Abstract
Background
Mitochondrial dysfunction and oxidative stress are pathophysiologic mechanisms implicated in experimental models and genetic forms of Parkinson’s disease (PD). Certain pesticides may affect these mechanisms, but no pesticide has been definitively associated with PD in humans.
Objectives
Our goal was to determine whether pesticides that cause mitochondrial dysfunction or oxidative stress are associated with PD or clinical features of parkinsonism in humans.
Methods
We assessed lifetime use of pesticides selected by mechanism in a case–control study nested in the Agricultural Health Study (AHS). PD was diagnosed by movement disorders specialists. Controls were a stratified random sample of all AHS participants frequency-matched to cases by age, sex, and state at approximately three controls: one case.
Results
In 110 PD cases and 358 controls, PD was associated with use of a group of pesticides that inhibit mitochondrial complex I [odds ratio (OR) = 1.7; 95% confidence interval (CI), 1.0–2.8] including rotenone (OR = 2.5; 95% CI, 1.3–4.7) and with use of a group of pesticides that cause oxidative stress (OR = 2.0; 95% CI, 1.2–3.6), including paraquat (OR = 2.5; 95% CI, 1.4–4.7).
Conclusions
PD was positively associated with two groups of pesticides defined by mechanisms implicated experimentally—those that impair mitochondrial function and those that increase oxidative stress—supporting a role for these mechanisms in PD pathophysiology.
Keywords: aging, agricultural epidemiology, environmental epidemiology, epidemiology, fungicides, herbicides, insecticides, persistent organic pollutants, pesticides
Mitochondrial dysfunction and oxidative stress have long been implicated as pathophysiologic mechanisms underlying Parkinson’s disease (PD) (Betarbet et al. 2000; Di Monte et al. 2002). Genetic forms of PD associated with mutations in the alpha-synuclein, PARKIN, PINK1, or DJ-1 genes may involve these mechanisms (Henchcliffe and Beal 2008). In experimental models, the pesticides paraquat, which causes oxidative stress, and rotenone, which inhibits mitochondrial complex I, both induce loss of nigral dopaminergic neurons and behavioral changes associated with human PD (Henchcliffe and Beal 2008). Yet despite decades of laboratory study, neither pesticide has been definitively associated with PD in humans. Previous studies have reported associations with paraquat, but results are inconsistent and, in general, studies included few exposed cases; evidence concerning rotenone is sparse (Table 1). To assess the relevance of experimental results to human PD, we investigated the association of PD with use of pesticides linked to complex I inhibition or oxidative stress in a population with well-characterized pesticide exposure.
Table 1.
Relationship of PD to paraquat or rotenone exposure in human populations.
| Study | Design | Method of assessing pesticide use | No. enrolled (cases, controls) | Finding | OR (95% CI) or p-value | Exposed n |
|---|---|---|---|---|---|---|
| Paraquat
| ||||||
| Sanchez-Ramos et al. 1987 | Case report | Medical history | 1 case | Symptoms “comparable to PD” in a 32-year-old farmer after 15 years of paraquat use | NA | 1 case |
| Hertzman et al. 1990 | Case–control | Specific question re: paraquat use | 57 cases | Association with PD | p = 0.0*a | 4 cases |
| 122 controls | 0 controls | |||||
| Semchuk et al. 1992 | Case–control | General questions re: pesticide use | 130 cases | One case with early-onset PD (< age 40 years) reported using paraquat (ages 26–31 years) | NA | 1 case |
| 260 cases | ||||||
| Hertzman et al. 1994 | Case–control | Specific question re: paraquat use | 127 cases | 1.11 (0.32–3.87) | 6 cases | |
| 245 controls | No association with PD | 5 controls | ||||
| 1.25 (0.34–4.63) | 6 cases | |||||
| 4 controls | ||||||
| Liou et al. 1997 | Case–control | Open-ended question re: pesticide use | 120 cases | No association with PD | 3.22 (2.41–4.31) | 31 cases |
| 240 controls | 22 controls | |||||
| Firestone et al. 2005 | Case–control | Checklistb | 250 cases | Association with PD | 1.67 (0.22–12.76) | 2 cases |
| 388 controls | 2 controls | |||||
| Kamel et al. 2007 | Case–control | Specific question re: paraquat use | 83 prevalent cases | Association with PD in prevalent cases | 1.8 (1.0–3.4) | 14 prevalent |
| 79,557 controls | 11,266 controls | |||||
| 78 incident cases | No association with PD in incident cases | 1.0 (0.5–1.9) | 11 incident | |||
| 55,931 controls | 7,382 controls | |||||
| Tanner et al. 2009 | Case–control | Specific question re: occupational paraquat use | 519 cases | Association with PD | 2.80 (0.81–9.72) | 9 cases |
| 511 controls | 4 controls | |||||
| Firestone et al. 2010 | Case–control | Checklistc | 404 incident cases | Added subjects to 2005 interim population | 0.9 (0.14–5.43) | 2 cases |
| 526 controls | No association with PD in reanalysis | 3 controls | ||||
| Rotenone
| ||||||
| Kamel et al. 2007 | Case–control | Specific question re: rotenone use in a supplementary questionnaire | 83 prevalent cases | Association of PD with ever use | 1.7 (0.6–4.7) | 4 prevalent |
| 79,557 controls | 671 controls | |||||
| 78 incident cases | Could not determine | 1 incident | ||||
| 55,931 controls | 565 controls | |||||
| Tanner et al. 2009 | Case–control | Specific question re: occupational rotenone use | 519 cases | No association with PD | 0.82 (0.05–13.34) | 1 case |
| 511 controls | 1 control | |||||
| Dhillon et al. 2008 | Case–control | General question re: “organic pesticides” | 100 cases | Greater use of “organic pesticides such as rotenone” in PD patients | 10.0 (2.9–34.3) | 27 cases |
| 84 controls | 3 controls | |||||
NA, not analyzed.
Four PD patients and no controls reported paraquat contact; OR could not be calculated;
Occupational pesticide exposures were identified from a checklist of common chemical agents and home-based pesticide exposures from a checklist of commercial brand name products.
Subjects reported exposures to various industrial toxicants identified from a checklist.
The Farming and Movement Evaluation study (FAME) is a case–control study nested in the Agricultural Health Study (AHS), a prospective study including 84,740 private pesticide applicators (mostly farmers), recruited in 1993–1997 in Iowa and North Carolina, and their spouses (Alavanja et al. 1996). Pesticide use is reported reliably by these applicators (Blair et al. 2002; Hoppin et al. 2002). We previously reported that self-reported PD was associated with increasing lifetime days of use of any pesticide, but no specific pesticide could be definitely implicated (Kamel et al. 2007). In this study, we assessed lifelong use of pesticides with toxicant mechanisms relevant to PD. Because 15–25% of self-reported PD diagnoses in community populations may be incorrect (Tolosa et al. 2006), we based diagnoses on in-person examination and consensus of two experts, minimizing diagnostic misclassification and enabling analyses of clinical features. We report here results from our investigation of pesticides previously associated with mitochondrial complex I inhibition (termed “complex I inhibitors”) or oxidative stress (termed “oxidative stressors”).
Methods
Case and control identification
From among AHS cohort members who were private pesticide applicators (mostly farmers) and their spouses, we identified persons suspected to have PD based on diagnoses from self-reports or state mortality files. We selected potential controls by stratified random sampling of all AHS participants. Cohort members who were ultimately diagnosed with PD or parkinsonism, dead, cognitively impaired, or seriously ill were not eligible to be controls. Controls were frequency-matched to cases by age (< 40, 40–49, 50–59, 60–64, 65–69, ≥ 70 years), sex, and state (Iowa or North Carolina) at a ratio of approximately three controls per case.
Living suspect cases and all potential controls were assessed during home visits. Movement disorder specialist neurologists (subsequently called “neurologists”) assessed all cases and 5% of controls. Neurologist-trained technicians assessed the remaining controls. The in-person assessment included factors used to diagnose PD and to distinguish PD from other disorders, including a standardized medical and neurological history, the Cognitive Abilities Screening Instrument (Teng et al. 1994), a scripted, standardized videotaped assessment of parkinsonism, a handwriting sample, medications, and the University of Pennsylvania brief smell identification test (Sensonics, Inc., Haddon Heights, NJ, USA) (Doty et al. 1996). Assessments by neurologists also included a standardized neurological examination, orthostatic hypotension assessment, the Unified Parkinson’s Disease Rating Scale (Fahn et al. 1987), and the Tremor Rating Scale (Fahn et al. 1993). Technician-assessed controls with signs suggesting parkinsonism were subsequently assessed by a neurologist.
Diagnosis was determined by agreement of two neurologists (C.M.T. and either G.W.R., C.M., A.C., or M.K.) after independent review of all available diagnostic information (medical records, in-person examination records, and videotaped examination) for all suspect cases and when PD was suspected in a control. Established criteria for PD and related disorders were used (Bain et al. 2000; Elble 2000; Gelb et al. 1999; Gilman et al. 1999; Hughes et al. 1992; Langston et al. 1992; Litvan et al. 1996; McKeith et al. 1996).
FAME was approved by institutional review boards for the Parkinson’s Institute, National Institutes of Health, Social and Scientific Systems, Inc. (Durham, NC, USA), the University of Iowa (Iowa field station), and Battelle, Inc. (North Carolina field station). All participants provided written informed consent.
Exposure assessment
We used computer-assisted telephone interviews to obtain detailed information on pesticide use from 14 years of age onward for 31 selected pesticides [Supplemental Material, Table 1 (doi:10.1289/ehp.1002839)] as well as covariate information including lifelong smoking and family history of PD. For subjects who were unable to complete interviews, we used proxy informants. Although all controls were living and competent when enrolled, some later became unable to participate and a proxy informant was used. We considered only pesticide use occurring before a reference date (cases: age at PD diagnosis; controls: median age of PD diagnosis for cases within the corresponding age-, sex-, and state-specific stratum). For each pesticide, we determined ever use (used one or more times) and lifetime days of use (determined for each farm job by multiplying years of use by average days of use per year, and then summing across jobs). Self-reported pesticide use falling outside U.S. Environmental Protection Agency (EPA) approval dates was not included. For cigarette smoking, we classified individuals who smoked > 100 cigarettes before their reference date as exposed (Grant et al. 2004). Family history of PD was positive if the subject reported PD in any first-degree relative. We dichotomized educational level as ≤ 12 years and > 12 years.
Statistical analyses
We compared participant characteristics using Fisher’s exact test or Pearson’s chi-square statistic for categorical data and the Wilcoxon rank-sum test for continuous data. For pesticides reported by ≥ 10 subjects, we evaluated associations between pesticide use and PD using unconditional logistic regression to derive odds ratios (ORs) and 95% confidence intervals (CIs) [Supplemental Material, Table 2 (doi:10.1289/ehp.1002839)]. To control potential confounding, we included reference age (tertiles 40–57, 58–65, 66–87 years), sex, state, and cigarette smoking (ever/never) in all models. We also examined whether adjusting for overall pesticide use or educational level changed point estimates (± 15%) for individual pesticides. To adjust for overall pesticide use, we categorized individuals who used pesticides on < 25 lifetime days as unexposed and others as exposed, using data reported at enrollment in the AHS (data from AHS data release version P1REL0506 and AHSREL0612; unpublished data).
We performed analyses for pesticide exposure groups classified by mechanism as complex I inhibitors or oxidative stressors, based on literature review (Degli Esposti 1998; Krieger 2001; Uversky et al. 2001) and information in public databases [Agency for Toxic Substances and Disease Registry (ATSDR) 2002; Extension Toxicology Network (EXTOXNET) 2002; National Center for Biotechnology Information 2002]. Because men were the primary users of pesticides, we repeated analyses of ever use and duration of use restricted to men. For each pesticide reported by > 30 men [Supplemental Material, Table 3 (doi:10.1289/ehp.1002839)], we assessed the effect of exposure duration by separately comparing less use (at or below the median number of cumulative days of use) or more use (use above the median) with never use of that pesticide.
We repeated primary analyses in subgroups defined by race/ethnicity (non-Hispanic whites, others), state, family history of PD, cigarette use, and respondent status (self-reported vs. proxy-reported pesticide data) and, in cases, PD duration. We performed analyses for rotenone and paraquat use truncated 5, 10, and 15 years before the index date to evaluate whether the association of PD with pesticide use was influenced by behavioral changes caused by undiagnosed disease. We also assessed the combination of paraquat plus any dithiocarbamate (ferbam, mancozeb, maneb, metam sodium, vegedex, zineb, ziram), because previous studies reported an association of PD with the combination of paraquat and the dithiocarbamate maneb (Costello et al. 2009; Thiruchelvam et al. 2000). We also repeated the primary analysis after including cases of atypical parkinsonism in our case group. For cases, we compared clinical features in persons who did or did not use paraquat or oxidative stressors as a group, or rotenone or complex I inhibitors as a group.
We used SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA) and SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) for statistical analyses. We used α ≤ 0.05 as a criterion for statistical significance.
Results
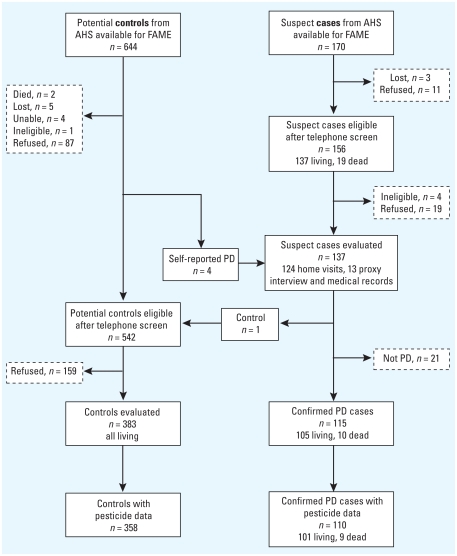
We identified 170 suspect cases and 644 potential controls and screened 156 (92%) and 542 (84%) of these, respectively (Figure 1). We conducted study evaluations in 137 (88%) of eligible suspect cases (including 4 initially indentified as potential controls who self-reported PD at screening) and 383 (71%) of eligible potential controls. Final diagnoses in suspect cases were PD (115), essential tremor/other tremor disorder (12), no neurologic diagnosis (5), dystonia (2), multiple system atrophy (2), and atypical parkinsonism not fulfilling any diagnostic criterion (1), the latter three cases subsequently termed “atypical parkinsonism.”
Figure 1.
Screening process of cases and controls. We identified 170 suspect cases and 644 potential controls and screened 156 (92%) and 542 (84%) of these, respectively. We conducted study evaluations in 137 (88%) of eligible suspect cases (including four initially identified as potential controls who self-reported PD at screening) and 383 (71%) of eligible potential controls. Suspect cases were evaluated in home visits or from medical records, matched controls in home visits. Final diagnoses in suspect cases were PD (115), essential tremor/other tremor disorder (12), no neurologic diagnosis (5), dystonia (2), multiple system atrophy (2), and atypical parkinsonism not fulfilling any diagnostic criterion (1). The latter three cases were subsequently termed “atypical parkinsonism.”
The analyses reported here include 110 PD cases and 358 controls who provided complete information on pesticide use and application practices. Cases and controls were similar for most characteristics assessed (Table 2), although cases were more likely to have impaired smell recognition or to have a family history of PD, and were less likely to smoke.
Table 2.
Characteristics of subjects.
| Characteristic | Cases | Controls |
|---|---|---|
| n | 110 | 358 |
| Age at FAME enrollment [years (mean ± SD)] | 70 ± 8 | 69 ± 8 |
| Men [n (%)] | 80 (73) | 265 (74) |
| Residence in Iowa (vs. North Carolina) [n (%)] | 79 (72) | 262 (73) |
| Non-Hispanic white (vs. other) [n (%)] | 107 (97) | 350 (98) |
| Pesticide applicator (vs. spouse) [n (%)] | 80 (73) | 267 (75) |
| Education > high school [n (%)] | 49 (45) | 178 (50) |
| PD in first-degree relative [n (%)] | 18 (16) | 22 (6)* |
| Smoked at least 100 cigarettes [n (%)] | 31 (28) | 141 (39)** |
| BSIT (mean score ± SD) | 5.5 ± 2.8 | 8.8 ± 2.3# |
| CASI (mean score ± SD) | 89.2 ± 8.5 | 93.1 ± 5.0# |
| Clinical features among casesa | ||
| Age at PD diagnosis [years (mean ± SD)] | 61 ± 9 | — |
| PD duration at FAME enrollment [years (median ± SD)] | 7 ± 6 | — |
| Resting tremor [n affected/n with data (%)] | 100/108 (93) | — |
| Bradykinesia [n affected/n with data (%)] | 105/110 (95) | — |
| Rigidity [n affected/n with data (%)] | 106/107 (99) | — |
| Postural reflex impairment [n affected/n with data (%)] | 61/92 (66) | — |
| Asymmetric onset [n affected/n with data (%)] | 102/104 (98) | — |
| Response to dopaminergic therapy (if prescribed) [n affected/n with data (%)] | 95/98 (97) | — |
Abbreviations: BSIT, Brief Smell Identification Test; CASI, Cognitive Abilities Screening Instrument.
Percentages for clinical features are based on numbers of cases with features divided by the total number of cases with available data; cases with missing data for a feature are excluded.
p < 0.005 (Fisher’s exact test).
p < 0.05 (Fisher’s exact test).
p < 0.001 (Wilcoxon rank-sum test).
To validate our approach to pesticide exposure assessment, we compared information on use of four pesticides collected at enrollment in the AHS with information collected for FAME. Despite an average lapse of 9 years, agreement was good [DDT (dichlorodiphenyltrichloroethane) 79%, 2,4-D (2,4-dichlorophenoxyacetic acid) 84%, paraquat 85%, rotenone 93%].
Ninety-eight percent of men and 44% of women had ever used pesticides. Information on 18 pesticides used by > 10 subjects is presented in Supplemental Material, Table 2 (doi:10.1289/ehp.1002839). Results from analyses in men were similar to those in men and women combined (Supplemental Material, Table 2). Information on pesticides linked to complex I inhibition or oxidative stress, regardless of the number of users, is presented in Table 3. Most of the pesticides in the oxidative stressor and mitochondrial inhibitor groups were used infrequently, limiting individual analyses; but in general, use of these chemicals was more common in cases. Use of paraquat (OR = 2.5; 95% CI, 1.4–4.7) or any of the group of oxidative stressors (OR = 2.0; 95% CI, 1.2–3.6) was associated with PD (Table 3). Similarly, use of rotenone (OR = 2.5; 95% CI, 1.3–4.7) or any of the group of complex I inhibitors (OR = 1.7; 95% CI, 1.0–2.8) was associated with PD (Table 3).
Table 3.
Association of PD with ever use of pesticides before diagnosis or reference date by mechanism.
| Pesticide | Cases (n = 110) [n (%)] | Controls (n = 358) [n (%)] | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Oxidative stressors
| ||||
| Paraquat | 23 (24) | 49 (14) | 2.5 (1.4–4.7) | 0.004 |
| Permethrin | 16 (16) | 41 (12) | 1.5 (0.77–2.9) | 0.244 |
| Carbon disulfide | 2 (2) | 3 (1) | 2.6 (0.41–16) | 0.313 |
| Chloranil | 1 (1) | 3 (1) | 1.6 (0.16–16) | 0.706 |
| Cyhalothrin | 1 (1) | 1 (0) | 3.8 (0.22–64) | 0.359 |
| Dichlone | 3 (3) | 8 (2) | 1.6 (0.40–6.2) | 0.517 |
| Mercury compounds | 2 (2) | 5 (1) | 1.4 (0.26–7.5) | 0.692 |
| Pybuthrin | 0 (0) | 6 (2) | NA | |
| Any oxidative stressor | 35 (40) | 93 (28) | 2.0 (1.2–3.6) | 0.012 |
| Mitochondrial complex I inhibitors
| ||||
| Benomyl | 7 (7) | 15 (4) | 1.9 (0.70–5.0) | 0.207 |
| Carbendazim | 1 (1) | 2 (1) | 2.2 (0.19–25) | 0.529 |
| Cyhalothrin | 1 (1) | 1 (0) | 3.8 (0.22–64) | 0.359 |
| Permethrin | 16 (16) | 41 (12) | 1.5 (0.77–2.9) | 0.244 |
| Pyridaben | 0 (0) | 1 (0) | NA | |
| Rotenone | 19 (19) | 32 (9) | 2.5 (1.3–4.7) | 0.005 |
| Thiabendazole | 3 (3) | 12 (3) | 0.8 (0.23–3.1) | 0.778 |
| Any complex I inhibitor | 36 (38) | 92 (27) | 1.7 (1.0–2.8) | 0.041 |
NA, not available. Analyses used logistic regression adjusted for reference age tertile, sex, state, and cigarette smoking.
Associations between ever use of paraquat or rotenone and PD were similar when exposures were truncated at 5, 10, or 15 years before the diagnosis or reference date [paraquat: 5 years prior OR = 2.7 (95% CI, 1.4–4.9), 10 years OR = 2.9 (95% CI, 1.6–5.5), 15 years OR = 3.1 (95% CI, 1.6–5.8); rotenone: 5 years prior OR = 2.3 (95% CI, 1.2–4.4), 10 years OR = 2.4 (95% CI, 1.3–4.6), 15 years OR = 2.4 (95% CI, 1.3–4.6]. Exposures to rotenone and paraquat were not correlated (r = 0.004), and ORs from a model that included both pesticides (paraquat OR = 2.6; 95% CI, 1.4–4.9; rotenone OR = 2.9; 95% CI, 1.5–5.5) were comparable with those from models without mutual adjustment (Table 3). For men who used paraquat plus any dithiocarbamate (7 cases, 16 controls), the OR compared with use of neither was 2.2 (95% CI, 0.79–5.9). Compared with never users, associations with PD were generally stronger among those who had used pesticides for more than the median number of lifetime days than among those who had used pesticides for fewer than the median number of days [Supplemental Material, Table 3 (doi:10.1289/ehp.1002839)].
Results from analyses stratified by race/ethnicity, cigarette use, state, or duration of disease (in cases) were not appreciably different between subgroups (data not shown). In an analysis restricted to 18 cases and 22 controls (28 men and 12 women) with a first-degree relative with PD, PD was not associated with ever exposure to rotenone (OR = 0.98) or paraquat (OR = 1.1). Adjustment for education or overall pesticide use did not appreciably alter effect estimates, nor did exclusion of 28 cases and 6 controls with proxy interviews or inclusion of 3 atypical parkinsonism cases.
We compared clinical features of cases who did or did not use paraquat or any oxidative stressor, or rotenone or any complex I inhibitor (Table 4). PD was diagnosed at a younger age among those who used oxidative stressors (mean age = 59 vs. 64 years, p = 0.02). Although postural reflex impairment appeared to be less frequent in those using either paraquat or rotenone, these differences were not statistically significant. Clinical features did not otherwise differ between groups. We were unable to determine whether exposure to both paraquat and rotenone was associated with unique clinical features because only five cases reported use of both pesticides (data not shown).
Table 4.
Clinical features of PD cases by use of paraquat or rotenone or mechanistic group.
| Paraquat
|
Any oxidative stressor
|
Rotenone
|
Any complex I inhibitor
|
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Exposed (n = 23) | Not exposed (n = 74) | Exposed (n = 35) | Not exposed (n = 53) | Exposed (n = 19) | Not exposed (n = 82) | Exposed (n = 36) | Not exposed (n = 59) |
| Age at PD diagnosis [years (mean ± SD)] | 59 ± 8 | 62 ± 9 | 59 ± 8 | 64 ± 9* | 65 ± 11 | 61 ± 8 | 61 ± 11 | 62 ± 8 |
| PD duration [years (mean ± SD)] | 8.6 ± 6 | 8.4 ± 6 | 8.2 ± 6 | 7.6 ± 5 | 7.7 ± 7 | 8.7 ± 5 | 8.2 ± 6 | 8.5 ± 5 |
| Clinical features [n with feature/n with data available (%)] | ||||||||
| Resting tremor | 21/22 (95) | 66/73 (90) | 31/34 (91) | 49/53 (92) | 17/18 (94) | 75/81 (93) | 32/35 (91) | 54/58 (93) |
| Bradykinesia | 22/23 (96) | 70/74 (95) | 33/35 (94) | 50/53 (94) | 17/19 (89) | 79/82 (96) | 34/36 (94) | 56/59 (95) |
| Rigidity | 23/23 (100) | 72/73 (99) | 33/34 (97) | 50/53 (94) | 18/19 (95) | 80/80 (100) | 34/35 (97) | 58/58 (100) |
| Postural reflex impairment | 10/21 (48) | 43/62 (69) | 18/31 (58) | 29/43 (67) | 7/15 (47) | 48/69 (70) | 16/28 (57) | 37/52 (71) |
| Asymmetric onset | 23/23 (100) | 69/71 (97) | 35/35 (100) | 49/51 (96) | 18/18 (100) | 77/79 (97) | 35/35 (100) | 54/56 (96) |
| Response to dopaminergic therapy (if prescribed) | 21/21 (100) | 63/65 (97) | 30/30 (100) | 46/48 (96) | 14/15 (93) | 73/74 (99) | 30/31 (97) | 53/54 (98) |
Difference between exposed versus not exposed p = 0.02.
Discussion
The pathogenesis of PD is thought to involve several critical abnormalities, each of which can be the result of genetic or environmental factors. Chief among these abnormalities are dysfunction of the mitochondrial respiratory chain, particularly complex I, and the production of reactive oxygen species (Henchcliffe and Beal 2008). To our knowledge, we have performed the first analysis of pesticides classified by presumed mechanism, rather than by functional categories (e.g., herbicides) or chemical class (e.g., organochlorines). We found significant associations of PD with use of groups of pesticides classified as complex I inhibitors or as oxidative stressors, providing support in humans for findings from decades of experimental work. In particular, PD was strongly associated with rotenone and paraquat, two individual pesticides used extensively to model PD in the laboratory.
This study provides strong evidence of an association between rotenone use and PD in humans. PD developed 2.5 times as often in those who reported use of rotenone compared with nonusers, and an association of similar magnitude was observed even when exposure was truncated up to 15 years before PD diagnosis. In our prior analysis of self-reported PD in the AHS, information on rotenone was available for a small subgroup, and nonsignificant association with PD (OR = 1.7; 95% CI, 0.6–4.7) was observed (Kamel et al. 2007), whereas in a multicenter, clinic-based case–control study distinct from the AHS, only two individuals were exposed and no association was observed (Tanner et al. 2009). In the only other report of rotenone-like compounds and PD, use of organic pesticides such as rotenone in the previous year was determined for PD clinic attendees, who reported current use more often than did cases with other neurologic diseases (Dhillon et al. 2008). This information cannot be used to assess etiology, because the study evaluated associations with rotenone use that occurred after PD had been diagnosed. In contrast, in the present population-based study, we evaluated rotenone use before PD diagnosis in cases and during a comparable time period in neurologically healthy controls.
Rotenone is a plausible cause of PD because of its mechanism of action. Like 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a toxicant known to cause parkinsonism in humans, rotenone directly inhibits mitochondrial complex I (Langston et al. 1983; Sherer et al. 2007). In experimental models, both MPTP and rotenone cause selective injury of dopaminergic neurons in the substantia nigra, a key pathological feature of PD (Greenamyre et al. 1999; Langston et al. 1984). Because rotenone is believed to have a relatively short environmental half-life and limited bioavailability, a relationship to human disease has been questioned (Hatcher et al. 2008; Li et al. 2005). However, recent work in rodent models indicated that a temporally limited exposure to rotenone later caused progressive functional and pathologic changes in the enteric nervous system of rodents, mimicking changes found in human PD; as in PD, these enteric nervous system changes preceded central nervous system pathology (Abbott et al. 2001; Braak et al. 2006; Drolet et al. 2009; Greene et al. 2009; Pan-Montojo et al. 2010). Chronic rotenone exposure in the laboratory has been reported to have additional effects associated with PD pathogenesis, including ones similar to changes observed in monogenic forms of PD (Henchcliffe and Beal 2008). Rotenone toxicity, therefore, provides a conceptual bridge, suggesting shared mechanisms for both sporadic and inherited forms of PD.
Although we report here findings for agricultural use of rotenone, the ubiquitous use of rotenone in both work and home settings that occurred until recently suggests that many people may have been exposed. Humans have used rotenone-containing plants as pesticides for centuries (Cabras et al. 2002). Because rotenone is plant derived, it has been considered an organic pesticide and was commonly used as a household insecticide in home gardening and agriculture, and to kill fish. For example, the California Department of Pesticide Regulation (2007) reported that almost 15,000 pounds of rotenone were used in 2007, not including home use. Rotenone was withdrawn from use in the European Union in 2007 (Schapira 2010), after which time most uses were voluntarily cancelled in the United States (U.S. EPA 2007). Other agents associated with mitochondrial complex I inhibition remain in common use. For example, permethrin is used in nonagricultural settings as an insect repellant, including use of permethrin-impregnated fabric for military uniforms and recreational clothing (Armed Forces Pest Management Board 2010).
Our study also extends prior research on paraquat. Experimentally, paraquat produces subcellular changes associated with PD, including increased production of reactive oxygen species, alpha-synuclein aggregation, and selective nigral injury (Dinis-Oliveira et al. 2006; Kuter et al. 2010; McCormack et al. 2002). Previously, we found an association between paraquat use and PD in prevalent but not incident self-reported cases in the AHS (Kamel et al. 2007) and a nonsignificant association between PD and occupational paraquat use in a multicenter case–control study (Tanner et al. 2009). Cumulative use was not assessed in either study. Only a few other studies have assessed associations between PD and paraquat use (Table 1). A study of 120 cases and 240 controls conducted in Taiwan (Liou et al. 1997) reported an OR of 3.22 (95% CI, 2.41–4.31) for PD in paraquat users compared with nonusers. Cumulative exposure was associated with greater risk, but paraquat use in the Taiwanese study was highly correlated with use of other herbicides. Although the inconsistency of findings in human populations has been used as a basis for suggesting that paraquat is not associated with PD (Li et al. 2005; Miller 2007), an alternative explanation is that few studies have had adequate size and sufficiently detailed exposure information to allow the association to be observed. Our findings, considered together with earlier results, suggest that paraquat use plays a role in human PD. Because paraquat remains one of the most widely used herbicides worldwide (Frabotta 2009), this finding potentially has great public health significance.
Parkinsonism in humans due to high-dose exposure to toxicants such as carbon monoxide or manganese has characteristic clinical features including less prominent tremor, more prominent postural instability, symmetric distribution of signs, and poor response to dopaminergic therapy (Tanner 1992). We did not observe such features in our cases. Cases who did or did not use rotenone, paraquat, or groups of pesticides with similar mechanisms were generally similar, suggesting that PD associated with these agents is clinically typical and indirectly supporting a role for pesticide exposure in the etiology of typical PD. We did note an earlier age at diagnosis in users of oxidative stressors and a suggestion of this in paraquat users specifically. Early age at onset is also a characteristic of genetic parkinsonism in which oxidative stress is a presumed pathophysiologic mechanism (alpha-synuclein, PINK-1, DJ-1, and PARKIN mutations) (Henchcliffe and Beal 2008; Klein et al. 2009).
In FAME, pesticide exposure was not associated with PD in individuals with a family history, although numbers were small. Interestingly, Hancock et al. (2008) similarly found pesticide exposure to be associated with PD risk only in those without an affected first-degree relative.
Our study has some limitations. First, because most participants were exposed to many pesticides, we cannot confidently exclude effects of agents other than those studied or rule out the possibility that our results are attributable to combined exposures. However, the associations that we observed remained after adjustment for overall pesticide use, and estimated effects of rotenone and paraquat were comparable after mutual adjustment. Future investigations of combinations of pesticides and of other mechanistic groups will be important. Second, we could not use laboratory measures of pesticides or their metabolites to estimate exposure. Such measures are not available for many of the pesticides we studied, and when available, they are poor predictors of past or long-term use. Thus, although we recognize that retrospective exposure assessment has limitations, it is often the best approach for studying lifelong exposure in an adult population in connection with a rare disease. Third, we included prevalent cases already diagnosed but still living at enrollment in the AHS; therefore, survivor bias is possible. However, our results were similar when only those with shorter disease duration were analyzed. Additionally, we were able to investigate only persons willing to participate. Thus, PD cases or controls in this study may not have been fully representative of the entire population. However, participation was good, partially allaying this concern. Finally, we selected pesticides presumed to act through specific toxic mechanisms, but for most pesticides there is very little information regarding toxic effects in humans, as most studies are directed toward effects on plant or animal pests. It is likely that we have misclassified some pesticides with regard to mechanism. However, the likely effect of any misclassification would be to attenuate an association with pesticides grouped according to a common mechanism.
Strengths include the size of the study; the focus on an agricultural cohort with many exposed individuals and wide variability in exposure; the quality of diagnosis, which was based on in-person assessment and agreement of movement disorders experts; and the completeness and reliability of the pesticide exposure information. An additional strength is the nested case–control design with an internal control group who had similar exposure opportunities as the cases and similar demographic and lifestyle characteristics, reducing the likelihood of bias or confounding. Use of pesticides in general was ubiquitous, of course, in applicators and relatively common among their spouses, and all participants may have had additional passive pesticide exposure. However, these features would all be likely to lower the chance of identifying any effect.
Our study helps connect the dots between basic research and human populations. Rotenone and paraquat have been linked experimentally to pathophysiological mechanisms implicated in human PD. Groups of pesticides linked to the mechanisms of mitochondrial dysfunction or oxidative stress were also associated with PD in our study, thus extending experimental work to provide strong evidence that these mechanisms play a role in PD in humans. Importantly, the potential for exposure to many of these pesticides, including rotenone and paraquat, extends well beyond the occupational setting. Many persons with nonoccupational pesticide exposures may be unaware of the presence of pesticides in their environments (Centers for Disease Control and Prevention 2009). The potential for exposure to other toxicants with similar mechanisms is even greater. To continue the interplay between human and experimental studies, future mechanistic studies of these pesticides should model exposure conditions similar to those occurring in humans, including chronic low-dose exposure, exposure to multiple agents, and assessment of gene–exposure interactions. Such work could provide new insights into the pathogenesis and ultimately the prevention of PD.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.1002839 via http://dx.doi.org/).
The authors thank the participants, the research team, T. Greenamyre for manuscript review, and J. Wright for editorial assistance.
This study was supported in part by the Intramural Research Program of the National Institutes of Health [National Institute of Environmental Health Sciences (NIEHS) grants Z01-ES044007 and Z01-ES049030, National Cancer Institute grant Z01-CP010119], NIEHS grant R01-ES10803, and James and Sharron Clark.
References
- Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armed Forces Pest Management Board. Permethrin – Impregnated Clothing. 2010. [[accessed 4 November 2010]]. Available: http://fhpr.osd.mil/factsheetDetail.jsp?fact=27.
- ATSDR (Agency for Toxic Substances and Disease Registry) Home page. 2002. [[accessed 15 January 2002]]. Available: http://www.atsdr.cdc.gov/
- Bain P, Brin M, Deuschl G, Elble R, Jankovic J, Findley L, et al. Criteria for the diagnosis of essential tremor. Neurology. 2000;54(11 suppl 4):S7. [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Blair A, Tarone R, Sandler D, Lynch CF, Rowland A, Wintersteen W, et al. Reliability of reporting on life-style and agricultural factors by a sample of participants in the Agricultural Health Study from Iowa. Epidemiology. 2002;13(1):94–99. doi: 10.1097/00001648-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Cabras P, Caboni P, Cabras M, Angioni A, Russo M. Rotenone residues on olives and in olive oil. J Agric Food Chem. 2002;50(9):2576–2580. doi: 10.1021/jf011430r. [DOI] [PubMed] [Google Scholar]
- California Department of Pesticide Regulation. Pesticide Use Reporting (PUR) 2007. [[accessed 12 September 2009]]. Available: http://www.cdpr.ca.gov/docs/pur/purmain.htm.
- Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. 2009. [[accessed 17 December 2009]]. Available: http://www.cdc.gov/ExposureReport/index.html.
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169(8):919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli Esposti M. Inhibitors of NADH-ubiquinone reductase: an overview. Biochim Biophys Acta. 1998;1364(2):222–235. doi: 10.1016/s0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Tarbutton GL, Levin JL, Plotkin GM, Lowry LK, Nalbone JT, et al. Pesticide/environmental exposures and Parkinson’s disease in East Texas. J Agromedicine. 2008;13(1):37–48. doi: 10.1080/10599240801986215. [DOI] [PubMed] [Google Scholar]
- Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson’s disease. Neurotoxicology. 2002;23(4–5):487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Navarro AS, Bastos ML, et al. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology. 2006;27(6):1110–1122. doi: 10.1016/j.neuro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) Laryngoscope. 1996;106(3 pt 1):353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- Drolet RE, Cannon JR, Montero L, Greenamyre JT. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol Dis. 2009;36(1):96–102. doi: 10.1016/j.nbd.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Elble RJ. Diagnostic criteria for essential tremor and differential diagnosis. Neurology. 2000;54(11 suppl 4):S2–S6. [PubMed] [Google Scholar]
- EXTOXNET (Extension Toxicology Network) Home page. 2002. [[accessed 3 February 2002]]. Available: http://pmep.cce.cornell.edu/profiles/extoxnet/
- Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. II. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s Disease and Movement Disorders. Baltimore: Williams & Wilkins; 1993. pp. 225–234. [Google Scholar]
- Firestone JA, Lundin JI, Powers KM, Smith-Weller T, Franklin GM, Swanson PD, et al. Occupational factors and risk of Parkinson’s disease: a population-based case-control study. Am J Ind Med. 2010;53(3):217–223. doi: 10.1002/ajim.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone JA, Smith-Weller T, Franklin G, Swanson P, Longstreth WT, Jr, Checkoway H. Pesticides and risk of Parkinson disease: a population-based case-control study. Arch Neurol. 2005;62(1):91–95. doi: 10.1001/archneur.62.1.91. [DOI] [PubMed] [Google Scholar]
- Frabotta D. Paraquat: Herbicide Helps No-till Operations. 2009. [[accessed 17 December 2009]]. Available: http://www.farmchemicalsinternational.com/magazine/?storyid=413.
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163(1):94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, MacKenzie G, Peng TI, Stephans SE. Mitochondrial dysfunction in Parkinson’s disease. Biochem Soc Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp Neurol. 2009;218(1):154–161. doi: 10.1016/j.expneurol.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, et al. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [Online 28 March 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29(6):322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Bowering D, Snow B, Calne D. Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am J Ind Med. 1990;17(3):349–355. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Snow B, Kelly S, Calne D. A case-control study of Parkinson’s disease in a horticultural region of British Columbia. Mov Disord. 1994;9(1):69–75. doi: 10.1002/mds.870090111. [DOI] [PubMed] [Google Scholar]
- Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Yucel F, Dosemeci M, Sandler DP. Accuracy of self-reported pesticide use duration information from licensed pesticide applicators in the Agricultural Health Study. J Expo Anal Environ Epidemiol. 2002;12(5):313–318. doi: 10.1038/sj.jea.7500232. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165(4):364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- Klein C, Schneider SA, Lang AE. Hereditary parkinsonism: Parkinson disease look-alikes—an algorithm for clinicians to “PARK” genes and beyond. Mov Disord. 2009;24(14):2042–2058. doi: 10.1002/mds.22675. [DOI] [PubMed] [Google Scholar]
- Krieger R. Handbook of Pesticide Toxicology. 2nd ed. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Kuter K, Nowak P, Golembiowska K, Ossowska K. Increased reactive oxygen species production in the brain after repeated low-dose pesticide paraquat exposure in rats. A comparison with peripheral tissues. Neurochem Res. 2010;35(8):1121–1130. doi: 10.1007/s11064-010-0163-x. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984;292(2):390–394. doi: 10.1016/0006-8993(84)90777-7. [DOI] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks DJ, Fahn S, Freeman TB, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- Li AA, Mink PJ, McIntosh LJ, Teta MJ, Finley B. Evaluation of epidemiologic and animal data associating pesticides with Parkinson’s disease. J Occup Environ Med. 2005;47(10):1059–1087. doi: 10.1097/01.jom.0000174294.58575.3e. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, et al. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48(6):1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, et al. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10(2):119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Miller GW. Paraquat: the red herring of Parkinson’s disease research. Toxicol Sci. 2007;100(1):1–2. doi: 10.1093/toxsci/kfm223. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. PubChem. 2002. [[accessed 23 January 2002]]. Available: http://pubchem.ncbi.nlm.nih.gov/
- Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5(1):e8762. doi: 10.1371/journal.pone.0008762. [Online 19 January 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ramos JR, Hefti F, Weiner WJ. Paraquat and Parkinson’s disease. Neurology. 1987;37(4):728. doi: 10.1212/wnl.37.4.728. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Complex I: inhibitors, inhibition and neurodegeneration. Exp Neurol. 2010;224:331–335. doi: 10.1016/j.expneurol.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42(7):1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, et al. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. J Neurochem. 2007;100(6):1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM. Occupational and environmental causes of parkinsonism. Occup Med. 1992;7(3):503–513. [PubMed] [Google Scholar]
- Tanner CM, Ross GW, Jewell SA, Hauser RA, Jankovic J, Factor SA, et al. Occupation and risk of parkinsonism: a multicenter case-control study. Arch Neurol. 2009;66(9):1106–1113. doi: 10.1001/archneurol.2009.195. [DOI] [PubMed] [Google Scholar]
- Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6(1):45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson’s disease? Brain Res. 2000;873(2):225–234. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5(1):75–86. doi: 10.1016/S1474-4422(05)70285-4. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) Reregistration Eligibility Decision for Rotenone. March 2007 EPA 738-R-07-005. 2007. [[accessed 17 November 2009]]. Available: http://www.epa.gov/pesticides/reregistration/REDs/rotenone_red.pdf.
- Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500(3):105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]