. doi: 10.1016/s0887-2333(00)00018-7. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Niemela J, Wedebye EB, Jensen GE. Screening of 397 chemicals and development of a quantitative structure-activity relationship model for androgen receptor antagonism. Chem Res Toxicol. 2008;21(4):813–823. doi: 10.1021/tx7002382. [DOI] [PubMed] [Google Scholar]
- Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117:1505–1513. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Lambright CR, Gray LE., Jr A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci. 2002;66(1):69–81. doi: 10.1093/toxsci/66.1.69. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831(1–2):110–115. doi: 10.1016/j.jchromb.2005.11.050. [DOI] [PubMed] [Google Scholar]