Abstract
Introduction
The P2X7 receptor (P2X7R) has an important role in inflammation and immunity, but until recently, clinical application has been limited by a lack of specific antagonists. Recent studies using P2X7R knockout (KO) mice and specific receptor antagonists have shown that the P2X7R is an important therapeutic target in inflammatory diseases.
Areas covered
We have reviewed the current literature on the role of the P2X7R in inflammatory diseases, focusing on potential therapeutic applications of selective P2X7R antagonists as an anti-inflammatory agent. Particular emphasis has been placed on the potential role of P2X7R in common inflammatory diseases. The latest developments in phase I and II clinical trials of P2X7R antagonists are covered.
Expert opinion
Recent studies using gene KO mice and selective P2X7R antagonists suggest that P2X7R is a viable therapeutic target for inflammatory diseases. However, efficacious P2X7R antagonists for use in clinical studies are still at an early stage of development. Future challenges include: identifying potential toxicity and side effects of treatment, timing of treatment initiation and its duration in chronic inflammatory conditions, optimum dosage, and development of a functional assay for P2X7R that would help to guide treatment.
1. Introduction
1.a. Purinergic receptors
An important early conceptual shift proposed by Burnstock in 1970 was that intracellular adenosine triphosphate (ATP) might have an additional role outside cells (extracellular ATP) as a signalling molecule and the neurotransmitter in so-called non-cholinergic, non-adrenergic (NANC) neurotransmission. Since then our understanding of the role of ATP as an extracellular messenger (purinergic cell signalling) that is not confined to the nervous system has increased dramatically; many roles for purinergic receptors in health and disease have now been identified.
In 1978, Burnstock sub-classified the purinergic receptors into P1 and P2 receptors: adenosine acting on P1 receptors, whereas ATP and its breakdown products, ADP and AMP, acting on P2 receptors. Burnstock and Kennedy later proposed a further sub-classification of the P2 receptors, dividing them into P2X and P2Y receptors: P2X receptors are inotropic ligand-gated non-selective cation channel receptors and P2Y receptors are G protein coupled receptors; there are currently seven P2X subtypes and eight P2Y subtypes [1].
The P2X7 receptor (P2X7R) is a distinct member of the P2X subclass, as its downstream signalling is coupled to pro-inflammatory cascades. It is a 595-amino acid polypeptide with two membrane-spanning domains and has a long intracellular C-terminus compared with the other P2X receptors [2]. This receptor is expressed on many types of cells, the most studied being macrophages and monocytes, and it has a key role in regulating cell survival and release of mature IL-1β and IL-18 cytokines [3].
1.b. IL-1β, IL-18, and caspase-1
IL-1 is a central player in the inflammatory cascade. It is produced by many types of cells, including activated monocytes and macrophages. It has a variety of effects on its target cells by activation of signal transduction pathways, such as MAPK and NF-κB, resulting in upregulation of several gene products in the inflammatory cascade, such as COX-2, IL-6, chemokines, and cellular adhesion molecules. IL-1 has two isoforms, IL-1α and IL-1β, which bind to the same receptors and are biologically active [4,5].
Inflammatory stimuli, especially LPS, engage with the TLR4 receptor of T cells, which activates MAPK and/or NFκB signalling cascades, resulting in the synthesis of pro-IL-1β. Pro-IL-1α is constitutively expressed and does not require TLR stimulation [6]. Both IL-1α and IL-1β are produced as 31 kDa precursors that are stored within the cell cytosol. The pro- IL-1β precursor remains within the cytosol and is cleaved to its mature form through the action of caspase-1 (or interleukin 1β Converting enzyme). Pro- IL-1β is also cleaved into its active form by other enzymes such as serine proteases (e.g., proteinase 3) and metalloproteinases (MMP-2 and MMP-9). IL-1α processing is via the Ca-dependent protease calpain [7]. IL-1α has similar biological activity in its precursor and cleavage product forms; in contrast, IL-1β is only active once it is cleaved to its 17 kDa mature form. Caspase-1 is crucial for the processing of intracellular pro-IL-1β; although extracellular pro-IL-1β can be processed by a number of different proteases during inflammation [3,8].
Caspase-1 is itself produced from the constitutively expressed 45 kDa pro-enzyme, pro-caspase, which is also found in the cytoplasm; it requires post-translational processing to form 20 and 10k Da forms of active caspase-1 [8]. Activation of caspase-1 occurs following assembly of an intracellular complex known as the ‘inflammasome’. The NALP3 inflammasome is a multiprotein complex containing NALP3 (crypopyrin), apoptosis-associated speck-like protein (ASC), and caspase-1, which oligomerise on cell activation [9]. Proteolytic activation of IL-1β occurs within the inflammasome protein complex. However, the exact mechanism of inflammasome formation and activation is not fully understood, since it can be triggered by different mechanisms in different cell types. Mature IL-1β may also be released into the extracellular space by exocytosis in small vesicles or as a result of loss of membrane integrity [10].
In addition to IL-1, IL-18 is a key mediator in the host response to infection and the inflammatory response [4,11]. IL-18 is also constitutively produced as a precursor, pro-IL 18 [11]. Pro-IL-18 is cleaved by either caspase-1 or proteinase-3 (cf. pro IL-1β above) into its active form, which is released into the extracellular space along with mature IL-1β. Extracellular release of active IL-1β and IL-18 is dependent on ATP-sensitive P2X7R activation. Contact with LPS alone is insufficient for extracellular release of IL-1β and IL-18; any one of a number of different stimuli, including extracellular ATP, nigericin, bacterial toxins, hypotonic stress, and T cells are usually required. The best-established stimulus to IL-1β and IL-18 post-translational processing and release is ATP acting via the P2X7R [3,11].
Experimental evidence supporting an attenuated inflammatory response with the use of P2X7R antagonists, or in P2X7R KO mice, may be directly related to the inhibition of IL-1β release. However, the attenuated inflammatory response may also be related to the lack of induction of other inflammatory mediators. IL-1β may induce or release nitric oxide, COX-2, superoxide products, and other pro- inflammatory mediators [6,12], which must be considered when interpreting experimental data.
1.c. P2X7R
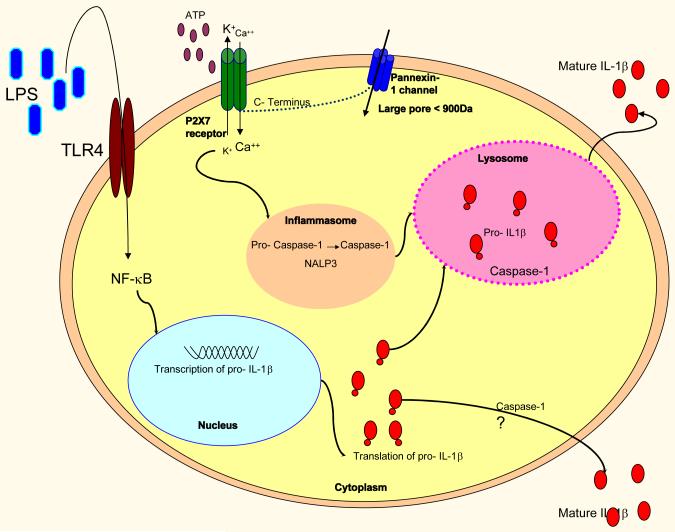
P2X7R expression was originally defined in macrophages and monocytes [13], and has since been characterised in several different cell types. The expression and activity of P2X7R in monocytes and macrophages is significantly upregulated in response to inflammatory stimuli, including LPS. Unlike other P2X receptors, the P2X7 receptor in distinct because its downstream signalling is coupled to pro-inflammatory cascades [2]. During active inflammation, the local concentrations of extracellular ATP are raised, which is thought to be mainly a consequence of cell damage and lysis. To activate the P2X7R in vitro, extracellular concentrations of ATP in the range of 1mM are required, in contrast to concentrations of ≤100μM needed to activate other P2 receptors. The ATP molecule binds to and activates P2X7, resulting in pore formation. This leads to K+ efflux from the cell, which is a crucial step in inflammasome assembly. Macrophages treated with ATP in medium containing KCl (rather than NaCl) failed to activate and release IL-1β, suggesting that an ATP-induced K+ efflux from the cell is necessary for release of mature IL-1β, IL-1α, and IL-18. In addition to K+ efflux from the cell, there is an influx of Ca2+, which is also required for the release of active IL-1β (Figure 1) [3,10]. Prolonged activation of the P2X7R results in irreversible pore formation and allows the non-selective passage of ions and hydrophilic solutes of up to 900Da; this can result in colloido-osmotic lysis and cell death by apoptosis or necrosis [3]. Pore formation is also thought to allow entry of bacterial products (pathogen associated molecular proteins, PAMPs) and extracellular ATP into the cell, which drives inflammasome formation [14].
Figure 1. Mechanism of action of the P2X7 receptor.
LPS engages with the Toll receptor 4 (TLR4). This results in activation of NFκB and nuclear transcription of IL-1β. IL-1β mRNA is subsequently translated into the precursor molecule pro- IL-1β within the cytoplasm [1]. IL-18 is transcribed and translated constitutively [2].
LPS ‘primes’ the P2X7R. On contact with ATP and/or LL37, the P2X7R forms a non-selective ion pore [2]. This allows the efflux of K+ and the influx of Ca2++ [3]. The change in membrane potential and intracellular Ca2+ and K+ results in the assembly of the inflammasome and drives the conversion of pro- caspase-1 into mature caspase-1. Pro- IL-1β and caspase-1 are incorporated into the lysosome. Pro-Il-1β is converted into mature IL-1β by the action of caspase-1 within the lysosome and is released into the extracellular environment [4].
Caspase-1-dependent processing of pro-IL-1β may occur in the cytosol following activation of P2X7-receptor and may be released via mechanisms independent of the lysosomal pathway (marked with ‘?’) [5,6]. The P2X7 receptor is coupled to a dye uptake pore, pannexin-1, which opens on prolonged stimulation of the P2X7R [7]. Opening of the pannexin pore allows the passage of hydrophilic solutes into the cell. This is thought to result in cell death [1].
- *Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The p2x7 receptor: A key player in il-1 processing and release. J Immunol 2006;176:3877-83. (A useful review of P2X7)
- Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol 2007;27:98-114.
- North RA. Molecular physiology of p2x receptors. Physiol Rev 2002;82:1013-67.
- Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by p2x7 receptor-mediated k+ release. Am J Physiol Cell Physiol 2004;286:C1100-8.
- Eder C. Mechanisms of interleukin-1beta release. Immunobiology 2009;214:543-53.
- Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci 2007;120:772-81.
- Pelegrin P, Surprenant A. The p2x(7) receptor-pannexin connection to dye uptake and il-1beta release. Purinergic Signal 2009;5:129-37.
Oxidised ATP (oATP) is a potent, but unselective, P2X7R antagonist and it can attenuate inflammatory responses independent of P2 receptor blockade [15], which must considered when interpreting much of the experimental data referred to in this review; oATP has been one of the most widely used P2X7R antagonist. More selective P2X7R antagonists exist and they have been used successfully in in vitro experiments (Table 1), and in Phase 1 and Phase 2 clinical trials (Table 2).
Table 1.
P2X7R and inflammatory diseases
| P2X7R and renal disease | |||||
|---|---|---|---|---|---|
| Reference | Disease/ Stimulation |
P2X7 expression (Cell type) |
in vivo/ in vitro |
Species | KO/ Antagonist |
| Turner [32] | None | None | In vivo | Rat | - |
| Harada [33] | TNF-α | Mesangial cells | In vitro | Rat | - |
| Gonclaves [35] | Unilateral ureteric obstruction |
Tubular epithelial cells | In vivo | Mouse | KO |
| Vonend [34] | HTN | Glomeruli | In vivo | Rat | - |
| DM | Glomerular podocytes | In vivo | Rat | - | |
| Turner [36] | Experimental glomerulonephritis |
Glomeruli and macrophages |
In vivo | Mouse Rat |
|
| Turner [36] | Lupus Nephritis | Glomerular | In vivo | Humans | - |
| Taylor [91] | Experimental Glomerulonephritis |
In vivo | Rat Mouse |
Selective antagonist A438079 KO |
|
| Gauer [92] | None | Collecting duct alpha- and beta-intercalated cells |
In vivo | Humans | - |
| P2X7R and respiratory disease | |||||
| Reference | Disease/stimulation |
P2X7 expression (Cell type) |
in vivo/ in vitro |
Species |
KO/ Antagonist |
| Mizuno [39] | Con A | Monocytes | In vitro | Humans | oATP |
| Lemaire [93] | Polymixin B | Alveolar macrophages | In vitro | Rat | oATP |
| Wareham [40] | ATP | Lung mast cells | In vitro | Human | AZ11645373 |
| Fernando [41] | ATP-mediated mycobacterial killing |
Macrophages | In vitro | Human | Loss of function SNP |
| Kolliputi [44] | Hyperoxia | Alveolar macrophages |
in vitro and
in vivo |
Mice | oATP |
| Dellinger [48] | Asthma | ‘Whole blood’ | in vivo | Human | - |
| Muller [46] | Acute and chronic asthmatic airways inflammation |
Dendritic cells Bronchial lavage macrophages and peripheral blood eosinophils of humans |
In vivo
In vitro |
Mice and humans |
KO oATP, KN62 (in mice) |
| Lucatelli [45] | Cigarette smoke (CS)-induced lung inflammation and emphysema |
Blood and airway neutrophils, alveolar macrophages, and in whole lung tissue |
In vivo | Mice | KO KN62 |
| Riteau [47] | Bleomycin-induced lung inflammation and fibrosis |
Respiratory epithelial cells |
In vivo
In vitro |
Mice | KO In vitro A438079 and A740003 |
| P2X7R and rheumatological disease | |||||
| Reference | Disease/stimulation |
P2X7 expression (Cell type) |
in vivo/ in vitro |
Species |
KO/ Antagonist |
| Caporali [53] | Patients with RA | Synoviocytes | In vivo | Human | - |
| Al- Shukaili [54] | Patients with RA | Peripheral blood mononuclear cells produced more IL-1β |
In vitro | Human | - |
| Lopez- Castejon [57] |
ATP-induced cathepsin release |
Macrophages (Human) Bone marrow-derived macrophages (mice) release of lysosomal cathepsins |
In vitro | Humans Mice |
Antagonist (AZ10573295 and AZ11648720) KO |
| Dell’ Antonio [94] |
Arthritis was induced by the local injection of Freund’s complete medium |
Peripheral nerve endings, and in epithelial cells |
In vivo | Rats | oATP |
| Christenson [26] | SAA purified from patients with RA suppresses a-CD95 (FAS) mAb-induced human neutrophil apoptosis |
Neutrophil | In vitro | Human | oATP |
Table 2.
Summary of Clinical studies (Phase I and Phase II) investigating P2X7 receptor antagonists
| Study design | Study aim | Patients included | Intervention | Primary Outcome Result |
|---|---|---|---|---|
| An Open, Randomised, 2- way Crossover Study (AZD9056; Astra Zeneca) (Study code: D1520C05285) [1] (2003) |
To establish the absolute bioavailability of AZD9056 in tablet formulation (200 mg) compared with an intravenous formulation (25 mg) |
12 healthy Volunteers | AZD9056 was provided as two 100 mg tablets for oral administration, and as a 100 mL intravenous (iv) infusion of 25 mg. |
The mean absolute bioavailability was estimated as 62% There was evidence of secondary peak at 5 hours post-dose in the mean plasma concentration profiles for both the tablet and the iv formulations. The geometric mean t½ was approximately the same for each formulation (18 hours for iv and 16 hours for oral). The average plasma clearance was 29 L/h, and the average volume of distribution was 735 L. The median tmax was shorter for the iv infusion (2 hours) than for the oral tablet (3 hours). |
| A Phase I, Double-Blind, Placebo-Controlled, Randomised, Group Comparative Study (AZD9056; Astra Zeneca) Study code: D1520C05216 [2] (2004) |
To Investigate the Safety, Tolerability, PK and PD of Multiple Ascending Doses of AZD9056 in Healthy Volunteers |
48 healthy volunteers | 11 patients were in the 100 mg dose group (Cohort A), and 12 each to two 400 mg dose groups (Cohorts B and C) and the 600 mg dose group (Cohort D) for 10 consecutive days. |
Thirteen subjects discontinued from the study. Two discontinuations were due to AEs (one in Cohort B and one in Cohort C). The subject in Cohort B subsequently experienced a SAE, and as a result the remainder of Cohort B were also discontinued from the study |
| An Open, Non- Randomised, Crossover Study (AZD9056; Astra Zeneca) (Study Code D1520C00002) [3] (2005) |
To Investigate the Effect of AZD9056 (50 mg Oral Dose for 5 Days) on Cytochrome P450 3A Using the Probe Drug Midazolam (7.5 mg Oral and 1 mg Intravenous Dose) |
12 male and female healthy volunteers |
AZD9056 administered orally as a single 50 mg tablet midazolam administered orally as a 7.5 mg tablet and as an IV bolus of 1 mg At Visit 2, subjects were administered a single 7.5 mg tablet of midazolam. Six hours later, they were administered 1 mg midazolam by iv bolus injection. At Visit 3, subjects were administered 50 mg AZD9056 for 5 days. On Day 5, they were also administered 7.5 mg oral midazolam and 1 mg iv midazolam, as for Visit 2. There was a washout period of 6 days between Visits 2 and 3. |
Results suggest weak inhibition of CYP3A by ADZ9056 The estimate of t½, (20.5 hours) suggests that steady state for AZD9056 would be achieved by Day 4 to 5 |
| Phase II RCT-, double blind, placebo- controlled, parallel group, multicentre international study (AZD9056; Astra Zeneca) (Study code: D1522C00001) [4] (2005) |
Primary outcome: Pain control Secondary outcomes: Safety and tolerability |
Patients with OA of the knee 404 patients enrolled and 108 patients were randomised (54 in each arm). 87 patients completed the study |
AZD9056 given for 28 days as a daily oral single dose (200mg for first 7 days followed by 400mg for next 21 days) |
No significant difference in pain assessment. Discontinuation rate due to intolerability similar. 1 serious AE in ADZ group (viral illness) AEs: GI disturbance, headache |
| Phase II Clinical study RCT-, double blind, placebo- controlled, parallel group, multicentre international study (CREATE Study, 2007) (AZD9056; Astra Zeneca) (Study code: D1520C05287) [5,6] (2006) |
Efficacy and tolerance ACR and DAS28 scores ESR Physician assessment of disease activity Patient assessment of physical function Pain, fatigue, and duration of morning stiffness |
75 patients with RA receiving background treatment with methotrexate and/ or sulphasalazine |
An ascending dose study to compare the efficacy and tolerance of 2 doses of orally administered ADZ9056 (100mg and 400mg) over placebo over a one month period |
ADZ9056 400mg was associated with significantly more patients achieving ACR 20, clinically relevant improvements in DAS28, HAQ, and reduced swollen and tender joints compared with placebo.* No significant improvement in systemic surrogate markers of disease activity (ESR, CRP). ADZ-9056 was well-tolerated-main AEs were gastrointestinal (nausea, diarrhoea, and vomiting) |
| A Phase 1, Double Blind Study NCT00446784 [7] (2007) (CE 224, 535; Pfizer) |
The Safety And PK Of Multiple Doses Of CE 224,535 PK of CE 224, 535 and methotrexate |
Subjects With Rheumatoid Arthritis Receiving Methotrexate |
Multiple doses of CE 224,535 administered to patients with RA who have had at least 4 weeks of methotrexate |
Study completed |
| A 2 Week, Randomized, Double Blind, Placebo And Positive Controlled, Parallel Group, Multicenter Study Of CE-224,535 NCT00418782 [8] CE 224, 535 (2007) |
To evaluate the analgesic and anti inflammatory efficacy and safety of CE 224,535 versus placebo and naproxen treatment in patients with OA knee pain. Safety, tolerability, PK and PD of oral multiple doses of CE 224,535 |
Subjects With Osteoarthritic Pain Of The Knee |
Administration of CE 224,535 or placebo and naproxen in patients with OA knee pain for 2 weeks |
Results of interim analysis indicate lack of efficacy when compared to placebo |
| Phase II RCT-, double blind, placebo- controlled, parallel group, multicentre international study (AZD9056; Astra Zeneca) (Study code: D1521C00002) [9] (2007) |
Primary outcome: Lung function tests, Exercise tolerance, Symptoms, Baseline dyspnoea, Inflammatory markers Secondary outcomes: Safety and tolerability |
Patients with moderate to severe COPD 190 patients were enrolled and 134 patients were randomised-66 to active treatment and 68 to placebo |
AZD9056 given for 28 days as a daily oral single dose (400mg) |
No difference in either primary or secondary outcomes |
| Phase IIa/ III Clinical study RCT- randomised, double blind, placebo- controlled, parallel group, multicentre international study (CE-224,535; Pfizer) NCT 00628095 [10] (2008) |
ACR 20, 50 and 70 scores DAS28 score PK Adverse events Components of ACR responses |
Patients with active RA who have not completely improved with methotrexate |
Twice daily oral CE- 224,535 500mg over a 12- week time period |
Study completed |
| A Phase 1, Randomized, 4-Period, 4-Sequence Cross-Over NCT00782600 [11] CE 224, 535 (2008) |
Study Of The PK Of 3 Durations Of Release Of A Controlled Release Formulation And A Single Dose Of An Immediate Release Oral Suspension Of CE-224,535 |
Normal Healthy Volunteers | Administration of a controlled release formulation and a single dose of an immediate release oral suspension of CE-224,535 |
Study completed |
| Phase IIb randomised, double blind, open arm comparator, parallel group, multicentre international study (AZD9056; Astra Zeneca) NCT00520572 [12] (2008) |
ACR 50 and ACR 70 response Disease activity score (DAS 28) Health assessment questionnaire- disability index (HAQ DI) |
Patients with RA with active disease who are receiving a background of methortexate or sulphasalazine |
Once daily oral ADZ9056 (4 doses- 50mg, 100mg, 200mg, and 400mg) administered for 6 months compared to patients receiving etanercept (25mg IM twice weekly) or placebo |
ACR 20 response at 6 months (treatment: ACR 20 score/ serious adverse events (%))- ADZ9056 50mg: 23/ 3.17 ADZ9056 100mg: 26/ 0 ADZ9056 200mg: 23/ 1.59 ADZ9056 400mg: 21/ 1.56 Placebo: 21/ 1.54 Etanercept: 42/ 3.13 |
| Phase I, single centre, non-randomised, open- label study (AZD9056; Astra Zeneca) NCT00736606 study [13] (2008) |
To assess the PK of both AZD9056 (steady state) and simvastatin (single dose) when co-administered in healthy volunteers |
12 healthy volunteers | One single dose of simvastatin 40mg 400mg once daily of oral ADZ9056 with a single dose of 40mg of simvastatin on day 7 |
Study completed |
| Phase I randomised double- blind, placebo- controlled, 2- period cross over study (AZD9056; Astra Zeneca) NCT00700986 [14] (2008) |
Retinal function as measured by electroretinography Visual acuity, contrast sensitivity, and colour vision Visual evoked potential Psychomotor testing |
12 healthy volunteers | Single dose of oral ADZ9056 800mg |
Study completed |
| Phase II RCT-, double blind, placebo- controlled, parallel group, multicentre international study (AZD9056; Astra Zeneca) (Study Code D8830C00002) [15] (2008) |
Primary outcome: Change in CDAI (Crohn’s disease activity index) Secondary outcomes: Reduction in CDAI by at least 70 points Time to remission Time in study SF-36 and IBD questionnaire |
Patients with active Crohn’s disease affecting the ileum and/ or colon 40 patients were enrolled and 30 patients were randomised-20 to active treatment and 10 to placebo |
AZD9056 given for 28 days as a daily oral single dose (200mg) |
Statistically significant decrease in CDAI in ADZ group* Proportion of patients in remission and with clinical response was greater in the ADZ group Significant improvement in the IBDQ score in the ADZ group AE: Abdominal pain (no difference between groups) |
| Phase I double blind, placebo-controlled study of EVT-401 (Evotec; 2008) [16] |
To evaluate the compound’s safety, tolerability, pharmacokinetic profile, and pharmaodynamic effects |
96 Healthy male volunteers | Single ascending dose study |
Safe and well-tolerated |
| An Open-Label Two-Part Randomized, Crossover Study NCT00838058 [17] CE 224, 535 (2009) |
The PK Of CE-224,535 Administered As Controlled And Immediate Release Formulations |
Healthy Volunteers | Four treatment periods to take either different forms of the pill(s) or the same form either after fasting or eating a meal |
Study completed |
| Phase I open- label, randomised, 2 cohort, 2 period crossover study (AZD9056; Astra Zeneca) NCT00908934 [18] (2009) |
To assess the relative bioavailability of a new formulation of ADZ9056 using PK variables Cmax and AUC compared to the previous phase III to phase IIb formulations of ADZ9056 |
24 healthy volunteers | ADZ9056 formulation Single dose of 50mg or 400mg of both the previous and new formulations |
Study completed |
| An Open-label, Randomized, 2-cohort, 2- period Crossover Study. (AZD9056; Astra Zeneca) (Study Code D1520C00004) [19] (2009) |
To Assess the Relative Bioavailability of the Phase III to the Phase IIb Formulation of AZD9056 in Healthy Male and Female Subjects |
24 Healthy subjects, aged ≥18 to ≤55 years |
Subjects received either 50mg or 400mg of ADZ9056 |
AZD9056 50 mg and 400 mg from both test and reference formulations appeared to be well tolerated |
| A Randomised, Double- Blind (with Open Comparator Etanercept Limb), Placebo- Controlled, Phase IIb, Multicentre Study (AZD9056; Astra Zeneca) (Study Code D1520C00001) [20] (2009) |
To Evaluate the Efficacy of 4 Doses of AZD9056 Administered for 6 Months on the Signs and Symptoms of Rheumatoid Arthritis in Patients with Active Disease Receiving Background Methotrexate or Sulphasalazine |
658 patients were enrolled into the study; 385 of whom were subsequently randomised to study treatment (64 patients assigned to etanercept and each of the AZD9056 dose levels and 65 patients to placebo |
4 doses of AZD9056 (50, 100, 200 and 400 mg) or etanercept for 6 months |
Each of the AZD9056 treatment groups was similar to the placebo group in terms of the ACR20 response rates at week 24. The observed effect in the open-label etanercept arm was clearly distinguishable from placebo and consistent with expectations. A similar pattern was seen for the ACR50 and ACR70. |
| Phase I randomised, single-blind, parallel assignment, safety study NCT00849134 [21] (GSK1482160; GlaxoSmithKline) (2010) |
To monitor the safety parameters, adverse events, PK and PK of single ascending doses of GSK1482160 and to make a preliminary assessment of the effect of food Measuring the amount of inhibition of inflammatory chemical release from blood following dosing with GSK1482160 |
32 healthy volunteers | Multiple doses of the drug (or placebo) with or without a high fat meal |
Study completed |
| Study design | Study aim | Patients included | Intervention | Primary Outcome Result |
Abbreviations
PK: Pharmacokinetics
PD: Pharmacodynamics
1. An open, randomised, 2-way crossover study to establish the absolute bioavailability of azd9056 in tablet formulation (200 mg) compared with an intravenous formulation (25 mg), in healthy volunteers (study code: D1520c05285) 2003.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10629864
2. A phase i, double-blind, placebo-controlled, randomised, group vcomparative study to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of multiple ascending doses of azd9056 in healthy volunteers (study code: D1520c05216). 2004.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10631407
3. An open, non-randomised, crossover study to investigate the effect of azd9056 (50 mg oral dose for 5 days) on cytochrome p450 3a using the probe drug midazolam (7.5 mg oral and 1 mg intravenous dose) (study code d1520c00002) 2005.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10775659
4. A randomised, double-blind placebo-controlled, parallel group study to assess the efficacy, safety and tolerability of azd9056 administered for 28 days in patients with osteoarthritis of the knee (study code: D1522c00001). 2005.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10745200
5. McInnes IB, Snell NJ, Perrett JH, Parmar H, Wang MM, Astbury C. Results of a phase ii clinical trial of a novel p2x7 receptor antagonist, azd9056, in patients with active rheumatoid arthritis (create study). 2007.Available at: http://acr.confex.com/acr/2007/webprogram/Paper8110.html
6. A randomised, double-blind, placebo-controlled, parallel group, ascending dose study to assess the activity, safety and tolerability of 2 doses of azd9056 for 4 weeks in patients with active rheumatoid arthritis receiving methotrexate and/or sulphasalazine (study code: D1520c05287). 2006.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10645408
7. A phase 1, double blind study of the safety and pharmacokinetics of multiple doses of ce 224,535 in subjects with rheumatoid arthritis receiving methotrexate. Clinicaltrials.Gov identifier: Nct00446784. 2007.Available at: http://clinicaltrials.gov/ct2/show?term=CE-+224%2C+535&rank=1
8. A 2 week, randomized, double blind, placebo and positive controlled, parallel group, multicenter study of ce-224,535 in subjects with osteoarthritic pain of the knee. Clinicaltrials.Gov identifier: Nct00418782. 2008.Available at: http://clinicaltrials.gov/ct2/show?term=CE-+224%2C+535&rank=5
9. Phase ii rct-, double blind, placebo-controlled, parallel group, multicentre international study (azd9056; astra zeneca) (study code: D1521c00002) 2007.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10775742
10. A phase 2a, randomized, double-blind, placebo-controlled, parallel-group study of ce-224,535, an antagonist of the p2x7 receptor, in the treatment of the signs and symptoms of rheumatoid arthritis in subjects who are inadequately controlled on methotrexate. Clinicaltrials.Gov identifier: Nct00628095. 2009.Available at: http://clinicaltrials.gov/ct2/show?term=CE224%2C535&rank=2
11. A phase 1, randomized, 4-period, 4-sequence cross-over study of the pharmacokinetics of 3 durations of release of a controlled release formulation and a single dose of an immediate release oral suspension of ce-224,535 in normal healthy volunteers. Clinicaltrials.Gov identifier: Nct00782600. 2009.Available at: http://clinicaltrials.gov/ct2/show?term=CE-+224%2C+535&rank=4
12. A randomised, double-blind (with open comparator etanercept limb), placebo-controlled, phase iib, multicentre study to evaluate the efficacy of 4 doses of azd9056 administered for 6 months on the signs and symptoms of rheumatoid arthritis in patients with active disease receiving background methotrexate or sulphasalazine. 2008.Available at: http://clinicaltrials.gov/ct2/show/NCT00520572?term=AZD9056&rank=5
13. A phase i, single centre, open-label study to assess the pharmacokinetics of both azd9056 (steady state) and simvastatin (single dose) when co-administered in healthy volunteers. Clinicaltrials.Gov identifier: Nct00736606. 2008.Available at: http://clinicaltrials.gov/ct2/show/NCT00736606?term=AZD9056&rank=4
14. A randomised, double-blind, placebo-controlled, 2-period cross-over study in healthy male volunteers, to investigate retinal function following a single 800mg oral dose of azd9056. Clinicaltrials.Gov identifier: Nct00700986. 2008.Available at: http://clinicaltrials.gov/ct2/show/NCT00700986?term=AZD9056&rank=3
15. Efficacy and safety of azd9056 200 mg once daily versus placebo in adult patients with active crohn’s disease – a randomized, pilot, double-blind, four week, parallel-group, multicentre, phase ii study (azd9056; astra zeneca) (study code d8830c00002) 2008.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10746961
16. Evotec announces phase i initiation with p2x7 antagonist. 2008.Available at: http://www.evotec.com/article/en/Adhoc-Releases/Ad-hoc-Evotec-Announces-the-Successful-Completion-of-the-First-Phase-I-Study-with-EVT-401/347?selected_category_id=
17. An open-label two-part randomized, crossover study of the pharmacokinetics of ce-224,535 administered as controlled and immediate release formulations in healthy volunteers. Clinicaltrials.Gov identifier: Nct00838058. 2009.Available at: http://clinicaltrials.gov/ct2/show?term=CE-+224%2C+535&rank=3
18. An open-label, randomized, 2 cohort, 2 period crossover study to assess the relative bioavailability of the phase iii to the phase iib formulation of azd9056 in healthy male and female subjects. Clinicaltrials.Gov identifier: Nct00908934. 2009.Available at: http://clinicaltrials.gov/ct2/show?term=AZD9056&rank=1
19. An open-label, randomized, 2-cohort, 2-period crossover study. (study code d1520c00004) 2009.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10776007
20. A randomised, double-blind (with open comparator etanercept limb), placebo-controlled, phase iib, multicentre study to evaluate the efficacy of 4 doses of azd9056 administered for 6 months on the signs and symptoms of rheumatoid arthritis in patients with active disease receiving background methotrexate or sulphasalazine (study code d1520c00001). 2009.Available at: http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10775953
21. A first-time-in-human randomised, single blind placebo-controlled study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics, of single escalating doses of gsk1482160, in male and female healthy subjects, and to make a preliminary assessment of the effect of food. Clinicaltrials.Gov identifier: Nct00849134. 2010.Available at: http://clinicaltrials.gov/ct2/show/NCT00849134?term=GSK1482160&rank=1
1.d. Genetics of the P2X7R in human disease
SNPs are the most common form of genetic variation in the human genome. SNPs are now widely used to identify genetic associations and thereby disease-causing genes, but may in themselves have altered gene function. The P2X7 gene is located on chromosome 12q24.31, spanning 53kb [16]; it is highly polymorphic with 32 identified SNPs within it. There are four non-synonymous P2X7 SNPs that form five haplotypes in 99.9% of Caucasians [17]. These SNPs may lead either to loss or gain of function mutations. For instance, the SNP rs1653624 results in the amino acid substitution of isoleucine to asparagine at residue 568. This substitution prevents trafficking and normal cell surface membrane expression of the receptor [17,18]; it is uncommon in Caucasian populations and has an estimated allelic frequency of 0.026. Population studies of mapping and linkage disequilibrium have revealed a P2X7 SNP (rs22309012) that renders increased susceptibility to either bipolar disorder or major depressive illness. However, the functional effect of this SNP on P2X7R is yet to be determined [19,20]. Other conditions linked to P2X7R gene polymorphisms include tuberculosis (TB) susceptibility, resistance to infection with Chlamydia trachomatis, and increased fracture risk in post-menopausal women [17].
1.e. The P2X7R and cell survival
There is evidence to support the role of the P2X7R in cell survival and growth. The growth-promoting function of P2X7R seems at odds with its established role in apoptosis; however, this receptor is expressed at a high level in some malignancies, which may be consistent with its growth-promoting role [21]. The apparent anti-apoptotic property of P2X7R is based on studies using oATP [21,23] and KN-93 [25]. Work by Adinolfi et al has demonstrated that basal activation of P2X7R (via the autocrine/paracrine release of extracellular ATP) promotes cell growth. P2X7R-transfected HEK293 cells have greater amounts of intracellular ATP, higher mitochondrial resting potential, and increased basal mitochondrial Ca2+ compared with HEK293 mock transfected cells. P2X7R transfected HEK293 cells are able to grow in the absence of serum [22]. This growth-promoting function of the P2X7R is dependent on pore formation, since it does not occur in cells transfected with a truncated form of the P2X7R that cannot form a pore. The same authors showed that in P2X7R transfected HeLa cells an ATP challenge resulted in mitochondrial fragmentation and subsequent cell death [23]. HEK293 P2X7R expressing cells were also resistant to apoptosis induced by C2-ceramide, staurosporin, or intracellular Zn+ chelation. This survival advantage was dependent on NFATc1 activation, the main intracellular growth- promoting pathway mediated by increases in ER Ca2+ concentration [23].
It is controversial whether P2X7R are expressed at the plasma membrane of neutrophils [24]. The anti- apoptotic property of the P2X7 receptor has been described in neutrophils of patients with rheumatoid arthritis (RA) [26]. Serum amyloid A (SAA) purified from the plasma of patients with RA, or recombinant SAA, suppressed both spontaneous and α-FAS (CD95) induced-neutrophil apoptosis of human neutrophils in vitro; oATP inhibited this SAA-mediated anti-apoptotic effect.
Nagaoka et al have also demonstrated an anti-apoptotic action of P2X7R [25]. LL-37, an antimicrobial cathelicidin peptide (see later) has been shown to suppress neutrophil apoptosis in association with reduced activity of caspase-3. This effect was blocked by oATP and KN93 (a more selective P2X7 receptor antagonist). Future work with more selective P2X7R antagonists will be needed to clarify the role of P2X7R in neutrophils.
Although these studies have increased our understanding of the potentially bi-functional (pro- and anti-apoptotic) P2X7R, it is still not clear if these effects can occur in all cell types expressing P2X7R, or what factors determine the balance between pro- and anti-apoptosis.
1.f. LL-37
Although ATP is a P2X7R agonist, causing IL-1β processing and its release from LPS-primed macrophages and monocytes, the concentration of ATP (0.5-5mM) required to induce IL-1β release in vitro is believed to be far higher than is normally present in the extracellular space of tissues, at least under physiological conditions [27]. There are several studies suggesting that human cathelicidin-derived peptide, LL-37, may be a P2X7R agonist. Cathelicidins are an integral part of the innate immune system. Human cationic antimicrobial protein-18 (hCAP18) is the only known cathelicidin in humans [28]. In neutrophils, hCAP18 is stored as a precursor molecule and cleaved by proteases in the neutrophilic granules to release the active C-terminal peptide LL-37 [29]. This is a potentially important finding, but further work is necessary to assess to what extent the effect of LL-37 is P2X7R-dependent.
In the presence of LL-37, the concentration of ATP required to activate P2X7 is approximately 4000-fold less than the usual concentration of ATP needed to release the same amount of IL-1β [30]. LL-37 has a well-established antimicrobial function, exerting direct antimicrobial activity against both Gram-positive and Gram-negative organisms, and neutralising LPS [31]. LL-37 treatment of LPS-primed monocytes leads to caspase-1 activation and an increase in IL-1β release via P2X7R in a dose-dependent manner [30]. LL-37 treatment of LPS-primed monocytes also results in a transient release of ATP, peaking after 5-10 minutes at a concentration of ~150nM [30]; the mechanism of this is not clear. LL-37-mediated release of IL-1β from monocytes may be independent of extracellular ATP. The addition of apyrase (to rapidly degrade ATP) to LL-37 primed monocytes did not significantly alter the effect on IL-1β release, and addition of exogenous ATP did not increase further the amount of IL-1β released.
2. P2X7R and inflammatory diseases
2.a P2X7R and renal disease
Within the rat kidney, P2X7R is expressed at very low levels under normal conditions [32]; however, several studies have reported upregulation of renal P2X7R expression under various pathological conditions in vivo and in vitro. Harada et al reported that rat P2X7R mRNA levels are very low in normal isolated glomeruli and in cultured mesangial cells [33]. P2X7R protein was detectable in unstimulated mesangial cells only by immuno-electron microscopy (EM). P2X7R mRNA was induced by TNF-α and linked to mesangial cell apoptosis.
P2X7R expression in renal diseases was first described in a rat model of hypertension and diabetes mellitus [34]. In normal rat kidneys, consistent with Harada’s earlier findings, P2X7R was detected at very low levels in glomeruli [33], whereas in a rat transgenic model of renin-dependent hypertension there was an increase in P2X7R expression at 12 weeks. Similarly, rats with streptozotocin-induced diabetes had significant P2X7R detectable in their glomeruli at 6 and 9 weeks. At the mRNA level this corresponded to a ten-fold increase in expression of P2X7R. Immunohistochemistry and immuno-electron microscopy studies of diabetic rats showed that P2X7R were expressed mainly in glomerular podocytes (also with some expression in endothelial cells and mesangial cells).
The role of P2X7R and TGF-β in extracellular matrix deposition was demonstrated in a mouse model of unilateral ureteral obstruction (UUO) [35]. Immunohistochemistry demonstrated P2X7R expression in the epithelial cells of the renal cortex in KO mice on day 7 only. Gonclaves et al demonstrated that myofibroblast number and collagen deposition were significantly reduced in P2X7R KO mouse compared with wildtype mice in the UUO model. There was a similar reduction in TGF-β protein in the KO mice. Furthermore, at day 14, kidneys from the P2X7R KO mice showed reduced numbers of infiltrating macrophages and had less tubular cell apoptosis. The tubulo-interstitial damage and subsequent fibrosis associated with UUO were attenuated in P2X7R KO mice, suggesting a role for tubular P2X7R in renal inflammation and fibrosis.
Taylor et al demonstrated perhaps the most striking evidence for a pathogenic role of P2X7R in a rodent model of experimental glomerulonephritis (GN). They confirmed that P2X7R is normally expressed at very low levels in rat and mouse kidney. In their mouse model of accelerated nephrotoxic nephritis, an increased expression of this receptor was demonstrated, along with an increase in glomerular apoptotic cells. In renal biopsies of patients with lupus nephritis, there was also increased glomerular, and some tubular, expression of P2X7R. In a rat model of antibody-mediated GN, the onset of glomerular P2X7R expression coincided with the onset of proteinuria [36].
Following this finding the same group of investigators demonstrated that P2X7R KO mice were significantly protected from antibody-mediated GN [37]. P2X7R KO mice exposed to nephrotoxic serum had reduced glomerular macrophage infiltration, less fibrin deposition, less glomerular thrombosis, a reduction in proteinuria, and protection of overall renal function (with a smaller rise in serum creatinine concentration). IL-1β levels were too low for quantification, but CCL2 (also known as MCP-1) levels were reduced in P2X7R KO mice compared with wildtype controls.
Treatment with a selective P2X7R antagonist (A438079) also reduced the glomerular expression of CCL2, the number of glomerular macrophages, the severity of glomerular injury, and proteinuria.
These data provide evidence for a pathogenic role of P2X7R in inflammatory GN, as well as a possible therapeutic role for a selective P2X7R receptor antagonist in the treatment of renal inflammatory diseases.
2.b. P2X7R and respiratory disease
As already mentioned, the P2X7R gene is located on chromosome 12q24, which also contains multiple genes linked to respiratory function [16,38], so it is perhaps not surprising that the P2X7R has been implicated in respiratory disease. Chronic inflammatory reactions in lung tissue are characterized by the formation of multinucleated giant cells (MGC), as seen in patients with sarcoidosis and TB. The ability of monocytes from patients with sarcoidosis and TB to form MGC in vitro is abolished by the presence of the non-selective P2X7R antagonist oATP, suggesting a role for P2X7R [39]. Polymixin B, which stimulates P2X7R pore formation in rat alveolar macrophages, caused a dose-dependent increase in the formation of MGC in vitro. The Polymixin B-stimulated alveolar cells increased in number and had a greater tendency to fuse with adjacent cells, resulting in a significant increase in MGC formation. Pre-treatment with oATP abrogated the stimulatory effect of polymixin B [40]. These findings suggest a role for P2X7R in MGC formation during chronic inflammatory reactions. Functional P2X7R have also been demonstrated in human lung mast cells.
P2X7R is important in the defence mechanism against mycobacterium tuberculosis [41]. In vitro studies have shown that mycobacteria can survive following phagocytosis by macrophages, and that the crucial bactericidal step is P2X7R-mediated apoptosis of the macrophage; having a loss-of-function SNP results in a 3.5-fold increase in the risk of latent TB reactivation in a South-East Asian population in Australia [41], and in a Mexican population [42].
Prolonged exposure to high oxygen concentrations results in inflammation of lung tissue and in acute lung injury [43], which is related to an increase in IL-1β levels. Murine alveolar macrophages exposed to hyperoxia in vitro and in vivo demonstrated activation of P2X7R, inflammasome formation, release of IL-1β and induction of caspase-1, and IL-1β cleavage [44]. This effect was enhanced by the addition of the P2X7R agonist ATP, and inhibited by the P2X7R antagonist oATP. The release of IL-1β was absent in vitro on silencing the short hairpin RNA of inflammasome components, demonstrating the role of the inflammasome in P2X7R-mediated inflammation in hyperoxia.
Extracellular ATP is upregulated in the airways of patients with chronic obstructive pulmonary disease (COPD), bronchial asthma, and pulmonary fibrosis, and may also contribute to the pathogenesis of these diseases [45-47]. P2X7R activity has been linked to virus-induced loss of asthma control in patients with mild- to moderate bronchial asthma and who were followed after the onset of a naturally occurring common cold through to convalescence [48]. Whole-blood P2X7R pore function inversely correlated with asthma symptoms. P2X7R pore function in whole blood was assessed by the fold change in Bz-ATP-stimulated YO-PRO-1 uptake relative to the buffer control. Furthermore, P2X7R pore activity from whole blood samples correlated positively with the peak in nasal lavage neutrophil counts, suggesting that this whole blood P2X7R pore functional assay may identify patients with asthma-associated loss-of-function P2X7R genotypes. Consistent with these findings, Muller et al demonstrated upregulation of P2X7R in bronchoalveolar lavage (BAL) macrophages and in peripheral blood eosinophils during acute and chronic asthmatic airways inflammation in mice and humans [46]. The functional significance of P2X7R was demonstrated by the use of the specific receptor antagonist KN62, which attenuated allergic airway inflammation in rats. P2X7R-deficient dendritic cells (DC) showed a reduced capacity to induce Th2 immunity in a DC-driven murine model of allergic airway inflammation. Inhibition of this receptor on haematopoietic cells (e.g. DCs or eosinophils) may prove a novel therapeutic target in asthma treatment.
Similar findings have been described in cigarette smoke (CS)-induced lung inflammation and emphysema in vivo. Extracellular ATP contributes to the development of CS-induced lung inflammation and emphysema through activation of P2X7R. Upregulation of P2X7R in blood and airway neutrophils, alveolar macrophages, and in whole lung tissue of mice with CS-induced lung inflammation, has been demonstrated in an in vivo model [45]. Selective intrapulmonary inhibition of P2X7R reduced CS-induced lung inflammation and prevented the development of emphysema. Consistent with this finding, P2X7R KO mice showed reduced pulmonary inflammation following acute CS exposure. These results raise the possibility that targeting P2X7R might also be a therapeutic option in the management of COPD.
Patients with pulmonary fibrosis have elevated levels of ATP in BAL samples. In a bleomycin-induced murine model of pulmonary inflammation and fibrosis, Riteau et al found increased levels of extracellular ATP in BAL fluid [47]. The use of apyrase resulted in a significant reduction in bleomycin-induced inflammatory cell recruitment, and in local IL-1β levels. Conversely, the use of the more stable analogue of ATP, ATPγS, increased inflammation. P2X7R KO mice were protected from bleomycin-induced lung inflammation and had reduced markers of tissue fibrosis, including lung collagen deposition. In vitro studies also demonstrated the release of ATP from pulmonary epithelial cells exposed to bleomycin. The release of ATP was reduced by selective P2X7R antagonists (A438079 and A740003) in vitro.
ATP has been implicated in an animal model of ventilator-associated lung injury [49]. High-pressure mechanical ventilation was found in vivo to increase broncho-alveolar lavage ATP concentrations, whereas lactate dehydrogenase concentrations were unchanged, which makes ATP release from cell lysis unlikely. Furthermore, intra-tracheal ATP increased lung water content, implicating nucleotides in mechanical ventilation-associated pulmonary oedema. However, a functional role for P2X7R in this setting requires further work.
2.c. P2X7R and rheumatological disease
High levels of purines and pyrimidine nucleotides have been identified in the synovial fluid of patients with rheumatoid arthritis (RA) [50]. Activated synovial fibroblasts (type B synoviocytes) are involved in the pathogenesis of cartilage damage in RA [51]. Nuceleotides have been shown to produce joint inflammation through the production of local cytokines, including IL-1β, TNF-α, IL-2, and IL-6 [52].
Functional P2X7R on human rheumatoid synoviocytes has been demonstrated in vivo [53]. BzATP, a relatively selective P2X7R agonist, induced an upregulation of IL-6 mRNA and protein expression, which was diminished in the presence of oATP. The functional capacity of P2X7R to form large pores was absent in this cell type, as demonstrated by the failure of BzATP to induce significant uptake of YO-PRO. The effect of BzATP-induced IL-1β production by synoviocytes has not been reported.
Peripheral blood mononuclear cells isolated from patients with RA produce significantly higher amounts of IL-1β in response to ATP in the presence of LPS [54]; although the degree of P2X7R upregulation after 24 hours of ATP incubation did not vary between mononuclear cells from normal controls and from patients with RA. However, it remains possible that mononuclear cells of patients with RA are more responsive to ATP stimulation due to a genetic polymorphism in the P2X7R gene.
Cathepsins are lysosomal proteases that play a role in inflammatory and degenerative arthropathies, leading to joint destruction [55]. Cathepsins are involved in the degradation of proteins expressed by the MHC class II processing pathway, and in the proteolytic removal of invariant chain (Ii), a critical regulator of MHC class II function. Mice lacking cathepsins show a defect in Ii degradation in antigen presenting cells, and presentation of exogenous proteins in vivo, conferring protection from collagen-induced arthritis [56].
P2X7R activation of mouse bone marrow-derived macrophages (BMDM) results in release of lysosomal cathepsins that degrade collagen matrix. The release of these cathepsins is inhibited by P2X7R antagonists (AZ10573295 and AZ11648720) and is absent in P2X7R KO mice, suggesting a functional role for P2X7R in their release [57]. However, the lysosomal release of cathepsins was found to be independent of release of IL-1β.
P2X7R antagonists have been shown to limit joint destruction and pain in a rodent model of RA. Arthritis was induced by the local injection of Freund’s complete adjuvant into the hind paws of rats. Administration of oATP into inflamed paws of rats with arthritis relieved oedema and inflammatory pain within 48 hours [58]. The reduction in inflammatory pain was mediated by downregulation of P2X7R in peripheral nerve endings and epithelial cells, and it was independent of immune cell recruitment.
Neutrophil apoptosis is an important part of the regulation of inflammation [59]. The role of the P2X7R is less well-established in neutrophils. P2X7R may have an additional and indirect role in the mediation of inflammatory arthritis via anti-apoptotic signalling in neutrophils. Serum amyloid A (SAA) protein is an acute phase reactant that often correlates with active joint inflammation, and it is elevated in many patients with RA [60]. SAA purified from patients with RA suppresses α-CD95 (FAS) mAb-induced human neutrophil apoptosis in vitro [26]; the P2X7R antagonist oATP prevented this inhibitory effect, which suggests that SAA promotes neutrophil survival via a P2X7R-dependent mechanism (see earlier - section 1.d.).
2.d. P2X7R and neurological disease
Microglial cells are the primary immune cells of the CNS. Unsurprisingly, they express a number of P2 receptors activated by nucleotides that release cytokines, including IL-1β and IL-6 [61]. Microglia play an important part in the immune system of the CNS by acting as scavenger cells. Activation of P2X7R by ATP in microglial cells results in the release of autolysosomes into the extracellular space, providing a mechanism for the clearance of intracellular pathogens [62].
P2X7R is upregulated around beta- amyloid plaques in a mouse model of Alzheimer’s disease [12]. P2X7R also plays a role in the generation of superoxide in microglia. Thus, P2X7R has been implicated in the pathophysiology of Alzheimer’s disease and other neurodegenerative conditions through ATP-mediated cortical cell death and superoxide release.
Wang et al have demonstrated a downregulation of P2X7R in oligodendrocyte precursor cells in hypoxic brain injury [63], which may indicate a role for this receptor in hypoxic brain injury. In a recent paper by Domercq et al supporting this idea, ATP released during ischemia, and subsequent activation of P2X7R, was linked to white matter loss following ischaemic injury [64].
P2X7R pathway disruption also results in amelioration of chronic inflammatory and neuropathic pain [65,66]. Work in P2X7 knock out mice has demonstrated the lack of an exaggerated response to mechanical stimuli (allodynia and hyperalgesia) during chronic inflammation in P2X7 KO mice [65]. In addition to the expected attenuation in IL-1β production, P2X7 KO mice had reduced IL-6, TNF α, and MCP-1 production. There has been extensive research on the anti-noiciceptive properties of P2X7R antagonists, which is beyond the scope of this article.
3. Conclusion
Laboratory and clinical research to date has provided promising results for the clinical application and safety of P2X7R antagonists. Further study of SNP of P2X7R in the susceptibility and severity to diseases, and their interaction with antagonist may be the next step. The challenges ahead are to identify appropriate therapeutic applications for P2X7R antagonists, increase our understanding of the physiology and pathophysiology of the P2X7R, and to develop more selective and potent P2X7R agonists and antagonists.
4. Expert opinion
Finding therapeutic agents that are able to act rapidly to prevent any irreversible organ damage in inflammatory diseases remains a challenge, as many patients suffer from inflammatory diseases that are resistant to current therapies. The need to find an efficacious agent with limited adverse effects, either as a first-line or adjunctive therapy, is important. There is growing evidence to support the role of P2X7R in inflammatory diseases (Table 1), particularly from studies of P2X7R KO mice. A large number of antagonist studies have been carried out using non-selective or poorly selective antagonists like oATP. Therefore, the results obtained with these antagonists need to be interpreted with some caution. Recently, intervention studies with more selective P2X7R antagonists, such as A438079, have provided results supporting the importance of P2X7R as a potential therapeutic target. Thus, more selective P2X7R antagonists are likely to prove promising therapeutic tools (Table 2).
4.1. P2X7R and sepsis
Sepsis is a common and serious clinical condition, with an incidence of 700,000 cases and a mortality of 200,000 cases per year in the United States [67]. The importance of capase-1 and secretion of mature IL-1β in sepsis have been studied in rodent models of sepsis [68,69], and studies suggest a key role for the regulation/inhibition of IL-1β release as a therapeutic strategy [70].
In an in vivo model of sepsis, LPS-induced vascular smooth muscle hyporeactivity has been shown to be mediated by P2X7R activation and subsequent IL-1β release, followed by an increase in iNOS expression, and increased levels of NO [71]. Therefore, P2X7R antagonism might be beneficial in states of vasodilatory shock.
However, a large Phase 3 randomised clinical trial has failed to support the benefit of the recombinant human IL-1 receptor antagonist in the treatment of patients with severe sepsis [72]. Although this finding does not preclude the use of a P2X7R antagonist in sepsis, as there are actions over and above IL-1β release that could be beneficial in sepsis. An immunomodulatory agent that can interfere with more than one arm of the sepsis response, such as a P2X7 receptor antagonist, may have promise if given to the right patient group and at the right time. For example, apoptosis of lymphocytes is associated with a worse outcome in sepsis [73,74] and blocking apoptosis has been shown to result in improved survival in experimental models of sepsis [74,75]. T lymphocytes express functional P2X7R [76] and blocking these receptors with P2X7R antagonists to prevent apoptosis might confer a survival advantage in sepsis.
There is also a significant association between sepsis severity, mitochondrial dysfunction, and outcome [77]. BzATP stimulation of P2X7R transfected HEK293 and HeLa cells results in loss of the mitochondrial membrane potential with subsequent fragmentation of mitochondria and cell death [22]. Mitochondrial fragmentation was absent in cells lacking P2X7R. Thus, it is plausible that a P2X7R antagonist could have a role in the preservation of mitochondrial function in sepsis.
4.2 P2X7R antagonist in renal disease
Renal P2X7R upregulation has been described in both acute and chronic diseases, including glomerulonephritis (GN), ureteric obstruction, diabetes mellitus, and hypertension [34-36]. P2X7R KO mice are protected against GN, and a selective P2X7 receptor antagonist (A438079) confers protection in a rat model of GN [37]. This supports a pathologic role for P2X7R in acute kidney injury in GN. In addition, mesangial cells express P2X7R in response to TNF-α, suggesting a role for P2X7R in inflammatory renal disease [33].
Under basal conditions, renal P2X7R may have a role in maintaining normal cell turnover through the regulation of cell growth and apoptosis [33,78]. The possible functional roles of the P2X7R in inflammation include apoptosis, cytokine production, loss of functional receptors, and fibrosis. There is good evidence to support a role for apoptosis of renal tubular cells in experimental models of ischaemic and endotoxic renal injury [79,80]. In experimental GN, there is increased glomerular expression of caspase-3 and pro-apoptotic genes p53 and bax, with no change in the anti-apoptotic gene bcl-2 [36,81]. Mesangial cells can also undergo apoptosis on exposure to BzATP in vitro [33]. Glomerular cell apoptosis may lead to scar formation as part of healing, with subsequent glomerulosclerosis [82,83]. Thus, blockade of P2X7R to prevent apoptosis in these conditions could be protective.
Urinary IL-18 is a sensitive marker of AKI in sepsis, ischaemia, and post-operatively [84]. Caspase-1-KO mice are protected from renal ischaemic injury, and effect that was dependent on impaired IL-18 processing and an associated decrease in renal neutrophil infiltration [85]. Caspase-1 inhibition resulted in decreased IL-18 protein and IFN-γ mRNA, a reduction in histological injury, preservation of renal function, and increased survival [86].
The role of IL-1β in renal ischaemic reperfusion injury and endotoxaemia is also well established. Furuchi et al demonstrated that IL-1β stimulation of renal tubular cells in vitro results in increased MCP-1 and MIP-1α production [87]. In vivo this is related to an increased inflammatory cell infiltrate in the kidney during ischaemia-reperfusion injury. In a GN model, P2X7R expression correlated with IL-1β gene expression, with a renal protective effect of P2X7R antagonism [36]. P2X7R blockade may decrease IL-1β and IL-18 production, leading to renal dysfunction in sepsis or ischaemia-reperfusion injury.
Caspase-1-deficient mice are protected from AKI in endotoxaemia [88]. This protective effect was independent of tubular cell apoptosis, neutrophil infiltration, caspase-3 activity, and calpain activity. This suggests that inhibition of IL-1β and IL-18 production may have renal protective effects over and above apoptosis and inflammatory cell infiltration. Schmidt et al have demonstrated that IL-1β administered to mice results in decreased renal function and expression of functional renal transporters, including the epithelial sodium channel (ENaC), the renal outer medullary potassium channel (ROMK), and the Na+/K+-ATPase [89]. LPS-induced downregulation of sodium transporters was unaltered in cytokine-deficient mice, supporting a role for IL-1β in renal dysfunction. Similar results were seen after ischaemia-reperfusion injury in the kidney.
Following an episode of inflammation, fibrosis often follows and P2X7R has been shown to promote TGF-β-induced fibrosis in a model of UUO [35]. Mesangial cell proliferation mediates glomerular scaring by secretion of extracellular matrix components and pro-inflammatory cytokines [83,90]. Prevention of scar formation and glomerulosclerosis may help to preserve renal structure and function.
Given the evidence that P2X7R plays a part in renal inflammation, it seems likely that blockade of P2X7R will help to preserve renal structure and function in renal inflammatory diseases.
4.3 P2X7R and antibody production
The role of P2X7R in IL-1 and IL-18 release and in cell death is now well established. The nephrotoxic nephritis model provides evidence that P2X7R may affect antibody production. P2X7R is primarily found on antigen presenting cells, including dendritic cells and macrophages [13]. In the accelerated nephrotoxic nephritis model of GN there was a small decrease in the (autologous) mouse anti-sheep IgG deposition in the glomeruli of P2X7R KO mice [37]. This raises the possibility that P2X7R is also involved in antibody production. This possibility requires further investigation with, for example, the use of an autoantigen-induced model of autoimmune GN. This may indicate that P2X7R is also a potential target in treating autoimmune disease.
4.4 Current use of P2X7R antagonists in clinical trials
Phase 1 and phase 2 clinical trials investigating the safety and efficacy of the P2X7R antagonists are currently underway (Table 2). There have not been any serious concerns regarding the safety profile of the existing P2X7R antagonists. Phase 2 clinical trials have investigated the use of P2X7R antagonists in inflammatory bowel disease, rheumatoid arthritis, and chronic obstructive airway disease. The main side effects have included gastrointestinal upset (diarrhoea and nausea), dizziness, and headaches. These results, though not conclusive, are encouraging. Current P2X7R antagonists have proven to be safe and well tolerated in humans. Although some of P2X7R antagonists have been withdrawn from further clinical development because of insufficient clinical efficacy, a new generation of antagonists is entering early stage clinical studies (Table 2).
Factors that may have limited efficacy up to now may be due to drug pharmacokinetics and pharmacodynamics. P2X7R polymorphisms may be another factor to consider. Until we know exactly where P2X7R sits in the inflammatory pathway, and what redundancy there might be, it is difficult to be sure in what situations antagonising this receptor will be most effective.
In future drug development, the affinity of P2X7R antagonists for different polymorphic forms of P2X7R may need investigation and correlation with efficacy. With a better understanding of the underlying disease processes and more drug development, the prospects for P2X7 targeted therapies are still encouraging.
Article Highlights.
The P2X7 receptor (P2X7R) has an important role in inflammation and immunity.
There are several emerging functions of the P2X7R that require further understanding
Recent studies using P2X7R knockout (KO) mice and specific receptor antagonists have shown that the P2X7R is an important therapeutic target in inflammatory diseases, including glomerulonephritis
Clinical trials of P2X7R antagonists for inflammatory arthritis, inflammatory lung disease, and inflammatory bowel disease have progressed to Phase I and Phase II
There are several other potential applications of the P2X7R antagonists, such as the amelioration of the pro- inflammatory phase of sepsis
Footnotes
Declaration of Interest
RJ Unwin and F Tam have been supported by Wellcome Trust project grants (076949/A/05/Z and 076949/B/05/Z) for the work on P2 receptors. F Tam has been supported by the Diamond Fund from Imperial College Healthcare Charity, and has received research project grants from Roche, AstraZeneca, Cyclacel Limited and Baxter Biosciences.
Nishkantha Arulkumaran declares no conflict of interest.
References
- 1.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 2.North RA. Molecular physiology of p2x receptors. Physiol Rev. 2002;82:1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 3.*Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The p2x7 receptor: A key player in il-1 processing and release. J Immunol. 2006;176:3877–83. doi: 10.4049/jimmunol.176.7.3877. (A useful review of how P2X7 receptor leads to activation of inflammasome of release of mature IL-1beta)
- 4.Dinarello CA. The il-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. [PubMed] [Google Scholar]
- 5.Bevilacqua MP, Stengelin S, Gimbrone MA, Jr., Seed B. Endothelial leukocyte adhesion molecule 1: An inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–5. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 7.Kavita U, Mizel SB. Differential sensitivity of interleukin-1 alpha and -beta precursor proteins to cleavage by calpain, a calcium-dependent protease. J Biol Chem. 1995;270:27758–65. doi: 10.1074/jbc.270.46.27758. [DOI] [PubMed] [Google Scholar]
- 8.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–74. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 9.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: First line of the immune response to cell stress. Cell. 2006;126:659–62. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 10.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–35. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2x7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of alzheimer’s disease. J Biol Chem. 2003;278:13309–17. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 13.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, et al. Nucleotide receptors: An emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 14.Pelegrin P, Surprenant A. The p2x(7) receptor-pannexin connection to dye uptake and il-1beta release. Purinergic Signal. 2009;5:129–37. doi: 10.1007/s11302-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigi RD, Kertesy SB, Aquilina G, Dubyak GR. Oxidized atp (oatp) attenuates proinflammatory signaling via p2 receptor-independent mechanisms. Br J Pharmacol. 2003;140:507–19. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buell GN, Talabot F, Gos A, Lorenz J, Lai E, Morris MA, et al. Gene structure and chromosomal localization of the human p2x7 receptor. Receptors Channels. 1998;5:347–54. [PubMed] [Google Scholar]
- 17.Fuller SJ, Stokes L, Skarratt KK, Gu BJ, Wiley JS. Genetics of the p2x7 receptor and human disease. Purinergic Signal. 2009;5:257–62. doi: 10.1007/s11302-009-9136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, et al. An ile-568 to asn polymorphism prevents normal trafficking and function of the human p2x7 receptor. J Biol Chem. 2003;278:17108–13. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- 19.Barden N, Harvey M, Gagne B, Shink E, Tremblay M, Raymond C, et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12q24.31 region points to p2rx7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:374–82. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- 20.Lucae S, Salyakina D, Barden N, Harvey M, Gagne B, Labbe M, et al. P2rx7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–45. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- 21.Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A, et al. P2x7 receptor expression in evolutive and indolent forms of chronic b lymphocytic leukemia. Blood. 2002;99:706–8. doi: 10.1182/blood.v99.2.706. [DOI] [PubMed] [Google Scholar]
- 22.**Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, et al. Basal activation of the p2x7 atp receptor elevates mitochondrial calcium and potential, increases cellular atp levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–72. doi: 10.1091/mbc.E04-11-1025. (This manuscript investigates the novel role of P2X7 in cell survival)
- 23.Adinolfi E, Callegari MG, Cirillo M, Pinton P, Giorgi C, Cavagna D, et al. Expression of the p2x7 receptor increases the ca2+ content of the endoplasmic reticulum, activates nfatc1, and protects from apoptosis. J Biol Chem. 2009;284:10120–8. doi: 10.1074/jbc.M805805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martel-Gallegos G, Rosales-Saavedra MT, Reyes JP, Casas-Pruneda G, Toro-Castillo C, Perez-Cornejo P, et al. Human neutrophils do not express purinergic p2x7 receptors. Purinergic Signal. 2010;6:297–306. doi: 10.1007/s11302-010-9178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human cap18/ll-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and p2x7. J Immunol. 2006;176:3044–52. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- 26.Christenson K, Bjorkman L, Tangemo C, Bylund J. Serum amyloid a inhibits apoptosis of human neutrophils via a p2x7-sensitive pathway independent of formyl peptide receptor-like 1. J Leukoc Biol. 2008;83:139–48. doi: 10.1189/jlb.0507276. [DOI] [PubMed] [Google Scholar]
- 27.North RA, Surprenant A. Pharmacology of cloned p2x receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–80. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 28.Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene fall39 and processing of the cathelin precursor to the antibacterial peptide ll-37 in granulocytes. Eur J Biochem. 1996;238:325–32. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 29.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide ll-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341(Pt 3):501–13. [PMC free article] [PubMed] [Google Scholar]
- 30.**Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel p2x7 receptor activator, the human cathelicidin-derived peptide ll37, induces il-1 beta processing and release. J Immunol. 2004;172:4987–94. doi: 10.4049/jimmunol.172.8.4987. (This manuscript describes novel role of LL37 as an agonist for P2X7R, and the close association between infection, inflammation, and the P2X7 receptor)
- 31.Durr UH, Sudheendra US, Ramamoorthy A. Ll-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–25. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected atp-sensitive p2 receptor subtypes in normal rat kidney: An immunohistological study. Cells Tissues Organs. 2003;175:105–17. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 33.*Harada H, Chan CM, Loesch A, Unwin R, Burnstock G. Induction of proliferation and apoptotic cell death via p2y and p2x receptors, respectively, in rat glomerular mesangial cells. Kidney Int. 2000;57:949–58. doi: 10.1046/j.1523-1755.2000.00911.x. (This paper demonstrates TNF-α induces P2X7R synthesis in mesangail cells)
- 34.**Vonend O, Turner CM, Chan CM, Loesch A, Dell’Anna GC, Srai KS, et al. Glomerular expression of the atp-sensitive p2x receptor in diabetic and hypertensive rat models. Kidney Int. 2004;66:157–66. doi: 10.1111/j.1523-1755.2004.00717.x. (This is the first study demonstrating the increased expression of P2X7R in two rodent models of renal disease)
- 35.**Goncalves RG, Gabrich L, Rosario A, Jr., Takiya CM, Ferreira ML, Chiarini LB, et al. The role of purinergic p2x7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 2006;70:1599–606. doi: 10.1038/sj.ki.5001804. (The potential importance of P2X7R in tubulointerstitial nephritis was demonstrated using P2X7R KO mice)
- 36.*Turner CM, Tam FW, Lai PC, Tarzi RM, Burnstock G, Pusey CD, et al. Increased expression of the pro-apoptotic atp-sensitive p2x7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant. 2007;22:386–95. doi: 10.1093/ndt/gfl589. (This is the first demonstration of increased expression of P2X7R in renal biopsies of patients and two rodent models of glomeruloenphritis.)
- 37.**Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, et al. P2x7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20:1275–81. doi: 10.1681/ASN.2008060559. (This paper describes the similar protective effects conferred by the P2X7 knock out gene and a selective receptor antagonist in experimental glomerulonephritis)
- 38.Raby BA, Silverman EK, Lazarus R, Lange C, Kwiatkowski DJ, Weiss ST. Chromosome 12q harbors multiple genetic loci related to asthma and asthma-related phenotypes. Hum Mol Genet. 2003;12:1973–9. doi: 10.1093/hmg/ddg208. [DOI] [PubMed] [Google Scholar]
- 39.Mizuno K, Okamoto H, Horio T. Heightened ability of monocytes from sarcoidosis patients to form multi-nucleated giant cells in vitro by supernatants of concanavalin a-stimulated mononuclear cells. Clin Exp Immunol. 2001;126:151–6. doi: 10.1046/j.1365-2249.2001.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wareham K, Vial C, Wykes RC, Bradding P, Seward EP. Functional evidence for the expression of p2x1, p2x4 and p2x7 receptors in human lung mast cells. Br J Pharmacol. 2009;157:1215–24. doi: 10.1111/j.1476-5381.2009.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB, et al. A polymorphism in the p2x7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2007;175:360–6. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 42.Nino-Moreno P, Portales-Perez D, Hernandez-Castro B, Portales-Cervantes L, Flores-Meraz V, Baranda L, et al. P2x7 and nramp1/slc11 a1 gene polymorphisms in mexican mestizo patients with pulmonary tuberculosis. Clin Exp Immunol. 2007;148:469–77. doi: 10.1111/j.1365-2249.2007.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, et al. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest. 1998;101:1970–82. doi: 10.1172/JCI1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolliputi N, Shaik RS, Waxman AB. The inflammasome mediates hyperoxia-induced alveolar cell permeability. J Immunol. 2010;184:5819–26. doi: 10.4049/jimmunol.0902766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.*Lucattelli M, Cicko S, Muller T, Lommatzsch M, de Cunto G, Cardini S, et al. P2x7 receptor signalling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0038OC. (This paper describes the similar protective effects conferred by the P2X7 knock out gene and a selective receptor antagonist in experimental lung inflammation)
- 46.*Muller T, Paula Vieira R, Grimm M, Durk T, Cicko S, Zeiser R, et al. A potential role for p2x7r in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0129OC. (This paper describes the expression of P2X7R on human macrophages obtained from BAL and the protective effects conferred by the P2X7 knock out gene and a selective receptor antagonist in experimental lung inflammation)
- 47.*Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, et al. Extracellular atp is a danger signal activating p2x7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.201003-0359OC. (This paper describes the similar protective effects conferred by the P2X7 knock out gene and a selective receptor antagonist in experimental lung inflammation)
- 48.Denlinger LC, Shi L, Guadarrama A, Schell K, Green D, Morrin A, et al. Attenuated p2x7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med. 2009;179:265–70. doi: 10.1164/rccm.200802-293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rich PB, Douillet CD, Mahler SA, Husain SA, Boucher RC. Adenosine triphosphate is released during injurious mechanical ventilation and contributes to lung edema. J Trauma. 2003;55:290–7. doi: 10.1097/01.TA.0000078882.11919.AF. [DOI] [PubMed] [Google Scholar]
- 50.Ryan LM, Rachow JW, McCarty DJ. Synovial fluid atp: A potential substrate for the production of inorganic pyrophosphate. J Rheumatol. 1991;18:716–20. [PubMed] [Google Scholar]
- 51.Pap T, Muller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000;2:361–7. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 53.Caporali F, Capecchi PL, Gamberucci A, Lazzerini PE, Pompella G, Natale M, et al. Human rheumatoid synoviocytes express functional p2x7 receptors. J Mol Med. 2008;86:937–49. doi: 10.1007/s00109-008-0365-8. [DOI] [PubMed] [Google Scholar]
- 54.Al-Shukaili A, Al-Kaabi J, Hassan B. A comparative study of interleukin-1beta production and p2x7 expression after atp stimulation by peripheral blood mononuclear cells isolated from rheumatoid arthritis patients and normal healthy controls. Inflammation. 2008;31:84–90. doi: 10.1007/s10753-007-9052-0. [DOI] [PubMed] [Google Scholar]
- 55.Wang D, Bromme D. Drug delivery strategies for cathepsin inhibitors in joint diseases. Expert Opin Drug Deliv. 2005;2:1015–28. doi: 10.1517/17425247.2.6.1015. [DOI] [PubMed] [Google Scholar]
- 56.Nakagawa TY, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin s null mice. Immunity. 1999;10:207–17. doi: 10.1016/s1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. P2x7 receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 2010;185:2611–9. doi: 10.4049/jimmunol.1000436. [DOI] [PubMed] [Google Scholar]
- 58.Dell’Antonio G, Quattrini A, Cin ED, Fulgenzi A, Ferrero ME. Relief of inflammatory pain in rats by local use of the selective p2x7 atp receptor inhibitor, oxidized atp. Arthritis Rheum. 2002;46:3378–85. doi: 10.1002/art.10678. [DOI] [PubMed] [Google Scholar]
- 59.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 60.Kokubun M, Imafuku Y, Okada M, Ohguchi Y, Ashikawa T, Yamada T, et al. Serum amyloid a (saa) concentration varies among rheumatoid arthritis patients estimated by saa/crp ratio. Clin Chim Acta. 2005;360:97–102. doi: 10.1016/j.cccn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonte C, Matteoli M, et al. Pathophysiological roles of extracellular nucleotides in glial cells: Differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005;48:144–56. doi: 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Takenouchi T, Nakai M, Iwamaru Y, Sugama S, Tsukimoto M, Fujita M, et al. The activation of p2x7 receptor impairs lysosomal functions and stimulates the release of autophagolysosomes in microglial cells. J Immunol. 2009;182:2051–62. doi: 10.4049/jimmunol.0802577. [DOI] [PubMed] [Google Scholar]
- 63.Wang LY, Cai WQ, Chen PH, Deng QY, Zhao CM. Downregulation of p2x7 receptor expression in rat oligodendrocyte precursor cells after hypoxia ischemia. Glia. 2009;57:307–19. doi: 10.1002/glia.20758. [DOI] [PubMed] [Google Scholar]
- 64.Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. P2x7 receptors mediate ischemic damage to oligodendrocytes. Glia. 2009 doi: 10.1002/glia.20958. [DOI] [PubMed] [Google Scholar]
- 65.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the p2x7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–96. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 66.McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, et al. P2x7-related modulation of pathological nociception in rats. Neuroscience. 2007;146:1817–28. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 67.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the united states: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Mehta VB, Hart J, Wewers MD. Atp-stimulated release of interleukin (il)-1beta and il-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–6. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 69.Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science. 1994;264:1319–22. doi: 10.1126/science.8191285. [DOI] [PubMed] [Google Scholar]
- 70.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, et al. Mice deficient in il-1 beta-converting enzyme are defective in production of mature il-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 71.Chiao CW, Tostes RC, Webb RC. P2x7 receptor activation amplifies lipopolysaccharide-induced vascular hyporeactivity via interleukin-1 beta release. J Pharmacol Exp Ther. 2008;326:864–70. doi: 10.1124/jpet.107.135350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Opal SM, Fisher CJ, Jr., Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase iii, randomized, double-blind, placebo-controlled, multicenter trial. The interleukin-1 receptor antagonist sepsis investigator group. Crit Care Med. 1997;25:1115–24. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, et al. Overexpression of bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–56. [PubMed] [Google Scholar]
- 74.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr., Hui JJ, Chang KC, et al. Sepsis-induced apoptosis causes progressive profound depletion of b and cd4+ t lymphocytes in humans. J Immunol. 2001;166:6952–63. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 75.Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of fas/fas ligand signaling improves septic survival: Differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74:344–51. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 76.Aswad F, Dennert G. P2x7 receptor expression levels determine lethal effects of a purine based danger signal in t lymphocytes. Cell Immunol. 2006;243:58–65. doi: 10.1016/j.cellimm.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 78.Adinolfi E, Callegari MG, Cirillo M, Pinton P, Giorgi C, Cavagna D, et al. Expression of the p2x7 receptor increases the ca2+ content of the endoplasmic reticulum, activates nfatc1, and protects from apoptosis. J Biol Chem. 2009;284:10120–8. doi: 10.1074/jbc.M805805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonegio R, Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 2002;11:301–8. doi: 10.1097/00041552-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Jo SK, Cha DR, Cho WY, Kim HK, Chang KH, Yun SY, et al. Inflammatory cytokines and lipopolysaccharide induce fas-mediated apoptosis in renal tubular cells. Nephron. 2002;91:406–15. doi: 10.1159/000064280. [DOI] [PubMed] [Google Scholar]
- 81.Yang B, Johnson TS, Thomas GL, Watson PF, Wagner B, Nahas AM. Apoptosis and caspase-3 in experimental anti-glomerular basement membrane nephritis. J Am Soc Nephrol. 2001;12:485–95. doi: 10.1681/ASN.V123485. [DOI] [PubMed] [Google Scholar]
- 82.Sugiyama H, Kashihara N, Makino H, Yamasaki Y, Ota A. Apoptosis in glomerular sclerosis. Kidney Int. 1996;49:103–11. doi: 10.1038/ki.1996.14. [DOI] [PubMed] [Google Scholar]
- 83.Lovett DH, Sterzel RB. Cell culture approaches to the analysis of glomerular inflammation. Kidney Int. 1986;30:246–54. doi: 10.1038/ki.1986.176. [DOI] [PubMed] [Google Scholar]
- 84.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 85.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, et al. Impaired il-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–52. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu HF, Liang D, Wang LM, Zhou N, Yao CW, Hong T, et al. Effects of specific interleukin-1beta-converting enzyme inhibitor on ischemic acute renal failure in murine models. Acta Pharmacol Sin. 2005;26:1345–51. doi: 10.1111/j.1745-7254.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 87.Furuichi K, Wada T, Iwata Y, Kokubo S, Hara A, Yamahana J, et al. Interleukin-1-dependent sequential chemokine expression and inflammatory cell infiltration in ischemia-reperfusion injury. Crit Care Med. 2006;34:2447–55. doi: 10.1097/01.CCM.0000233878.36340.10. [DOI] [PubMed] [Google Scholar]
- 88.Wang W, Faubel S, Ljubanovic D, Mitra A, Falk SA, Kim J, et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol Renal Physiol. 2005;288:F997–1004. doi: 10.1152/ajprenal.00130.2004. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol. 2007;18:1072–83. doi: 10.1681/ASN.2006050454. [DOI] [PubMed] [Google Scholar]
- 90.Tam FW, Smith J, Morel D, Karkar AM, Thompson EM, Cook HT, et al. Development of scarring and renal failure in a rat model of crescentic glomerulonephritis. Nephrol Dial Transplant. 1999;14:1658–66. doi: 10.1093/ndt/14.7.1658. [DOI] [PubMed] [Google Scholar]
- 91.Taylor SR, Gonzalez-Begne M, Dewhurst S, Chimini G, Higgins CF, Melvin JE, et al. Sequential shrinkage and swelling underlie p2x7-stimulated lymphocyte phosphatidylserine exposure and death. J Immunol. 2008;180:300–8. doi: 10.4049/jimmunol.180.1.300. [DOI] [PubMed] [Google Scholar]
- 92.Gauer S, Sichler O, Obermuller N, Holzmann Y, Kiss E, Sobkowiak E, et al. Il-18 is expressed in the intercalated cell of human kidney. Kidney Int. 2007;72:1081–7. doi: 10.1038/sj.ki.5002473. [DOI] [PubMed] [Google Scholar]
- 93.Lemaire I, Falzoni S, Leduc N, Zhang B, Pellegatti P, Adinolfi E, et al. Involvement of the purinergic p2x7 receptor in the formation of multinucleated giant cells. J Immunol. 2006;177:7257–65. doi: 10.4049/jimmunol.177.10.7257. [DOI] [PubMed] [Google Scholar]
- 94.Dell’Antonio G, Quattrini A, Dal Cin E, Fulgenzi A, Ferrero ME. Antinociceptive effect of a new p(2z)/p2x7 antagonist, oxidized atp, in arthritic rats. Neurosci Lett. 2002;327:87–90. doi: 10.1016/s0304-3940(02)00385-3. [DOI] [PubMed] [Google Scholar]