Abstract
Differences in brain responses to aversive visceral stimuli may underlie previously reported sex differences in symptoms as well as perceptual and emotional responses to such stimuli in patients with Irritable Bowel Syndrome (IBS). The goal of the current study was to identify brain networks activated by expected and delivered aversive visceral stimuli in male and female patients with chronic abdominal pain, and to test for sex differences in the effective connectivity of the circuitry comprising these networks. Network analysis was applied to assess the brain response of 46 IBS patients (22 men and 24 women) recorded using [15O] water positron emission tomography during rest/baseline and expected and delivered aversive rectal distension. Functional connectivity results from partial least squares analyses provided support for the hypothesized involvement of 3 networks corresponding to: 1) visceral afferent information processing (thalamus, insula and dorsal anterior cingulate cortex, orbital frontal cortex), 2) emotional arousal (amygdala, rostral and subgenual cingulate regions, and locus coeruleus complex) and 3) cortical modulation (frontal and parietal cortices). Effective connectivity results obtained via structural equation modeling indicated that sex-related differences in brain response are largely due to alterations in the effective connectivity of emotional-arousal circuitry rather than visceral afferent processing circuits. Sex differences in the cortico-limbic circuitry involved in emotional-arousal, pain facilitation and autonomic responses may underlie the observed differences in symptoms, and in perceptual and emotional responses to aversive visceral stimuli.
Introduction
Irritable bowel syndrome (IBS) is one of the most common functional pain disorders, characterized by chronic abdominal pain or discomfort (related to visceral hyperalgesia) associated with altered bowel habits (related to autonomic dysregulation). Affected patients show frequent comorbidity with anxiety, (Mayer et al., 2001) as well as symptom-related anxiety (e.g. fears and worry over expected abdominal pain and discomfort (Labus et al., 2007). Altered brain gut interactions during a visceral stimulus and during its expectation have been suggested as an important pathophysiological mechanism for IBS, and functional brain imaging studies have identified brain regions and circuits which may be responsible for these alterations (Mayer et al., 2006).
Like many other syndromes characterized by chronic physical or emotional pain and discomfort, IBS is significantly more common in women (Chang and Heitkemper, 2002) and sex-related differences in the perceptual and emotional responses of IBS patients to aversive visceral stimuli have been reported (Chang et al., 2006b; Heitkemper et al., 2003; Mayer et al., 2004; Tillisch et al., 2005). The greater subjective responses in female IBS patients may be related to sex differences in brain responses to visceral stimuli (Berman et al., 2000; Naliboff et al., 2003; Berman et al., 2006). For example, female patients showed greater activation of limbic and paralimbic regions, including the amygdala and the closely connected anterior cingulate cortex (ACC) while male patients demonstrated greater activation of the insula (INS). Although activation analyses have suggested possible regional differences in central processing between healthy controls and IBS, and between male and female IBS patients (reviewed in Mayer et al., 2006), they are limited in their ability to describe more complete system-level models of the functional neurocircuitry that may be involved.
The current study applied network analyses to test the general hypothesis that at least 3 networks can be identified as operating during an expected and a delivered aversive visceral stimulus including: a) a network central to processing of visceral afferent information (“homeostatic-afferent” network i.e., thalamus, posterior (p) and anterior (a)INS and dorsal (d) ACC, orbital frontal cortex (OFC) (Craig, 2003b,c; Mayer et al., 2006)), b) a network involved in arousal, and emotion-related pain amplification (“emotional-arousal” network i.e., amygdala, ACC subregions and locus coeruleus complex (LCC) (Pezawas et al., 2005; Stein et al., 2007; Valentino et al., 1999)), and c) a network representing the mediating influence of cortical regions (“cortical-modulatory” network i.e., frontal, parietal) on a) and b) (Mayer et al., 2005; Naliboff et al., 2006). Even though these networks overlap and share some of the same regions, we decided to reduce the complexity of the model and discuss the 3 networks separately.
Based on the proposed role of the amygdala in cognitive and affective modulation of pain (Carrasquillo and Gereau, 2007; Neugebauer et al., 2004) and consistent reports of sex-related differences in amygdala responses in healthy subjects (Cahill, 2006) and IBS patients (Naliboff et al., 2003), we further hypothesized that most of the sex-related differences in brain response are in the effective connectivity of the “emotional-arousal” network, and less in the “cortical-modulatory” and “homoeostatic-afferent” networks. Specifically, we tested the following hypotheses regarding the activity within nodes, and the connectivity between nodes within the 3 networks: 1) Across conditions, male and female IBS patients show similar activity/connectivity in the “homeostatic-afferent” processing network. 2) Across conditions, the activity/connectivity of amygdala-related network(s) shows sex differences. 3) There are sex-related differences in the effective connectivity of the “emotional-arousal” network during both conditions. Specifically, we expected female patients to show greater activity/connectivity or engagement of the amygdala-related networks. Parts of these results have been published in abstract form (Labus et al., 2005; Labus et al., 2006).
Methods
Data from a previously published [15O] water positron emission tomography (PET) neuroimaging study (Naliboff et al., 2003) were analyzed. The sample included 46 men (n=22) and women (n=24) with a diagnosis of IBS (Rome I criteria) (Thompson et al., 1994). All patients were free from centrally acting medication for at least 30 days preceding the PET scan. Patients had no history of substance abuse or psychiatric illness. On average, women were 41.5(10.8) years old and reported a 6 month symptom severity of 13.4(12.7) on a 20-cm Verbal Descriptor Visual Analogue Scale (VDVAS). Men were 39.8 (10.8) years old and reported similar levels of symptom severity (12.7(2.6)). Women had more psychological symptoms than men (57.6(9.8) vs 49.2(10.6), p<.01) as assessed by the General Severity Index of the SCL90-R questionnaire (Derogatis, 1983). However, means for both men and women were below the clinically significant cut-off scores.
The experimental protocol has been described in detail previously (Naliboff et al., 2003). Briefly, PET counts (Siemens/CTI 953 tomograph, Siemens-Computer Technology, Knoxville TN) from 31 contiguous axial planes were summed over 90 seconds after three intravenous administrations of 25 mCi [15O] water to construct volume images reflecting regional cerebral blood flow (rCBF) during 1) an initial resting baseline period (BL), 2) an expected moderate (45 mmHg) rectal balloon distension (INF), and finally 3) an expected but undelivered distension (EXP). In the original study, these 3 conditions were repeated following a train of repeated sigmoid colon distensions. Data analyzed in the current study were only taken from the initial 3 conditions, prior to the sigmoid stimulus. During the INF scan, the rectal balloon was inflated to 45 mmHg pressure for 60 seconds. This pressure has been shown to be associated with a subjective rating of discomfort rather than pain.(Posserud et al., 2007) Men and women rated this stimulus at similar levels of intensity on a 20-cm VDVAS (Mean (SD) 11.9(3.2) and 12.3(3.0), respectively). Intensity ratings for EXP were also similar between men (3.8 (4.1)) and women (3.2(3.6)). All scans were preprocessed (SPM99, Wellcome Trust Centre for the Study of Cognitive Neurology, London, UK) by realignment to the initial scan for each subject, registration into the standardized space of the average MRI brain image provided by the Montreal Neurological Institute (MNI space), spatial smoothing with an isotropic 12 mm FWHM Gaussian filter, and reslicing to 4 mm isotropic voxels.
Statistical Analysis
Overview
Statistical analyses were performed in steps that will be detailed below. First, a multivariate task partial least squares (PLS) (McIntosh et al., 1996; McIntosh et al., 2004; McIntosh and Lobaugh, 2004) identified spatially distributed patterns of regions activated during INF and EXP relative to BL in males and females. These results, in combination with relevant theoretical and neurobiological information from earlier studies, indicated that bilateral amygdala and right thalamus were reliably involved in the brain response during expectation and detection of aversive visceral stimuli. These results, in combination with relevant theoretical and neurobiological information from earlier studies, indicate that bilateral amygdala, and thalamus were involved in the brain's response during EXP, and detection of aversive visceral stimuli, respectively.
In particular, consistent reports of sex-related differences in amygdala responses have been reported during anticipation of aversive emotional stimuli in healthy subjects (Buchel et al., 1998; Cahill, 2006; Mackiewicz et al., 2006; Sarinopoulos et al., 2006) and IBS patients(Berman et al., 2008; Naliboff et al., 2003) and amygdala activity is associated with the cognitive and affective modulation of pain (Carrasquillo and Gereau, 2007; Neugebauer et al., 2004).
Several studies have demonstrated thalamic activation during rectal distension in healthy controls and IBS patients.(Baciu et al., 1999; Kwan et al., 2005; Mertz et al., 2000; Naliboff et al., 2001; Ringel et al., 2003; Silverman et al., 1997; Verne et al., 2003; Wilder-Smith et al., 2004; Yuan et al., 2003) Indeed greater activations of the thalamus has been shown with visceral stimuli compared with somatic stimuli in IBS(Verne et al., 2003). Visceral afferent information ascends in the lamina I spino-thalamic tract to a specific thalamo-cortical relay nucleus (VMpo) in posterolateral thalamus, which in turn projects to a discrete portion of dorsal posterior insular cortex. This ascending afferent lamina I pathway in primates and humans also provides a direct thalamo-cortical pathway (by way of MDvc in medial thalamus) that activates the dorsal anterior cingulated cortex.
A seed PLS was then applied to test for group- and condition-specific differences in the functional connectivity of the amygdala and thalamus with the rest of the brain (whole-brain activity). Finally, the effective connectivity of a hypothesized neural network comprised of “emotional-arousal,” “homeostatic-afferent”, and “cortical-modulatory” circuitry was specified and tested for sex differences using structural equation modeling.
Partial least squares analyses
PLS is a multivariate statistical technique that is analogous to principal components analysis (PCA), but the solutions are restricted to the part of the covariance structure that is attributable to conditions or groups in an experimental design (task PLS), behavioral measures (behavioral PLS), or the activity within a specific brain region (seed PLS). Behavioral and seed PLS are functional connectivity analyses that provide a test for hypotheses regarding the brain regions comprising neural networks. PLS analyses were implemented using freely available code (http://www.rotman-baycrest.on.ca:8080) in Matlab7.01 (Mathworks, Natick, MA).
Task PLS
First, a “nonrotated” task PLS extracted spatial patterns where brain activity changed during INF and EXP relative to the BL scan. A matrix comprising four a priori nonorthogonal experimental design contrasts (main effects of condition: BL–INF, BL–EXP, and the interaction of sex with each main effect) was correlated with a data matrix containing the normalized signal intensity measure at each voxel (i.e., rCBF adjusted for global CBF by dividing each voxel by the mean voxel activity of the whole-brain image) to produce a “cross-block covariance” matrix comprising four latent variables (LVs) or spatial patterns of brain activity related to the specified design contrasts. The data matrix comprised 138 (46 subjects × 3 conditions) rows and one column for each voxel. The design matrix comprised 138 rows × 4 columns for the design contrasts. Unlike its “rotated” counterpart, a “nonrotated” PLS does not employ singular value decomposition (SVD) as an exploratory tool to extract LVs corresponding to experimental patterns (e.g., design contrasts) accounting for the maximum amount of independent variance in the data. Instead, the columns of the “cross-block covariance” matrix are considered the brain LVs and the squared singular value for each LV is the sum of the squares of the “cross-block covariance” matrix column (McIntosh et al., 1996; McIntosh and Lobaugh, 2004).
The significance of each LV was assessed via nonparametric permutation testing using 500 permutations. This resampling technique, which reassigns the order of the conditions for each observation, determines whether the effect represented by the LV is significantly different from random noise and is thought to approximate a mixed model design in terms of optimal sensitivity and level of inference without increasing false positive rates (McIntosh and Lobaugh, 2004; Strother et al., 2002). The exact number of times the permuted singular values exceeded the observed singular value was computed and p <.05 was considered significant. The numerical weights of the voxels comprising the brain LV are called “saliences” and can be positive or negative, indicating the magnitude and direction in which each voxel covaries with the corresponding design contrast.
The reliability of the brain regions comprising a LV was determined via bootstrap estimation. Specifically, the standard error for each voxel salience was calculated from a distribution of saliences derived from resampling subjects 100 times with replacement and recalculating the “nonrotated” task PLS on each sample. The ratio of the observed salience to the bootstrap standard error, which is approximately equivalent to a z score, was then calculated to determine reliability. Voxels were considered reliable if the bootstrap ratio (BSR) > |±3.62| (p <.0001).
The regions comprising a LV are reported in terms of clusters of voxels. A cluster within the LV was defined as at least 20 reliable contiguous voxels and represented by its peak voxel, defined as the voxel with the highest BSR. Where a cluster comprised several brain regions, the local maximum within each brain region was identified and reported. Regions were characterized in terms of anatomical region and Brodmann Area (BA). The main findings are summarized and the detailed reports are provided as supplemental material.
Seed PLS
Seed PLS was applied to identify distributed patterns of activity that were functionally connected (correlated) with a specified brain region (“seed”) and compare these patterns between group and conditions. Seed PLS is identical to a “rotated” task PLS except that the columns of the design matrix comprise normalized rCBF activity from one or more voxels representing the activity of brain regions of interest during each condition. This matrix is then correlated with the data matrix to produce a “cross-block correlation” matrix. SVD is performed to extract commonalities and differences in this correlation matrix (McIntosh, 1999) and permutation testing provides an assessment of significance. For the seed PLS, voxels comprising the LVs were considered reliable if the BSR exceeded |±2.33| (p <.01). The experimental effects are depicted graphically by plotting the correlation of the seed voxel(s) activity with the latent variable score within group and condition. The salience associated with each region is interpreted in terms of positive and negative correlation with the seed. When group differences are observed using a seed PLS, the voxel salience of regions in the cluster report and projection plot represents group differences in correlations. Criteria for a voxel to be chosen as a seed included: 1) functional relevance supported by prior IBS research, 2) functional relevance to the hypothesized networks, and 3) significant and reliable functional connectivity empirically supported by the task PLS. Based upon these criteria, bilateral amygdala and right thalamus were selected as seed voxels.
Effective connectivity
Seed PLS identifies a pattern of brain regions functionally related to regions of interest. However, the correlation of two areas with a third does not reflect mutual correlation of the areas (Stephan, 2004). Instead, path analysis using a structural equation modeling framework (McIntosh and Gonzalez-Lima, 1991; McIntosh and Gonzalez-Lima, 1994; McIntosh et al., 1994) was applied to simultaneously quantify the interactions among brain regions and to test for sex differences in the effective connectivity of a hypothesized network.
Effective connectivity analyses require a priori specification of a structural (anatomical) model for testing. The structural model represents the hypotheses about the causal relations between brain regions. Guided by the principle of parsimony, the nodes of the network to be characterized and tested were selected based upon: 1) a fundamental role in the networks hypothesized to be operating during response to an aversive pelvic visceral stimulus (i.e., “homeostatic-afferent”, “emotional-arousal”, “cortical-modulatory”), 2) a reliable loading on the sex-related network of regions revealed by the seed PLS, and 3) consistency with functional neuroimaging studies providing information on the neural circuitry of pain (Petrovic and Ingvar, 2002; Ploghaus et al., 2003; Ploghaus et al., 2001; Porro, 2003; Porro et al., 2003; Price, 2000) and emotion (Pezawas et al., 2005; Stein et al., 2007). The causal structure among the brain regions comprising the network was supported by neuroanatomical studies (Amodio and Frith, 2006; Augustine, 1996; Cavada et al., 2000; Craig, 2002b, 2003a, c; Kringelbach, 2005; LeDoux, 2000; Ongur and Price, 2000; Price, 2003, 2005; Van Hoesen, 1995; Vogt, 2005; Vogt et al., 2005). Although reciprocal top-down (descending) and bottom-up (ascending) connectivity between regions is well-known, there are mathematical restrictions on the number of reciprocal pathways that can be specified for a given model using structural equation modeling (SEM) (Berry, 1984). Therefore, we modeled most paths between structures as unidirectional, emphasizing top-down connectivity between regions relevant for testing hypotheses regarding cortical modulation of the “homeostatic-afferent” and “emotional-arousal” networks, and bottom-up connectivity to test the hypotheses regarding ascending visceral input.
Having established a structural model, path analysis via a SEM framework was performed using Amos 6.0 (Arbuckle, 2005). Residual variances, representing external input into the system (e.g., unspecified regions, psychological characteristics, hormonal milieu), were fixed at 35% (McIntosh and Gonzalez-Lima, 1994) of the observed regional variances within group and condition. SEM is a multivariate covariance-based analysis that determines the path coefficients or coupling between brain regions in a specified network model. Expressed in terms of neural pathways, a path coefficient is the direct proportional influence that one brain region has on another via a direct anatomical connection, controlling for all other regions in the model. Path or beta coefficients reflect how a one unit change in one area influences activity in the region to which it projects. Normalized rCBF activity from each scan was extracted from the most reliable voxel of each region selected as a node of the network and entered into a path analysis.
Sex differences in the effective connectivity of the network were tested using multi-group tests for invariance (Joreskog, 1971). Specifically, sex differences in the circuitry of the network were localized using pair-wise comparisons between a completely unconstrained model and a partially constrained model using a chi square difference test with 1 degree of freedom. Sequentially, each path of interest was restricted to be equal across sex and tested against a completely unconstrained model (e.g., all parameters estimated freely). Significance indicates that a pathway should be freely estimated in a model rather than constrained to be equal and denotes significant differences between males and females in the effective connectivity of the brain regions. These differences can involve both the sign and magnitude of the coefficient. Differences in sign reflect a reversal or qualitative change in regional interactions. Changes in magnitude reflect increase or decrease in the strength of the coupling between regions. Chi-square- and t-test for group differences and path coefficients were interpreted as significant at p<.05.
Results
Level 1 analysis: Task PLS
Task PLS was employed to identify distributed patterns of regions that relate to the brain's response to aversive visceral stimulus and its expectation. Significant LVs were found for both main effect contrasts, but not the interaction effect contrasts involving sex.
BL vs. INF
Common INF-related network
The first significant LV (LV1) from the task PLS represented a pattern of brain regions that maximally distinguished between the BL and INF scans for both females and males. This LV explained approximately 35% of the variance in the “cross-block covariance” matrix. Permutation testing indicated significance at p <.0001. Reliable increases in brain activity were evident during the response to an aversive visceral stimulus in a network of brain regions that included known “homeostatic-afferent” (thalamus, anterior INS (aINS), dACC, medial OFC (mOFC)), and PFC and parietal (BA 40) “cortical-modulatory” regions (see supplemental Table 1). As expected, the thalamus and INS contributed significantly to the discrimination of BL and INF. The thalamus was selected as a seed voxel to test for sex differences in the functional connectivity of a “homeostatic-afferent” network.
BL versus EXP
Common EXP-related network
The second significant LV (LV2) from the task PLS characterized a functional pattern of regions that maximally differentiated BL from EXP for both females and males. LV2 accounted for 28% of the variance in the “cross-block covariance” matrix and permutation testing indicated significance at p <.0001. Greater activity was observed during EXP in limbic and paralimbic regions (bilateral amygdala, parahippocampal regions, infragenual cingulate (iACC) (BA 25) and posterior cingulate cortex (PCC) (BA 30)), posterior dorsal INS (pdINS), dorsal prefrontal cortex (dPFC) (BA 10), right parietal (BA 40) regions and visual cortex (see supplemental Table 2). Deactivations during EXP were observed in the right mPFC and the left OFC. As expected strong bilateral amygdala activation was evident and considered the most appropriate seed to test for sex differences in the functional connectivity of an “emotional-arousal” network.
Level 2 analyses: Seed PLS
Seed PLS was used to identify networks operating in concert with the thalamus and amygdala regions identified in the Task PLS and to determine the influence of condition and sex on these networks.
Common thalamo-centric network
Seed PLS with the right thalamus region (MNI -4,-8, 4), identified by the task PLS as being reliably activated during INF as compared to BL, revealed a network of regions correlating with the right thalamus and operating similarly for men and women across all conditions (see supplemental Figure 1). The projection plot in Figure 1 depicts this common thalamo-centric network of regions which explained 43% of the variance in the right thalamus-whole-brain activity “cross-correlation” matrix. Permutation testing indicated significance at p <.001. The network included “homeostatic-afferent” regions (bilateral thalamus, dACC/ mid-cingulate cortex (MCC) (BA 24)), and bilateral INS (left aINS, right pdINS)) as well as sensory (BA 1/40), motor (BA 6, 3), midbrain (ventral tegmental area), and prefrontal pain and affect modulating regions (PFC, OFC) (BA 44, 45, 47) (for details, see supplemental Table 3). In general, “homeostatic-afferent” regions (left thalamus, aINS, MCC), midbrain, prefrontal and parietal cortical regions (BA 40, BA 44, 45, 47) and sensory (BA 1) regions demonstrated positive correlations with the right thalamus whereas motor, premotor areas (BA 4, 6) demonstrated negative correlations (for details, see supplemental Table 3). To further test the hypothesis regarding sex differences in the “homeostatic-afferent” network, we performed an addition seed PLS using the aINS identified in the task PLS but again results did not reveal a significant sex-related network (not shown).
Figure 1. Projection plot of the common thalamo-centric network.
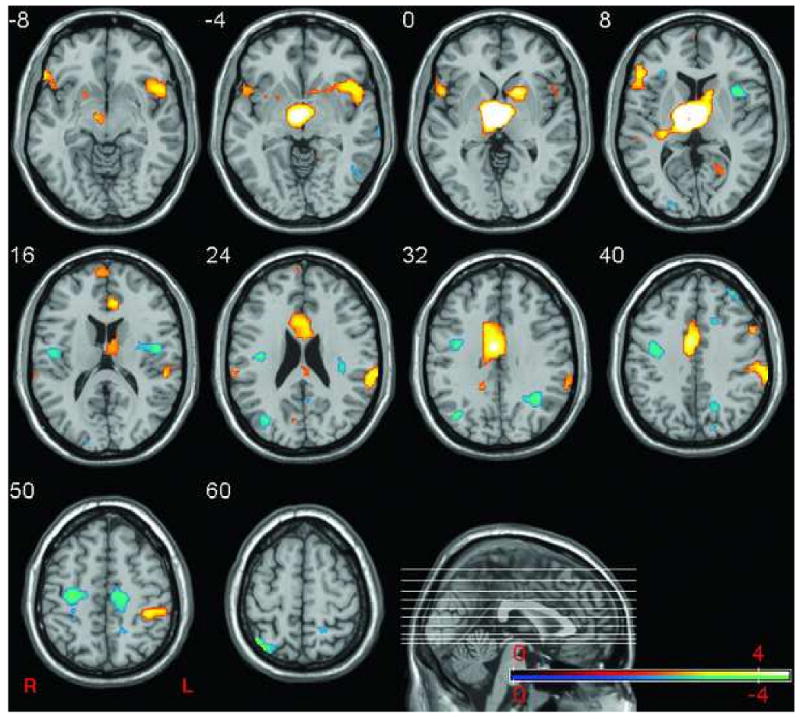
The plot depicts the network of brain regions that reliably correlated with the right thalamus (MNI, 4, 8, 4) across conditions and sex. Blue regions indicate regions with negative salience and correlated negatively with the thalamus. Red regions denote areas of positive salience that correlated positively with the thalamus. Sagittal section shows location of selected axial slices in the Z-plane plane of MNI space.
Amygdalo-centric networks
Common amygdalo-centric network
Based on the a priori hypothesis regarding the role of the amygdala and a related “emotional-arousal” network in central pain modulation, a seed PLS was performed with the amygdalae (MNI ±20,-5,-17) identified as more activated during EXP as compared to BL in the task PLS. PLS seeded with the amygdala bilaterally revealed two significant amygdalo-centric networks. The first LV (LV1) revealed a network of regions correlated with the amygdalae that operated similarly for females and males across all conditions (with the possible exception of the left amygdala during baseline for females since the 95% confidence interval of the average correlation includes zero) (see supplemental Figure 2). The projection plot in Figure 2 depicts the common amygdalo-centric network. This network accounted for 36% of the variance in the amygdalae-whole-brain activity “cross-correlation” matrix (p <.001). This network of regions included known regions of the “homeostatic-afferent” network (left thalamus, pons, bilateral ventral pINS), limbic and paralimbic (hippocampus, MCC) and OFC and PFC regions (BA 9, 10, 46, 11/47) (for details, see supplemental Table 4). In general, across conditions and sex, OFC, dlPFC, dPFC, mPFC, and MCC correlated negatively with the bilateral amygdala activity, consistent with known cortico-limbic inhibitory influences. In contrast, the bilateral mOFC, INS, and pons demonstrated positive correlations with the amygdalae.
Figure 2. Projection plot of the common amygdalo-centric network.

The plot shows the network of brain regions that correlated with the right and left amygdala (MNI ±20,-5,-17) similarly across conditions and sex. Red denotes regions of positive salience that correlated positively with the amygdalae whereas blue denotes regions of negative salience that correlated negatively with the bilateral amygdala activity. Sagittal section shows location of selected axial slices in the Z-plane of MNI space.
Sex-specific amygdalo-centric network (LV2)
The second LV (LV2) from the bilateral amygdala seed PLS revealed a network of regions that demonstrated a consistent relationship with the amygdale across conditions (again with the possible exception for females during BL) but operated differently for females and males (see supplemental Figure 3). That is, this network of regions showed strong sex differences in functional connectivity with the amygdalae. The projection plot in Figure 3 depicts this sex-related amygdalo-centric network that accounted for 16% of the variance in the bilateral amygdala-whole-brain activity “cross-correlation” matrix. Permutation testing indicated significance at p <.01. The regions comprising this network included “homeostatic-afferent” (bilateral thalamus, bilateral pINS), somatosensory (BA 40/7, BA 3/2/1, PCC), prefrontal (mPFC/mOFC (BA 10/11/47)), iACC and supragenual ACC (sACC) and pontine (PAG and LCC) regions. On average and across conditions, the mOFC, iACC, sACC, and PAG/LCC demonstrated more positive correlations with the amygdala for females but were characterized by either less positive or more negative correlations in males (see supplemental Table 5). Similarly, “homeostatic-afferent” regions (thalamus, pINS) and somatosensory regions were more positively correlated with the amygdala for men, but showed either less positive or more negative correlations with the amygdala bilaterally for women.Table 1 shows a summary of the various networks identified by the task and seed PLS.
Figure 3. Projection plot of sex-related amygdalo-centric network.
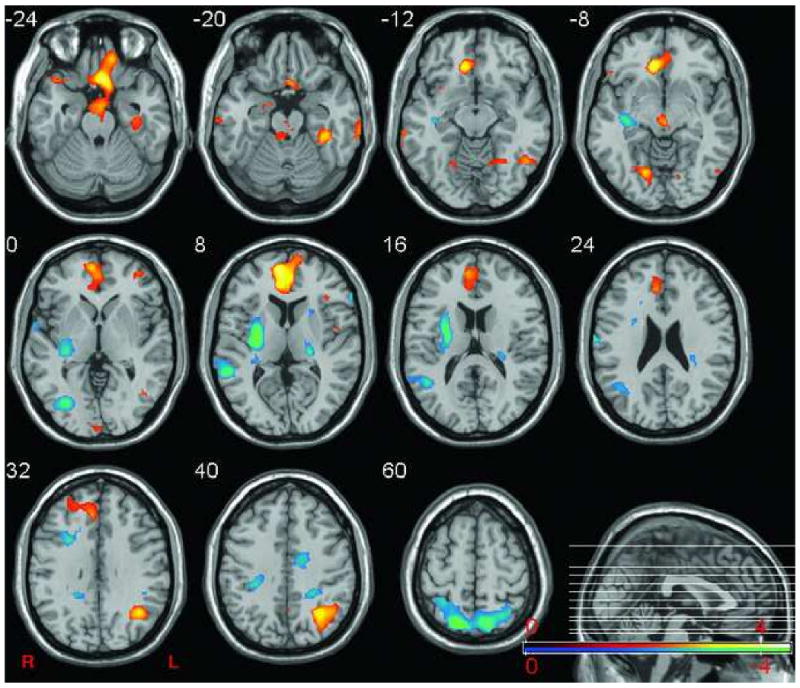
The plot shows the network of regions that reliably correlating with amygdalae (MNI ±20,-5,-17) across conditions. For females, blue regions indicate regions correlated negatively with the bilateral amygdala activity and yellow regions denote areas that correlated positively with the amygdalae. For men, blue regions indicate regions correlated positively with the amygdalae and yellow regions denote areas that correlated negatively. Sagittal section shows location of selected axial slices in the Z-plane of MNI space.
Table 1. Summary of the networks identified by the task and seed PLS.
| Network | PLS | Design matrix | LV | Variance explained | P | Sex difference |
|---|---|---|---|---|---|---|
| INF-related | Task | INF-BL | 1 | 35 % | <.0001 | No |
| EXP-related | EXP-BL | 2 | 28% | <.0001 | No | |
| Common thalamo-centric | Seed | thalamus | 1 | 43% | <.001 | No |
| Common amygdalo-centric | Seed | B amygdala | 1 | 36% | <.001 | No |
| Sex-related amygdalo-centric | 2 | 16% | <.01 | Yes |
Abbreviations: BL- baseline, INF- inflation, EXP- expectation, LV- latent variable, P- probability, PLS-partial least squares
Effective Connectivity
As described in the methods, brain regions comprising the network to be tested were selected from the sex-related amygdalo-centric network (LV2). The connectivity between the nodes of the regions were specified to permit assessment of sex differences in the effective connectivity of networks hypothesized to be involved in response to aversive pelvic stimuli. As can be seen in Figure 4, the network model to be tested comprised “emotional-arousal” (amygdala → iACC →sACC→ amygdala→ pons/LCC→amygdala, pons/LCC → sACC, pons/LCC → iACC), “homeostatic-afferent” (thalamus→ pINS, thalamus→sACC, thalamus→mOFC, pINS→mOFC) and “cortical-modulatory” (mOFC→iACC, mOFC→sACC, mOFC→ amygdala, iACC→ pINS, pINS→amygdala) circuitry.
Figure 4. Network nodes and schematic neural circuitry underlying responses to expectation and experience of aversive visceral stimulus.

The structural model for the proposed network to be tested was comprised of “emotional-arousal” (orange), “homeostatic-afferent” processing (blue) and “cortical-modulatory” circuitry (green). The table inset on the right gives MNI coordinates for network nodes shown in Fig. 4, left. Abbreviations: BA- Brodmann areas, Amyg- amygdala, iACC- infragenual cingulate cortex, INS- insula, LCC- locus coeruleus complex, mOFC- medial orbital frontal cortex, sACC- supragenual anterior cingulate cortex, Thal- thalamus, ROI- region of interest.
“Emotional-arousal” network
As shown in Figure 5 and Table 1, in general during all 3 conditions (BL, EXP, INF), the female subjects showed stronger coupling between nodes of the “emotional-arousal” network. The majority of the significant sex differences in this circuitry were evident during EXP (Fig. 5, right panel, Table 1). During EXP, males and females differentially engaged the amygdala-iACC-sACC-pons/LCC circuitry resulting in significant sex differences. Furthermore, the amygdala→iACC and amygdala →pons circuits showed consistent sex differences across all conditions with stronger positive coupling for females and negative or nonsignificant connectivity in males.
Figure 5. Estimated effective connectivity of the proposed network comprising the “homeostatic-afferent”, “emotional-arousal”, and “cortical-modulatory circuits”.

The operation of the proposed network (as estimated by the completely unconstrained model) during BL, INF and EXP (columns) is presented for females and males (rows). The beta coefficients (effective connectivity) are depicted by the thickness and color of the arrows. Solid arrows represent a parameter estimate that was considered significantly different from zero whereas dashed lines represent nonsignificant coefficients. Red arrows represent positive coupling whereas blue arrows represent negative coupling. The legend depicts the magnitude of the coefficients associated with each thickness. Abbreviations: Amyg- amygdala, iACC- infragenual cingulate cortex, INS- insula, LCC- locus coeruleus complex, mOFC- medial orbital frontal cortex, n.s.- nonsignificant, sACC- supragenual anterior cingulate cortex, Thal- thalamus.
“Homeostatic-afferent network”
As shown in Figure 5 and Table 1, there were only a small number of significantly activated circuits among the ascending input from the thalamus and pINS projections to sACC and mOFC in either female or male subjects, reflecting only minor group differences in effective connectivity. INS connectivity to mOFC was consistently negative during all 3 conditions in males and more positive in women, but a statistically significant group difference was only found during the EXP condition (Figure 5,Table 1). The connectivity between the thalamus and mOFC was more negative in females compared to men but no significant group differences were observed.
“Cortical-modulatory network”
There were only a few significant group differences in cortical-subcortical-modulatory circuits (see Figure 5, Table 1). While males consistently showed greater positive connectivity between the pINS→amygdala, with the strongest connections during BL and EXP, females showed weaker connectivity or lack of engagement of this circuit. These group differences were statistically significant for the BL and EXP conditions. In addition, females showed a strong positive connectivity between mOFC→ amygdala during the INF and EXP conditions whereas males demonstrated weak negative connectivity in this circuitry, resulting in a significant group difference.
Discussion
The primary goal of the current study was to identify brain networks activated by expected and delivered aversive pelvic visceral stimuli in male and female patients with chronic abdominal pain, and to test for sex differences in the hypothesized circuitry within these networks. Multivariate network analyses using partial least squares and structural equation modeling provided support for the involvement of regions comprising “homeostatic-afferent”, “emotional-arousal” and “cortical-modulatory” networks. While the brain of male and female patients showed a great degree of similarity in its response to the experimental stimuli, significant sex-related differences in the functional and effective connectivity of brain regions were demonstrated. To our knowledge, this is the first demonstration of such sex-related differences in the effective connectivity of brain networks in an experimental pain paradigm.
Brain networks activated similarly in males and females in response to the delivery or expectation of an aversive visceral stimulus
Using task PLS, we confirmed previous findings of distinct activations of brain regions during INF and EXP (reviewed in Mayer et al, 2006). Delivery of the aversive stimulus was associated with greater activity in regions closely connected to the thalamus, such as bilateral aINS and dACC as well as modulatory cortical regions including the mOFC and PFC. In contrast, the EXP of the stimulus without actual delivery, was characterized by greater activation of brain regions closely connected to the amygdala (parahippocampal regions, iACC, pINS and OFC) some of which are part of a well characterized “emotional-arousal network” (Pezawas et al., 2005; Stein et al., 2007). The parietal cortex (BA40) showed greater activation during both EXP and INF conditions, consistent with the hypothesized role of this region in a distributed network, allocating attentional resources to novel sensory information (Mesulam, 1998). Further supporting such a role of parietal cortex as part of an attentional network is our recent demonstration of decreased activation of this posterior attentional network during both the delivery and expectation of an aversive visceral stimulus as subjects become more familiar with the experimental paradigm (Naliboff et al., 2006).
Common (e.g., sex-unrelated) thalamo-centric and amygdalo-centric networks activated during both delivery and expectation of an aversive visceral stimulus
Seed PLS demonstrated the activation of the respective brain regions within the context of thalamo-centric and amygdalo-centric networks operating similarly between men and women across the nonINF (BL, EXP)and INF conditions. The functional connectivity of the common thalamo-centric network generally agreed with known neuroanatomical models (Craig, 2002a, 2003b). On the other hand, the functional connectivity within the amygdalo-centric network generally agreed with the postulated model of emotional regulation and arousal circuits (Pezawas et al., 2005; Stein et al., 2007). Thus, while our findings confirm previous observations (using SPM and region of interest analyses) by our group and others on brain regions activated during aversive pelvic visceral distension (reviewed in Mayer et al, 2006), they formally demonstrate for the first time that these regions are activated as part of distinct functional brain networks.
Sex-related activation of a network during delivery and expectation of an aversive visceral stimulus
In addition to the common networks identified in the seed PLS, where connectivity to the seed region was unrelated to the patient's sex, we identified a sex-related amygdalo-centric network operating during both the INF and nonINF (BL, EXP) conditions. The regions comprising this network supported the involvement of “homeostatic-afferent”, “emotional-arousal” and “cortical-modulatory” circuitry during expected and delivered aversive visceral stimuli.
Effective connectivity modeling supported the hypothesis that the most consistent sex-related differences occur within an “emotional-arousal” network. For example, women showed consistently stronger connectivity between the pons/LCC and the mOFC during all conditions, while males consistently showed much weaker engagement of this circuit. Even though the spatial resolution of the scanner limits our ability to precisely localize the source of the pontine activation to the LCC, ascending noradrenergic projections from the LCC to the (medial PFC/mOFC) would be a plausible neuroanatomical correlate of this connectivity (Valentino et al., 1999). Similarly, while women showed consistently strong and positive connectivity between amygdala and pons/LCC and iACC, these connections were all weaker and sometimes negative in men. The strongest and most consistent sex difference in the connectivity between these regions was seen during the EXP condition (Figure 5).
The connectivity within the major nodes of the “homeostatic-afferent” (thalamus, pINSand dACC) and “cortical-modulatory” networks showed only few sex-related differences. Men demonstrated consistently negative coupling of ascending connections from the pINS to mOFC during all three conditions, while women showed more variable connections (Figure 5). However, significant group differences in the input to the mOFC from the pINS were observed only during the EXP condition.
Possible correlation of findings with previously reported SPM analyses
Using SPM analysis of [15O] water PET data, we have previously reported sex-related differences in the brain's response to an aversive visceral stimulus, despite overall similarity of activated regions (Berman et al., 2000; Berman et al., 2006; Naliboff et al., 2003). When comparing male and female IBS patients, greater mid-pINS and aINS activation was found during INF in male patients in two different samples(Berman et al., 2000; Naliboff et al., 2003) and greater activation of mOFC, dACC and amygdala in female patients in one study (Naliboff et al., 2003). The greater aINS activation in men was replicated in a third sample of healthy men and women using fMRI (Berman et al., 2006). In the current analysis, significant sex differences were observed in the connectivity of the pINS with the amygdala (during BL and EXP), and of the pINS with the mOFC (during EXP). These group differences were related to men showing a greater positive connectivity between the pINS and amygdala during the nonINF conditions, and a lack of coupling between the pINS and mOFC during EXP. When viewed together, one may speculate that in male IBS patients, the consistently greater activation of the INS during INF and sex-related differences in amygdala connectivity seen in the current analysis may underlie the greater sympathetic nervous system responses previously reported in male IBS patients (Tillisch et al., 2005). Future studies with simultaneous recording of sympathetic nervous system activity are needed to confirm this hypothesis. On the other hand, the greater activation of the amygdala seen in the previous SPM analysis in women may be related to the greater positive connectivity that the amygdala receives from OFC and to the absence of the feedback inhibition from sACC to the amygdala during the EXP condition (Figure 5).
Possible correlation of findings with sex differences in behavioral responses
Using validated psychophysiological techniques, we have previously reported that women with IBS show greater subjective emotional and perceptual responses to colorectal distension than male patients or healthy men or women (Chang et al., 2006a). One may speculate that the sex-related differences in the connectivity of the “emotional-arousal” network seen in the current study may be related to these greater emotional and perceptual responses previously reported. However, in the current study, ratings were only obtained 10 min after each stimulus (BL, EXP, INF), and these limited post hoc perceptual ratings did not reveal any sex differences (Naliboff et al., 2003). Although the possibility of sex differences in ascending visceral afferents may exist (Verne and Price, 2002), PLS seeded with known ascending visceral pain regions (thalamus, anterior INS) indicated common functional connectivity between men and women. Sex differences in thalamus connectivity were only revealed by taking the amygdala influence into account. Thus, our findings are most consistent with the hypothesis that sex-related differences are primarily a consequence of differences in the engagement of “emotional-arousal” networks and to a lesser degree, “cortical-modulatory” input to this network, and that ascending information from homeostatic afferents are not driving the observed sex differences.
Strengths and limitations of connectivity modeling of brain responses
Path analysis via an SEM framework was used to test for sex differences in hypothesized circuitry, rather than to derive the best-fitting network model. We consider this approach reasonable given the small sample size and the limited number of data points available in our data. Although determining the best-fitting model (i.e., causal structure of the network) is highly desirable, inferences regarding group differences in effective connectivity have been shown to be valid regardless of the model fit (Protzner and McIntosh, 2006). Also of note, the current effective connectivity analysis did not allow for the precise estimation of network functioning during each condition due to biased parameter estimation in large networks with small samples. Greater precision of estimates can be obtained by sacrificing the completeness of a model (reducing the number of nodes and connections) or with greater sample sizes. In addition, the proposed model is incomplete since the external input into the current model (represented in the error terms, contextual factors such as psychological and hormonal milieu) remains to be incorporated. Also, many of the regions comprising the network have reciprocal connections with each other but could not be modeled due to mathematical constraints (Berry, 1984). Arguably other key brain regions and circuitry may remain to be delineated as well. Constructing and validating the most parsimonious model of effective connectivity during experience of aversive visceral stimuli was beyond the scope of this paper.
Although the current approach enabled testing of important hypotheses regarding the sex differences in effective connectivity of neural networks in IBS patients, connectivity analyses with PET data have several limitations, including its limited temporal and spatial resolution. For example, the spatial resolution of PET precludes localization of small brainstem nuclei and we can only speculate on the involvement of such regions as the LCC. Also, as in the current study, brain responses acquired with [15O] water PET are usually averaged over a 60-120 second period to achieve an acceptable signal to noise ratio (SNR). Such averaging results in the convolution of multiple neural processes, presumably occurring at different time scales (i.e., homeostatic-afferent processing, emotional-arousal, cortical modulation) in the overall picture of neural activation, and ignores the temporal engagement of this circuitry. For example temporal resolution of the postulated sequential processing of visceral afferent information within different INS subregions cannot be achieved reliably in the current data set. Network analyses with data obtained with fMRI, MEG and EEG would be prudent as this methodology can more realistically capture the temporal dynamics of the proposed circuitry.
Conclusions
In summary, the results of formal network analyses suggest the activation of distinct yet overlapping networks concerned with the processing of ascending visceral information, emotional-arousal (induced by expectation or actual experience of the stimulus) and modulatory cortical influences. While there was common activation of these networks in both sexes, network functioning during expectation was uniquely characterized by sex differences in the cortico-limbic circuits involved in emotional-arousal, pain facilitation and autonomic responses. These sex differences in specific brain circuits may have implications for a better understanding of IBS pathophysiology and for the reported sex differences in the effectiveness of certain pharmacologic treatments. Our findings also suggest, that despite multiple peripheral and spinal mechanisms proposed for sex differences in pain sensitivity, the peripheral signal from the gut in IBS patients appears to contribute little to overall sex differences at the level of the brain. Further work will be necessary to confirm the functional relevance of these networks via correlation with exogenous measures of attention, arousal, vigilance, emotional factors, and autonomic functioning. Despite these limitations, effective connectivity analyses permitted the first assessment of how brain regions interact with one another in the context of larger neural networks in patients with IBS.
Supplementary Material
Table 2. Effective connectivity parameter estimates and between-group comparisons of homeostatic-afferent, emotional-arousal, and cortical-modulatory circuits.
| Baseline | Inflation | Expectation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Circuitry | Connectivity | Fem Beta |
Male Beta |
χ² Δ | Fem Beta |
Male Beta |
χ²Δ | Fem Beta |
Male Beta |
χ²Δ |
| Homeostatic-afferent | Thal → sACC | 0.15 | -0.28 | 1.5 | -0.08 | -0.21 | 0.1 | -0.57 | 0.14 | 3.4 |
| Thal → INS | 0.13 | 0.08 | 0.1 | -0.09 | 0.11 | 1.7 | 0.20 | 0.01 | 0.7 | |
| Thal → mOFC | -0.17 | 0.43 | 1.7 | -0.64 | -0.15 | 1.8 | -0.68 | -0.03 | 2.1 | |
| INS → mOFC | -0.21 | -0.66 | 0.4 | -0.08 | -0.32 ** | 3.8 | 0.25 ** | -0.08 | 9.7 | |
| Emotional-arousal | sACC → Amyg | -0.01 | -0.29 * | 1.6 | 0.07 | -0.11 | 1.0 | 0.54 ** | -0.44 * | 17.4 |
| iACC → sACC | 0.02 | 0.43 ** | 1.8 | -0.08 | 0.60 ** | 4.4 | -0.16 | 0.59 ** | 7.1 | |
| Amyg → iACC | 0.34 ** | -0.16 * | 13.2 | 0.28 | -0.01 | 1.7 | 0.32 ** | -0.19 * | 12.7 | |
| Amyg → Pons | 0.48 | 0.16 | 0.6 | 0.75 * | 0.36 | 0.5 | 0.96 ** | -0.05 | 7.3 | |
| Pons → Amyg | 0.05 | 0.08 | 0.1 | -0.07 | 0.08 | 2.0 | 0.13 * | 0.11 | 0.0 | |
| Pons → iACC | -0.21 ** | -0.02 | 9.9 | 0.05 | -0.03 | 0.8 | -0.25 ** | 0.02 | 9.5 | |
| Pons →sACC | -0.09 | 0.10 | 1.9 | -0.13 | 0.16 | 3.5 | -0.30 * | 0.26 ** | 11.9 | |
| Pons→mOFC | 0.54 ** | 0.12 | 4.8 | -0.58 | 0.21 | 3.6 | -0.81 | 0.23 | 5.1 | |
| Cortical-Modulatory | mOFC → iACC | 0.08 | 0.11 | 0.1 | 0.27 ** | 0.06 | 2.8 | 0.10 | -0.06 | 1.0 |
| iACC → INS | -0.21 * | -0.06 | 0.8 | 0.03 | -0.26 * | 3.4 | 0.07 | -0.07 | 0.5 | |
| INS → Amyg | -0.20 | 0.60 | 4.5 | 0.03 | 0.26 | 1.2 | 0.08 | 0.93 ** | 9.0 | |
| mOFC → Amyg | 0.08 | 0.25 | 0.8 | 0.58 ** | -0.16 | 21.1 | 0.52 * | -0.20 * | 16.6 | |
Critical values for the chi square difference test (Δχ2) are 3.84, p <05, 6.64, p <.01, and 10.8, p <.001. Bolded values indicate significant chi- squared difference statistics.
Significant beta coefficients are denoted by =p <.05
Significant beta coefficients are denoted by p <.01.
Abbreviations: Amyg- amygdala, BA- Brodmann areas, Fem-female, iACC- infragenual cingulate cortex, INS- insula, LCC- locus coeruleus complex, mOFC- medial orbital frontal cortex, sACC- supragenual anterior cingulate cortex, Thal- thalamus.
Acknowledgments
Grant Support: Supported in part by grants from the National Institutes of Health (K08 DK071626 (JSL), P50 DK064539 (EAM), RO1 DK 48351(EAM)) the Office of Research in Women's Health (ORWH) (P50 DK064539 (EAM)) and the National Center for Complementary and Alternative Medicine (NCCAM)(R24 AT002681),
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. Amos™ 6.0 User's Guide. SPSS; 2005. [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baciu MV, Bonaz BL, Papillon E, Bost RA, Le Bas JF, Fournet J, Segebarth CM. Central processing of rectal pain: a functional MR imaging study. AJNR Am J Neuroradiol. 1999;20:1920–1924. [PMC free article] [PubMed] [Google Scholar]
- Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff B, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller J, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. Journal of Neuroscience. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- Berry WD. Nonrecursive Causal Models. Sahe Publications, Inc; Newbury Park: 1984. [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci. 2007;27:1543–1551. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- Chang L, Mayer EA, Labus JS, Schmulson M, Lee OY, Olivas TI, Stains J, Naliboff BD. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006a;291:R277–284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD. Gender, age, society, culture, and the patient's perspective in the functional gastrointestinal disorders. Gastroenterology. 2006b;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002a;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002b;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003a;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003b;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends in Neurosciences. 2003c;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90R. Administration, scoring and procedures manual — II. Towson, MD: 1983. [Google Scholar]
- Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5:56–65. doi: 10.1177/1099800403005001006. [DOI] [PubMed] [Google Scholar]
- Joreskog KG. Simultaneous factor analysis in several populations. Psychometrika. 1971;36:409–426. [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology. 2005;65:1268–1277. doi: 10.1212/01.wnl.0000180971.95473.cc. [DOI] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Berman SM, Suyenobu B, Chang L, Naliboff BD. Characterization of a sex-dependent brain network during anticipation of visceral discomfort. Society for Neuroscience; Washington, DC: 2005. Program No. 193.117. [Google Scholar]
- Labus JS, Mayer EA, Berman SM, Suyenobu B, Fallon J, Mandelkern M, Chang L, Naliboff BD. Sex-specific differences in a brain network functioning during anticipation of rectal discomfort in Irritable Bowel Syndrome Patients (IBS) Gastroenterology. 2006;130 [Google Scholar]
- Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Reviews in Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc Natl Acad Sci U S A. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8:451–463. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Craske MG, Naliboff BD. Depression, anxiety and the gastrointestinal system. Journal of Clinical Psychiatry. 2001;62:28–36. [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7:523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural modeling of functional neural pathways mapped with 2-deoxyglucose: effects of acoustic startle habituation on the auditory system. Brain Res. 1991;547:295–302. doi: 10.1016/0006-8993(91)90974-z. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Human Brain Mapping. 1994;2:2–22. [Google Scholar]
- McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, Rapoport SI, Horwitz B. Network analysis of cortical visual pathways mapped with PET. J Neurosci. 1994;14:655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23 1:S250–263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern M, Chang L, Mayer EA. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci. 2003;7:197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro CA. Functional imaging and pain: behavior, perception, and modulation. Neuroscientist. 2003;9:354–369. doi: 10.1177/1073858403253660. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cettolo V, Francescato MP, Baraldi P. Functional activity mapping of the mesial hemispheric wall during anticipation of pain. Neuroimage. 2003;19:1738–1747. doi: 10.1016/s1053-8119(03)00184-8. [DOI] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–8989. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–1123. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Price JL. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. J Comp Neurol. 2005;493:132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- Protzner AB, McIntosh AR. Testing effective connectivity changes with structural equation modeling: what does a bad model tell us? Hum Brain Mapp. 2006;27:935–947. doi: 10.1002/hbm.20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel Y, Drossman DA, Turkington TG, Bradshaw B, Hawk TC, Bangdiwala S, Coleman RE, Whitehead WE. Regional brain activation in response to rectal distension in patients with irritable bowel syndrome and the effect of a history of abuse. Dig Dis Sci. 2003;48:1774–1781. doi: 10.1023/a:1025455330704. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos I, Dixon GE, Short SJ, Davidson RJ, Nitschke JB. Brain mechanisms of expectation associated with insula and amygdala response to aversive taste: implications for placebo. Brain Behav Immun. 2006;20:120–132. doi: 10.1016/j.bbi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stephan KE. On the role of general system theory for functional neuroimaging. J Anat. 2004;205:443–470. doi: 10.1111/j.0021-8782.2004.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother SC, Anderson J, Hansen LK, Kjems U, Kustra R, Sidtis J, Frutiger S, Muley S, LaConte S, Rottenberg D. The quantitative evaluation of functional neuroimaging experiments: the NPAIRS data analysis framework. Neuroimage. 2002;15:747–771. doi: 10.1006/nimg.2001.1034. [DOI] [PubMed] [Google Scholar]
- Thompson GW, Drossman DA, Richter J, Talley NJ, Thompson GW, Corazziari E, Whitehead WE. The Functional Gastrointestinal Disorders. Little,Brown; Boston: 1994. Functional bowel disorders and functional abdominal pain; pp. 115–174. [Google Scholar]
- Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: Pharmacological target for pelvic visceral dysfunction. Trends in Pharmacological Sciences. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. Anatomy of the medial temporal lobe. Magn Reson Imaging. 1995;13:1047–1055. doi: 10.1016/0730-725x(95)02012-i. [DOI] [PubMed] [Google Scholar]
- Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Current Rheumatology Reports. 2002;4:322–328. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YZ, Tao RJ, Xu B, Sun J, Chen KM, Miao F, Zhang ZW, Xu JY. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J Gastroenterol. 2003;9:1356–1360. doi: 10.3748/wjg.v9.i6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


