Abstract
Compared to young adults, older adults show not only a reduction in true memories but also an increase in false memories. We investigated the neural bases of these age effects using functional magnetic resonance imaging and a false memory task that resembles the Deese–Roediger–McDermott (DRM) paradigm. Young and older participants were scanned during a word recognition task that included studied words and new words that were strongly associated with studied words (critical lures). During correct recognition of studied words (true memory), older adults showed weaker activity than young adults in the hippocampus but stronger activity than young adults in the retrosplenial cortex. The hippocampal reduction is consistent with age-related deficits in recollection, whereas the retrosplenial increase suggests compensatory recruitment of alternative recollection-related regions. During incorrect recognition of critical lures (false memory), older adults displayed stronger activity than young adults in the left lateral temporal cortex, a region involved in semantic processing and semantic gist. Taken together, the results suggest that older adults’ deficits in true memories reflect a decline in recollection processes mediated by the hippocampus, whereas their increased tendency to have false memories reflects their reliance on semantic gist mediated by the lateral temporal cortex.
INTRODUCTION
One of the most frequent cognitive complaints in older adults is poor memory for everyday events. Supporting this casual observation, research has shown a decline in episodic memory performance across the lifespan (for reviews, see Prull, Gabrieli, & Bunge, 2000; Zacks, Hasher, & Li, 2000). Furthermore, in addition to exhibiting deficits in true memories, older adults are also more prone to false memories than young adults (Tun, Wingfield, Rosen, & Blanchard, 1998; Koutstaal & Schacter, 1997; Norman & Schacter, 1997). Although the neural bases of age-related deficits in true memory has been previously investigated using functional neuroimaging (for a review, see Dennis & Cabeza, 2008), to our knowledge, no neuroimaging study has examined the age-related changes in brain activity that are associated with false memories. This was the goal of the present functional magnetic resonance imaging (fMRI) study.
We investigated true and false memory retrieval in the scanner using a false memory task that resembles the Deese–Roediger–McDermott (DRM) paradigm (Roediger & McDermott, 1995; Deese, 1959). In the typical DRM paradigm, participants study lists of words in which all the words in the list are semantically related to a word that is not presented (the critical lure). At test, participants show a tendency to falsely recall or recognize the critical lure, and this tendency is stronger for older than for young adults (Watson, McDermott, & Balota, 2004; Balota et al., 1999; Tun et al., 1998). Age-related increases in false memories have been attributed to both a deficit in memory for item-specific details (LaVoie & Faulkner, 2000; Tun et al., 1998; Koutstaal & Schacter, 1997; Norman & Schacter, 1997; Spencer & Raz, 1995) and to an increased reliance on semantic gist (Balota et al., 1999; Tun et al., 1998).
Behavioral studies have demonstrated that older adults are impaired in memory for item-specific details, and neuroimaging evidence has associated this deficit with a dysfunction of the hippocampus (Cabeza, 2006). Compared to young adults, older adults show difficulties in retrieving the context (Bayen, Phelps, & Spaniol, 2000; Spencer & Raz, 1995; Park & Puglisi, 1985) and perceptual features of studied items (Bastin & Van der Linden, 2003). Retrieval of such item-specific details is defined as “recollection,” whereas memory in the absence of item-specific contextual information is referred to as “familiarity.” Neuropsychological and neuroimaging studies indicate that recollection and familiarity are two separate, dissociable mechanisms and depend on different brain regions (Yonelinas, 2002). In the case of true memories, aging has been shown to impair recollection more so than familiarity (Bastin & Van der Linden, 2003; Davidson & Glisky, 2002; Parkin & Walter, 1992). Lesion and functional neuroimaging studies have associated recollection with the hippocampus (Eichenbaum, Yonelinas, & Ranganath, 2007; Yonelinas, 2002; Aggleton & Brown, 1999), which is a region that shows significant atrophy in older adults (Raz, Rodrigue, Kennedy, & Acker, 2007; Raz et al., 2005; Raz, Rodrigue, Head, Kennedy, & Acker, 2004). Linking behavioral and neuroscientific evidence, functional neuroimaging studies have associated older adults’ recollection deficits with reductions in hippocampal activity (Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006; Cabeza et al., 2004).
Despite the aforementioned deficits in recollection and hippocampal functioning during episodic memory tasks, older adults compared to young adults often show increased recruitment of other brain regions such as frontal lobes (e.g., Dennis, Daselaar, & Cabeza, 2006; Gutchess et al., 2005; Cabeza et al., 2004) or cortical medial-temporal lobe (MTL) regions (e.g., rhinal cortex: Daselaar, Fleck, Dobbins, et al., 2006). The idea that older adults compensate for declining processes with the recruitment of resources not typically recruited by young adults is a common finding in the aging literature (for a review, see Dennis & Cabeza, 2008). However, direct evidence linking performance and neural compensation is scarce. Although the current study will investigate age deficits in hippocampal-mediated recollection, it will also investigate the notion of compensation within the retrieval network, specifically related to recollection and retrieval of item-specific details.
As noted above, however, older adults’ memory impairments are assumed to reflect not only a deficit in memory for item-specific details associated with true memories but also greater reliance on semantic gist associated with false memories (Balota et al., 1999; Kensinger & Schacter, 1999; Koutstaal & Schacter, 1997). According to fuzzy trace theory, two different kinds of memory traces are created during encoding: item-specific traces and gist traces (Schacter, Verfaellie, & Pradere, 1996; Brainerd & Reyna, 1990). Item-specific traces retain the distinctive features of the individual items, whereas gist traces retain only the general meaning of the event, lacking any perceptual details or information pertaining to the encoding event.1 In the case of DRM lists, item-specific traces are stored for the details associated with each item in the list, and a gist trace is stored for the general semantic theme of the list. During retrieval, recovery of truly encoded item-specific traces should result in the successful recognition of studied items, whereas recovery of gist traces may result in the tendency to respond “old” not only to studied items but also to the nonstudied critical lures, which strongly matches the semantic theme of the studied list. The fuzzy trace theory can account for age-related increases in false memories by postulating that older adults have a deficit in memory for item-specific traces but not for gist traces (Tun et al., 1998).
Given that semantic processes are relatively well preserved in older adults (Light, 1992; Light & Burke, 1988; Salthouse, 1982), they may rely more on these processes to compensate for deficits in episodic memory. Although relying on semantic gist may, at times, enhance true memories, it may also lead to false memories when lures are semantically associated to encoded items. Functional neuroimaging studies have associated semantic processing with the left temporal cortex (for reviews, see Thompson-Schill, Kan, & Oliver, 2006; Wise & Price, 2006). Supporting the role of this area in processing semantic gist, patients with semantic dementia (and damage to this region) are impaired at extracting and/or utilizing semantic gist (Simons, Verfaellie, et al., 2005). Finally, there is also some evidence that older adults show enhanced activation in this region during lexical decision tasks (Whiting et al., 2003; Madden et al., 2002). In sum, increased false memories in older adults may reflect greater reliance on semantic gist processes mediated by the left temporal cortex.
The current study used fMRI and a categorized word-list task, which, similar to the DRM paradigm, has been studied extensively in the context of false memories and fuzzy trace theory (Brainerd & Reyna, 2007; Budson et al., 2006; Brainerd, Wright, Reyna, & Mojardin, 2001; Brainerd, Reyna, & Mojardin, 1999) in order to investigate the effects of aging on retrieval activity associated with both true and false memories. As illustrated by Figure 1, on each encoding trial, participants studied a “mini word-list” comprising four instances (e.g., horse, chicken, sheep, goat) of a semantic category (e.g., farm animals). During the memory test, participants performed an old–new recognition test with confidence ratings that included studied words (targets: e.g., horse, chicken) as well as novel words from studied categories (critical lures: e.g., cow, pig). At test, participants not only responded with their memory for the word but also how confident they were in their decision. Because we were interested in assessing age differences in retrieval processes most impaired in aging (i.e., recollection), we focused on high confidence responses. When assessing memory with confidence ratings, recollection has been associated with responses assigned the highest level of confidence (Daselaar, Fleck, & Cabeza, 2006; Yonelinas, 2001) and contrasts between high and low confidence responses have been related to recollection (see Diana, Yonelinas, & Ranganath, 2007). Thus, the current analysis approach of contrasting high versus low confidence hits was adopted to isolate memory processes associated with recollection-based retrieval processes (see Methods for additional reasoning). In keeping with the analysis for true retrieval, a similar analysis was used in assessing age differences in false retrieval. We recognize that in the case of false memories in particular, high versus low confidence memories may include a high level of familiarity compared to the true memory contrast; we address this issue in our interpretation of results. Specifically, we defined “true retrieval activity” (TRA) as greater activity for high than low confidence “old” responses to targets, and “false retrieval activity” (FRA) as greater activity for high than for low confidence “old” responses to critical lures.
Figure 1.
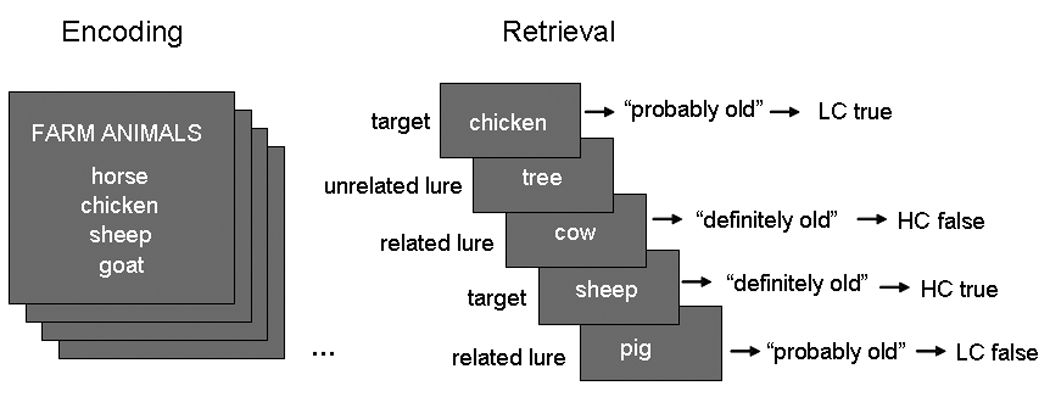
During encoding, participants were presented with short DRM lists. At retrieval, they viewed words from the list (targets), new words from different, unpresented categories (unrelated lures), and new words from presented categories (related lures). Participants were asked to make a recognition with confidence decision for each word presented at retrieval.
On the basis of the aforementioned evidence and the fuzzy trace theory of false memories, we predicted that compared to young adults, older adults would show reduced TRA in the hippocampus but increased FRA in the left temporal cortex. Additionally, we investigated the idea suggested by previous functional neuroimaging studies that older adults may compensate for deficits in a network component by relying more on other components of the same network (for a review, see Dennis & Cabeza, 2008). Thus, we explored the possibility that older adults would compensate for TRA reductions in the hippocampus by showing greater TRA in other regions associated with recollection, such as retrosplenial, posterior parietal, or left prefrontal regions.
METHODS
Participants
Sixteen young adults and 17 older adults participated in the experiment. They were healthy, right-handed, native English speakers, with no history of neurological or psychiatric disorders. All participants gave informed consent to a protocol approved by the Duke University Institutional Review Board. Due to scanner error resulting in missing data, two older adults were excluded from analyses; in addition, five young and one older adult were excluded from the analyses due to a sparse number of trials (<10) in one of the four conditions of interest (high-confidence true, low-confidence true, high-confidence false, and low-confidence false trials). Thus, the reported results are based on the data from 11 young [6 women; mean age: 23.45 (3.30) years] and 14 older adults [5 women; mean age: 68.41 (6.50) years]. In a separate session from the scanning session described below, all older adults completed a battery of neuropsychology tests derived from the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition). Results and group characteristics are reported in Table 1.
Table 1.
Demographics: Older Adults
| Mean | SD | |
|---|---|---|
| Education (years) | 17 | 2.18 |
| Shipley Vocabulary | 37.5 | 1.65 |
| CANTAB | ||
| Spatial working memory: A self-ordered task which also assesses heuristic strategy | 0.61 | 1.23 |
| Pattern recognition memory: A test of visual pattern recognition memory | 0.64 | 0.77 |
| Reaction time: A latency task with a comparative history | 0.29 | 0.94 |
| Rapid information processing: A test of sustained attention | 0.06 | 0.92 |
| Spatial span: A computerized version of the Corsi Blocks task | 1.11 | 0.98 |
| Intra–extra dimensional set shifting: A computerized analog of the Wisconsin Card Sorting Test | 0.38 | 0.43 |
| Paired associates learning: Assesses visual memory and new learning | 0.33 | 0.79 |
Mean and standard deviation for age, years of education, Shipley vocabulary score, and several standard (z) scores from the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Behavioral Methods
The present encoding task was an adaptation of the DRM paradigm (Roediger & McDermott, 1995). Materials consisted of 72 categorical six-word lists selected from category norms (Yoon et al., 2004; Battig & Montague, 1969). Each list consisted of the six most typical instances (e.g., cow, pig, horse, chicken, sheep, goat) of a category (e.g., farm animals), with minor exceptions. In each list, the third to the sixth most typical instances were used as encoding stimuli (targets); the first and the second most typical instances were used as critical lures (related lures) in the test phase and were not included in the study phase. Additionally, semantically unrelated words, matched in letter number, frequency, and concreteness to the category words, were used as control words (unrelated lures) in the test phase. The categories were carefully chosen so that their instances did not overlap. Thus, both “farm animal” and “wild animal” categories were included in the stimulus set, but “four-legged animal” was not included. To make sure there was minimal associative overlap between the categories, the probability that the related lures would be generated as an associative response to the other categories (e.g., the probability that “cow” would be generated as an associative response to “wild animal”) was examined using the “frequency of 1st occurrence” calculation from Carolyn Yoon’s normative database (http://agingmind.cns.uiuc.edu/Cat_Norms/; Yoon et al., 2004). The associative response probability was less than 1% in 10,211 out of 10,224 [72 (category number) × 2 (two related lures per category) × 71 (72 − 1)] examined word pairs (i.e., target word and related lure) and less than 5% in the remaining 13.
The study phase consisted of a single scan of 82 trials/lists: 72 encoding trials and 10 “catch” trials. Each encoding trial simultaneously showed a category name followed by four category members (see Figure 1). For each “catch” trial, only three of the four examples belonged to the category. Each encoding trial was presented for 4 sec, followed by a fixation cross for 2 sec. The participants’ task was to decide whether all four or only three examples belonged to the category. Responses were made by pressing one of two keys on a response box using the first two fingers of the right hand. The words were displayed in colors to promote the encoding of sensory/perceptual information (Cabeza, Rao, Wagner, Mayer, & Schacter, 2001). Each trial consisted of all words presented in one of five randomly assigned colors.
The test phase, which began approximately 10 min after completion of the study phase, consisted of six scans. There were a total of 288 targets, 144 related lures, and 144 unrelated lures across all six scans. Trials were presented in a predetermined, pseudorandom order. In each trial, a word was shown for 2 sec, followed by a fixation cross for 1 sec. All trials were separated using an intertrial fixation period, which varied randomly from 1.5 to 4.5 sec, allowing for event-related fMRI analyses. All words in the test phase were displayed in white color against black background. Participants responded by pressing one of four keys according to whether the word was judged to be “definitely old,” “probably old,” “probably new,” or “definitely new” (see Figure 1). Responses were made with the right hand and the mapping between response and keypresses/finger was held constant across all trials (with the index finger assigned to “definitely old” through the small finger assigned to “definitely new”).
fMRI Methods
Images were collected using a 4-T GE scanner. Stimuli were presented using liquid crystal display goggles (Resonance Technology, Northridge, CA), and behavioral responses were recorded using a four-button fiber-optic response box (Resonance Technology). Scanner noise was reduced with earplugs, and head motion was minimized using foam pads and a headband. Anatomical scanning started with a T2-weighted sagittal localizer series. The anterior (AC) and posterior commissures (PC) were identified in the midsagittal slice, and 34 contiguous oblique slices were prescribed parallel to the AC–PC plane. High-resolution T1-weighted structural images were collected with a 500-msec repetition time (TR), a 14-msec echo time (TE), a 24-cm field of view (FOV), a 2562 matrix, 68 slices, and a slice thickness of 1.9 mm. Functional images were acquired using an inverse spiral sequence with a 1500-msec TR, a 31-msec TE, a 24-cm FOV, a 642 matrix, and a 60° flip angle. Thirty-four contiguous slices were acquired with the same slice prescription as the anatomical images. Slice thickness was 3.75 mm, resulting in cubic 3.75 mm3 isotropic voxels.
fMRI analyses focused on data collected from the retrieval phase; data from the encoding phase were reported in a previous publication (Dennis, Kim, & Cabeza, 2007). Preprocessing and data analyses were performed using SPM2 software implemented in Matlab (www.fil.ion.ucl.ac.uk/spm/). After discarding the first six volumes, the functional images were slice-timing corrected and motion-corrected, and then spatially normalized to the Montreal Neurological Institute (MNI) template and spatially smoothed using an 8-mm isotropic Gaussian kernel, and resliced to a resolution of 3.75 mm3 isotropic voxels.
True and False Retrieval Analysis
Trial-related fMRI activity was first modeled by convolving a vector of the onset times of each trial with a canonical hemodynamic response function within the context of the general linear model, as implemented in SPM2. Confounding factors (head motion, magnetic field drift) were also included in the model. No participant moved more than 3 mm in any direction either within or across runs. Thus, no data were eliminated in either age group due to motion artifacts. Trials were coded based upon memory status: (1) high-confidence true retrieval, (2) low-confidence true retrieval, (3) high-confidence false retrieval, and (4) low-confidence false retrieval. Trials related to misses and correct rejections were also modeled, but treated as effects of no interest. For each participant, Statistical Parametric Maps pertaining to the effects of interest were calculated and subsequently integrated across participants using a random-effects model for each age group.
In order to identify TRA, direct contrasts were made between high- and low-confidence true retrieval and, to identify FRA, contrasts were made between high- and low-confidence false retrieval. Although previous studies have tended to use correct rejections or misses as the baseline in identifying memory success, we prefer the high versus low contrast in this instance for several reasons. First, as correct rejections are actually true memories, they present a confound in memory success when used to contrast both hits (true vs. true contrast) and false alarms (false vs. true contrast). Second, as correct rejections also involve novelty detection, they have been shown to elicit MTL activity (e.g., Daselaar, Fleck, & Cabeza, 2006; Gonsalves, Kahn, Curran, Norman, & Wagner, 2005; Grunwald, Lehnertz, Heinze, Helmstaedter, & Elger, 1998). Using misses as the baseline actually presents the same problem as using correct rejections, in that misses for false memories are actually true memories (correct rejections). Third, given that confidence was generally greater for true than for false memories, the hit-correct rejection contrast would tend to confound differences in memory veridicality with differences in confidence. The high versus low confidence contrast, on the other hand, provides an excellent control for these issues as it subtracts out (a) differences in stimuli (e.g., normative familiarity, category association strength) by comparing list items to list items for true memories and critical lures to critical lures for false memories, and (b) response processing (e.g., error detection) by comparing correct to correct responses for true memories and incorrect to incorrect responses for false memories, thus placing true and false memories on a comparable scale.
For assessing common areas of activation associated with retrieval across age groups, a conjunction map was created thresholding each age group’s random effects of the true retrieval contrast at p = .05 and 10 voxels (joint probability = .05 * .05 = .0025). This procedure yielded an activation map containing only those voxels that showed true retrieval in both age groups. A similar analysis was conducted for false retrieval. These results are reported in Table 3.
Table 3.
Common Areas of Activation for True (TRA) and False (FRA) Retrieval
| Young | Old | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H | BA | x | y | z | t | x | y | z | t | |
| TRA—Common Areas | ||||||||||
| Frontal | ||||||||||
| Superior | L | 8 | −38 | 24 | 44 | 4.01 | −34 | 21 | 44 | 2.95 |
| Ventromedial | ||||||||||
| PFC | R | 11/47 | 34 | 33 | −2 | 4.06 | 19 | 29 | −14 | 5 |
| Anterior cingulate | M | 32/10 | −8 | 37 | −5 | 5.32 | 8 | 44 | 1 | 8.51 |
| Hippocampus/PHG | L | −26 | −19 | −12 | 4.58 | −19 | −8 | −19 | 4.22 | |
| R | 30 | −23 | −8 | 3.7 | 30 | −19 | −5 | 3.43 | ||
| Posterior cingulate/Precuneus | M | 31/24 | −8 | −16 | 39 | 5.78 | 0 | −9 | 39 | 6.24 |
| Inferior parietal | L | 39/40 | −41 | −58 | 24 | 2.97 | −49 | −50 | 24 | 6.29 |
| Cuneus | M | 19/18 | 8 | −87 | 29 | 3.89 | −4 | −87 | 32 | 7.85 |
| FRA—Common Areas | ||||||||||
| Frontal | ||||||||||
| Fronto-polar | M | 10 | 0 | 45 | 12 | 5.25 | −8 | 55 | −3 | 4.84 |
| Superior | L | 8 | −34 | 21 | 51 | 3.65 | −34 | 24 | 44 | 3.69 |
| Cingulate gyrus | R | 23 | 11 | −39 | 23 | 2.52 | 19 | −39 | 23 | 2.4 |
| Inferior parietal | L | 7/40/39 | −30 | −50 | 31 | 6.9 | −41 | −64 | 42 | 4.64 |
The table reports common areas of activation for both young and old groups associated with true retrieval and false retrieval (conjunction analysis at p = .05 with a minimum cluster size ≥10 in each age group).
PFC = prefrontal cortex; PHG = parahippocampal gyrus; BA = Brodmann’s area; T = statistical t value; H = hemisphere; Talairach and Tournoux (T&T) coordinates are reported.
In order to identify regions that showed significant group differences, we used a multiple contrast approach. We first identified those regions that showed significant between-group effects at p < .05 with a minimum of 10 contiguous voxels by directly contrasting the statistical maps of older and young adults (The voxelwise probability for false positive activation within these regions is p = .033; Forman et al., 1995). We then also required that those regions showed a significant within-group effect at p < .005 with minimum cluster size of 10 voxels. This was done by inclusively masking the between-group contrast (e.g., TRA for young greater than old) with the main effects of the group contrast (e.g., TRA in young). (The voxelwise probability for false positive activation within these regions is p = .00013; Forman et al., 1995.) Results of age effects are reported in Table 4.
Table 4.
Age Differences Associated with True and False Retrieval
| H | BA | x | y | z | t | |
|---|---|---|---|---|---|---|
| TRA—Group Differences | ||||||
| Y > O | ||||||
| MTL | ||||||
| Hippocampus/PHG | L | −26 | −27 | −18 | 2.36 | |
| Posterior PHG | L | 19/30 | −30 | −44 | −1 | 3.73 |
| Frontal | ||||||
| Medial PFC | R | 10 | 19 | 59 | 1 | 3.37 |
| Ventrolateral PFC | R | 45/47 | 26 | 33 | 2 | 2.78 |
| Ventromedial PFC | M | 25 | −4 | 29 | −17 | 3.75 |
| Rostral PFC | M | 25 | 4 | 14 | −13 | 2.62 |
| Insula | R | 45 | 0 | 3 | 4.05 | |
| Superior temporal gyrus | R | 20 | 41 | −1 | −16 | 2.47 |
| Posterior cingulate | L | 31 | −11 | −20 | 40 | 3.29 |
| Supramarginal gyrus | L | 40 | −45 | −32 | 30 | 3.12 |
| Postcentral gyrus | R | 7 | 26 | −41 | 62 | 2.84 |
| L | 7 | −15 | −44 | 72 | 3.74 | |
| Precuneus | R | 7 | 19 | −50 | 31 | 3.13 |
| O > Y | ||||||
| Retrosplenial cortex | M | 23/31 | −4 | −47 | 16 | 2.65 |
| Superior medial PFC | M | 8/9 | −4 | 35 | 37 | 4.11 |
| Thalamus | M | 0 | 7 | 0 | 3.12 | |
| Postcentral sulcus | M | 4 | 0 | −37 | 65 | 2.51 |
| Occipito-temporal cortex | M | 18 | 0 | −62 | 10 | 2.93 |
| M | 18 | 0 | −70 | 4 | 3.62 | |
| Cuneus | L | 19/7 | −23 | −83 | 29 | 3.24 |
| FRA—Group Differences | ||||||
| Y > O | ||||||
| Frontal | ||||||
| Superior medial PFC | L | 9 | −11 | 35 | 33 | 4.32 |
| Orbito-frontal cortex | L | 47/10 | −30 | 51 | −9 | 3.09 |
| Dorsolateral PFC | L | 9 | −34 | 24 | 37 | 3.63 |
| L | 44/45 | −45 | 19 | 17 | 2.87 | |
| R | 9/6 | 30 | 6 | 38 | 3.02 | |
| Precuneus | L | 19/7 | −23 | −61 | 31 | 3.67 |
| O > Y | ||||||
| Middle temporal gyrus | L | 37 | −38 | −26 | −8 | 3.06 |
| Fronto-polar cortex | M | 25 | −8 | 11 | −10 | 2.61 |
| Caudate | R | 19 | 22 | 6 | 2.83 | |
| Amygdala | L | 34 | −15 | 0 | −10 | 2.98 |
| Insula | L | −38 | −18 | 4 | 2.56 |
The table reports age differences in regions significant at p < .05, uncorrected, with a minimum cluster size ≥10; inclusively masked with the primary analysis of interest (e.g., young true retrieval, old true retrieval, young false retrieval, or old false retrieval) at p > .005 with a minimum cluster size ≥10.
PFC = prefrontal cortex; PHG = parahippocampal gyrus; BA = Brodmann’s area; t = statistical t value; H = hemisphere; Talairach and Tournoux (T&T) coordinates are reported.
Individual Trial Analysis
To examine the compensatory relationship between different recollection network regions in older adults, we conducted a second analysis based on individual trial activity. As a first step, we created a general linear model in which each individual trial was modeled by a separate covariate, yielding different parameter estimates for each individual trial, for each individual subject (see Daselaar, Fleck, & Cabeza, 2006; Rissman, Gazzaley, & D’Esposito, 2004). As a second step, mean cluster activity for high-confidence TRA was extracted for all voxels within the MTL ROIs identified in the age group comparisons described above (specifically, a hippocampal and a retrosplenial cluster; see fMRI results). Correlations were calculated both across participants and within participants (across trials).
RESULTS
Behavioral Results
Table 2 (column a) breaks down the hit rate by confidence (e.g., dividing all low-confidence hits by all old items and doing the same for high-confidence hits). Adding the two confidence-based hit rates provides the total hit rate for each age group (i.e., 0.74 for young and 0.72 for older adults). The same analysis was done for related lures and unrelated lures. Unpaired t tests on each measure revealed no significant age-related differences in recognition performance or response bias (d′). However, in order to better understand age differences in classifying an item as “old,” we conducted the analyses shown in Table 2 (column b). Here we broke down all the trials to which participants responded “old” by trial type (targets, related lures, unrelated lures) and confidence (high, low) (e.g., proportion of high confidence targets was calculated by dividing all high confidence “old” response to a target by the total number of “old” responses). From this latter analysis, we found that older adults made more high-confidence “old” responses to related lures than did younger adults (0.13 and 0.09, respectively). The significance of this age difference in false memories was confirmed by an unpaired t test [t(23) = 2.15, p < .05]. No other age difference was found to be significant. These results are consistent with previous studies investigating age differences using the DRM paradigm, indicating that older adults exhibit an increased propensity for making false memories. Finally, confirming that the time allotted for responding was sufficient, both age groups responded well with the 3-sec response interval [mean reaction time (RT): 1.46 sec for young and 1.71 sec for older adults; Table 2 (column c) breaks down RT by response type].
Table 2.
Behavioral Results
| (a) Based on ‘‘Old’’ Items | (b) Based on “Old” Responses | (c) RT | ||||
|---|---|---|---|---|---|---|
| Y | O | Y | O | Y | O | |
| LC | ||||||
| Targets | 0.26 (0.10) | 0.24 (0.09) | 0.23 (0.07) | 0.22 (0.11) | 1.48 (0.14) | 1.80 (0.18) |
| Related lures | 0.32 (0.14) | 0.27 (0.12) | 0.15 (0.05) | 0.12 (0.06) | 1.53 (0.17) | 1.81 (0.18) |
| Unrelated lures | 0.15 (0.11) | 0.13 (0.08) | 0.07 (0.04) | 0.06 (0.03) | 1.54 (0.23) | 1.88 (0.25) |
| HC | ||||||
| Targets | 0.48 (0.16) | 0.48 (0.12) | 0.44 (0.01) | 0.45 (0.11) | 1.21 (0.14) | 1.43 (0.19) |
| Related lures | 0.18 (0.14) | 0.28 (0.16) | 0.09 (0.04) | 0.13 (0.05) | 1.29 (0.16) | 1.56 (0.23) |
| Unrelated lures | 0.04 (0.05) | 0.04 (0.04) | 0.02 (0.02) | 0.02 (0.01) | 1.23 (0.70) | 1.55 (0.49) |
LC = low confidence; HC = high confidence; RT = reaction time; Y = young; O = old; bolded scores represent a significant age difference (p < .05); mean and standard deviations are reported.
fMRI Results
Regions showing TRA and FRA in both age groups are listed in Table 3. Common TRA regions included the anterior and posterior cingulate cortex, bilateral hippocampus and parahippocampal areas, and several regions within the prefrontal cortex (PFC). Common FRA regions included the inferior parietal cortex, the fronto-polar cortex, the superior frontal cortex, and cingulate regions.
Age Differences in TRA
Regions showing age-related differences in TRA are listed in the top panel of Table 4. Consistent with our predictions, older adults showed weaker TRA than young adults in the hippocampus. This effect was specific to TRA as indicated by a significant three-way interaction (p < .01) in a 2 (young vs. old) × 2 (high vs. low confidence) × 2 (true vs. false) ANOVA performed on data from the hippocampal cluster. At the same time, older adults exhibited stronger TRA than young adults in the retrosplenial cortex. The age-related increase in the retrosplenial cortex was significant for true but not for false memory. The retrosplenial cortex is a region strongly associated with recollection, hence, it may help older adults compensate for hippocampal deficits.
To test this compensation account, we investigated functional connectivity between the hippocampus and the retrosplenial cortex. We calculated correlations between activity in these regions across participants and across trials in older adults (see Methods). Both analyses yielded a significant negative correlation between the two regions [across participants: r = −.57, p < .05; across trials: r = −.47, p < .001]. Thus, supporting the compensation account, those older adults who showed the weakest hippocampal activation also showed the strongest retrosplenial activation, and the activity in these two regions fluctuated in opposite directions across trials. Confirming age differences in the recruitment of these regions, the correlation in young adults failed to reach significance.
Age-related TRA reductions were also found in other brain regions including the posterior parahippocampal gyrus, the posterior cingulate, and the right PFC, whereas age-related increases in TRA were also observed in the medial PFC, thalamus, and occipito-temporal cortex. These results are consistent with the idea that older adults exhibit deficits in recollection processes.
Age Differences in FRA
Regions showing age-related differences in FRA are listed in the bottom panel of Table 4. Confirming our second prediction, older compared to younger adults exhibited greater FRA activity in the left middle temporal gyrus. Given that this region is associated with semantic processing, this activation is consistent with the idea that increased false memories in older adults reflect greater reliance on semantic gist. This effect was specific to FRA as indicated by a significant three-way interaction (p < .01) in a 2 (young vs. old) × 2 (high vs. low confidence) × 2 (true vs. false) ANOVA performed on data from the left temporal cortex.
Older compared to young adults also exhibited increased FRA activity in the fronto-polar cortex, caudate, amygdala, and insula regions. Fronto-polar activity has been associated with several functions, including the directing of attention between sensory input internal thoughts (e.g., Gilbert, Frith, & Burgess, 2005; Christoff & Gabrieli, 2000). On the other hand, young compared to older adults exhibited increased FRA activity in the precuneus and several regions of the PFC. This activity may reflect greater processing of familiarity in younger adults, leading to false memories.
DISCUSSION
The current study investigated the effects of aging on brain activity associated with true and false retrieval using a categorized word-list task. Behavioral results showed that older adults exhibited poorer memory performance than young adults, specifically reflected in their increased propensity to make high-confidence false alarms to critical lures. Regarding TRA, the results showed an age-related decrease in hippocampal activity coupled with an age-related increase in retrosplenial activity. Given that both regions have been associated with recollection processes, these findings suggest that older adults compensated for hippocampal deficits by additional recruitment of the retrosplenial cortex. Regarding FRA, the results yielded an age-related increase in activity in the left middle temporal gyrus, a region that has previously been associated with semantic processing. Taken together, our results suggest that older adults’ memory impairment reflects the combination of a deficit in retrieving item-specific details for studied items, coupled with greater reliance on semantic gist leading to false memories.
Effects of Aging on True Retrieval Activity
Compared to young adults, older adults exhibited a reduction in TRA in a number of brain regions including the hippocampus (see Figure 2A). Evidence from lesion studies and functional neuroimaging studies has strongly linked the hippocampus to memory for item-specific details and recollection (for reviews, see Eichenbaum et al., 2007; Yonelinas, 2002; Brown & Aggleton, 2001). Behavioral studies have clearly demonstrated that older adults are particularly impaired in context memory and recollection (for reviews, see Dennis & Cabeza, 2008; Cabeza, 2006; Zacks et al., 2000). These data fit with evidence that the hippocampus shows substantial structural decline not only in Alzheimer’s disease (AD) but also in healthy aging (for a review, see Dennis & Cabeza, 2008). Finally, linking the effects of aging on behavior and brain structure, recent functional neuroimaging studies have associated recollection deficits in older adults with reduced hippocampal activity (Daselaar, Fleck, Dobbins, et al., 2006; Cabeza et al., 2004). The current results support previous findings suggesting that hippocampal dysfunction is a key component of age-related memory impairments.
Figure 2.
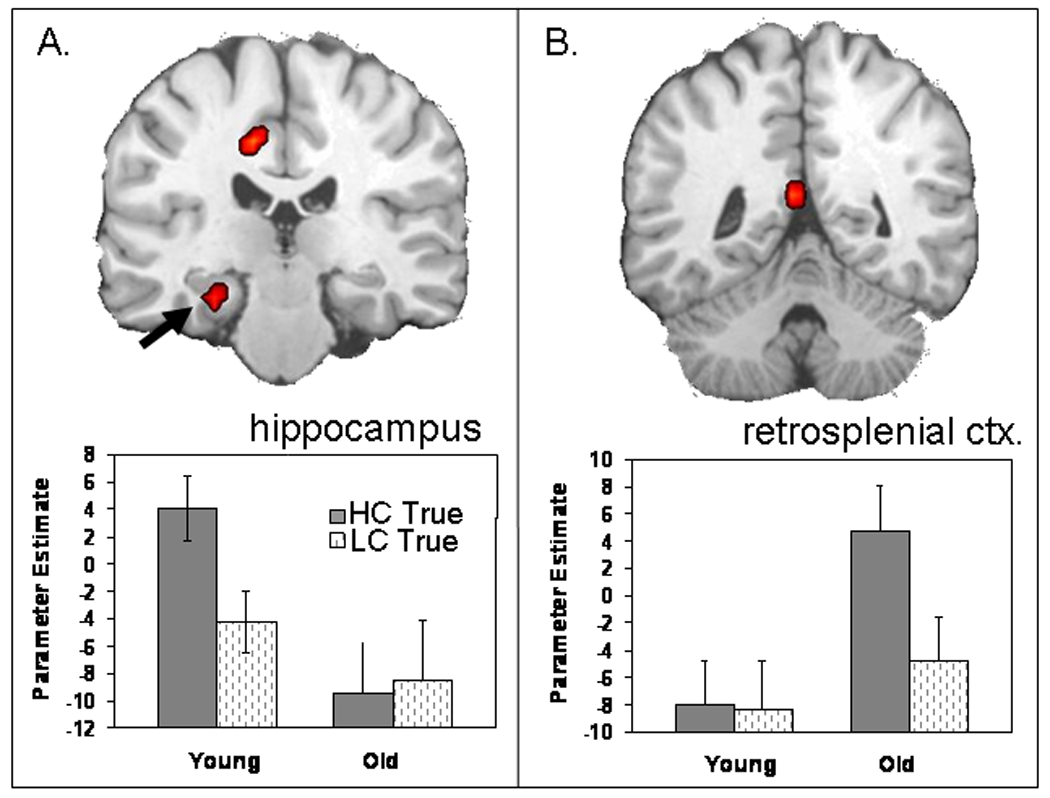
Age-related differences in TRA. The hippocampus (A) shows an age-related decrease in true retrieval, whereas the retrosplenial cortex (B) shows an age-related increase in true activity. Bar graphs represent functional activation (and standard error) associated with high- and low-confidence true memory for both age groups (see Table 4 for coordinates).
Despite their weaker hippocampal activity, older adults exhibited greater activity than young adults in the posterior midline, and particularly, in the retrosplenial cortex (see Figure 2B). The retrosplenial cortex is a region frequently activated during episodic retrieval (for reviews, see Squire, Stark, & Clark, 2004; Cabeza & Nyberg, 2000), and recent fMRI studies have associated its role specifically with recollection (Daselaar, Fleck, & Cabeza, 2006; Yonelinas, Otten, Shaw, & Rugg, 2005). Anatomically, the retrosplenial cortex is directly connected to medial-temporal lobe regions, including the hippocampus (Kobayashi & Amaral, 2003). Moreover, functional connectivity analyses in young adults have shown that the hippocampus and the retrosplenial cortex operate together as a network during recollection (Daselaar, Fleck, & Cabeza, 2006). Thus, although older adults failed to recruit the hippocampus as much as young adults during true memory retrieval, they recruited another important component of the recollection network, namely, the retrosplenial cortex, to a greater extent than young adults. This finding suggests that older adults may compensate for hippocampal deficits by relying more on the retrosplenial cortex.
To test this compensation account, we investigated functional connectivity between the hippocampus and the retrosplenial cortex in older adults. We calculated correlations in TRA between these regions across participants (collapsed across trials) and across trials (within-participants). Both analyses yielded significant negative correlations between high-confidence TRA in the hippocampus and the retrosplenial cortex in older adults. In other words, those elderly who showed the weakest hippocampal activations also showed the strongest retrosplenial activations (see Figure 3A), and activity in these two regions fluctuated in opposite directions across trials (see Figure 3B). Moreover, as this compensatory activity was associated with high-confidence true retrieval, results speak directly to recollection-related processing. These results strongly support the compensation account of the age-related increase in retrosplenial activity.
Figure 3.
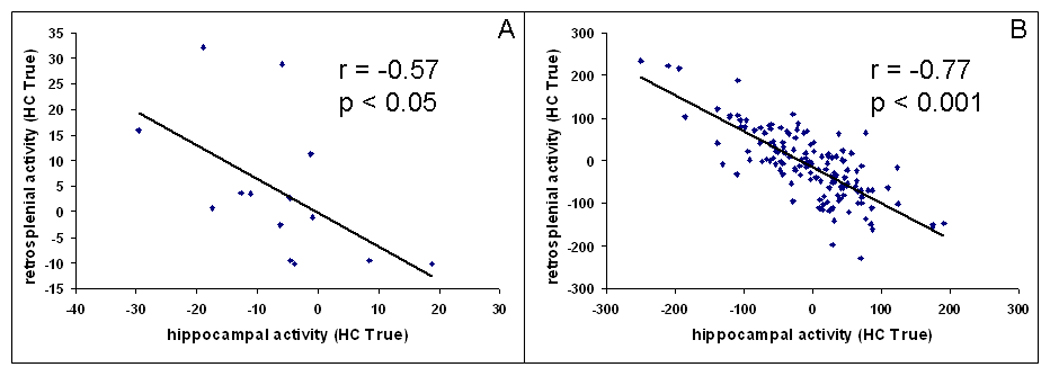
Negative correlation between activity associated with high-confidence true memory in the hippocampus and in the retrosplenial cortex in older adults (A) across participants and (B) across trials (in one randomly chosen participant).
The correlation between the hippocampus and the retrosplenial cortex was not significant in younger adults. The lack of a negative correlation in younger adults suggests that compensatory recruitment of the retrosplenial cortex occurs only when hippocampal functions are impaired. Alternatively, it may reflect limited variability in hippocampal activity within the young group.
An open question is why aging impaired hippocampal function but not retrosplenial function. As noted above, these two regions are closely related components of the recollection network, and they are linked by direct anatomical connections. Thus, it is interesting that aging had opposite effects on these two regions, dissociating their roles in episodic retrieval. It is notable that in two separate studies examining retrieval processes in healthy aging, we found compensatory activity in extended MTL regions, outside of the hippocampus (current: retrosplenial cortex and Daselaar, Fleck, & Cabeza, 2006; Daselaar, Fleck, Dobbins, et al., 2006: rhinal cortex). Although these cortical MTL regions are the first affected in AD (Braak & Braak, 1996; Braak, Braak, & Bohl, 1993), they appear to be those utilized in healthy older adults to offset declining hippocampal function. Contrary to atrophy seen in AD, healthy aging is marked by greater atrophy within the hippocampus proper compared to more cortical regions (e.g., entorhinal or retrosplenial cortex) (e.g., Raz et al., 2005). Thus, it may be the case that as volume loss influences functional loss in the hippocampus in healthy older adults, less affected neighboring cortical regions increase their functionality in a compensatory fashion. Further research regarding differences between the memory functions of the hippocampus and cortical MTL regions and their sensitivity to aging is warranted.
Finally, age-related reductions in hippocampal activity for TRA are consistent with results from the encoding data of this same study (Dennis et al., 2007). During encoding, older adults also exhibited reduced activity in the hippocampus associated with subsequent retrieval success (i.e., encoding items that were subsequently remembered compared to those forgotten). Taken together, results from the two studies indicate that age-related deficits in hippocampal function affect episodic memory processes both during encoding and during retrieval.
Effects of Aging on False Retrieval Activity
Behavioral results replicated previous false memory studies in which older adults showed significantly greater false memory for related lures than did young adults (Tun et al., 1998; Koutstaal & Schacter, 1997).
Confirming our predictions, older compared to young adults showed greater FRA in the left middle temporal gyrus (see Figure 4), a region previously associated with semantic processing (Wise & Price, 2006; Tyler et al., 2003; Kable, Lease-Spellmeyer, & Chatterjee, 2002; Martin, 2001). Activity in this region may be engaged in the recovery of semantic information associated with the critical lure—such as category membership or associated category exemplars. As previously noted, the role of this region in mediating semantic gist is supported by evidence from patients with semantic dementia who show both damage to this region and impairments in extracting and/or utilizing semantic gist (Simons, Verfaellie, et al., 2005). Age-related increases in left lateral temporal activity for FRA are consistent with results from the encoding data of the same study (Dennis et al., 2007). During encoding, older adults also showed age-related increases in this region associated with the production of subsequent false alarms to related lures. Results support theoretical accounts of age-related increases in false memories stemming from increased gist or semantic processing.
Figure 4.
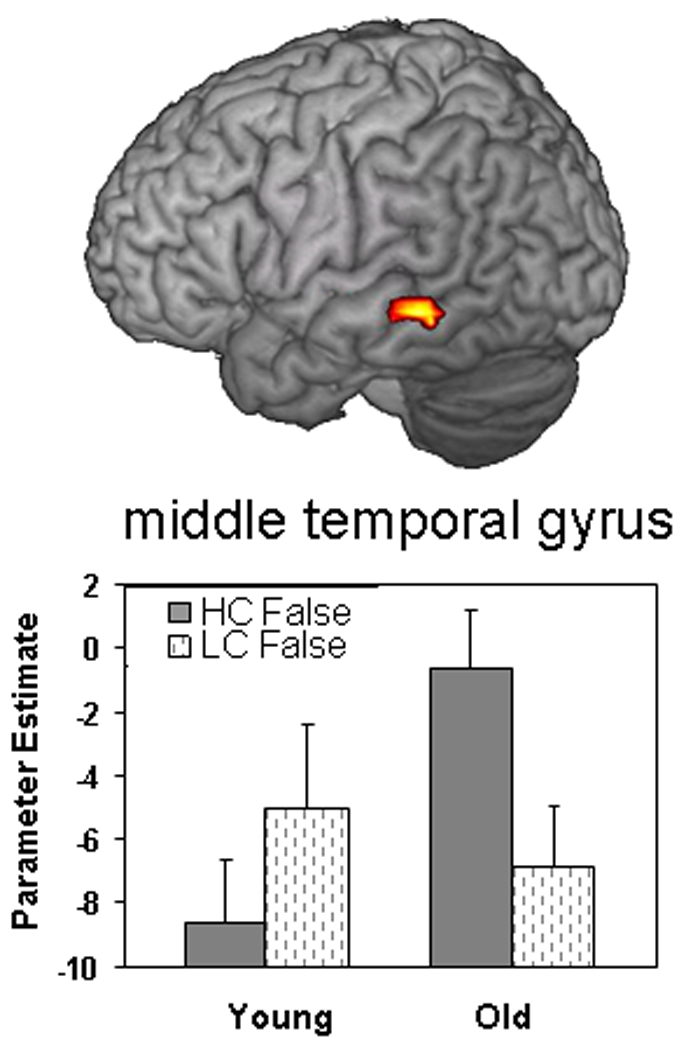
Age-related increase in false retrieval in the left middle temporal gyrus. Bar graphs represent functional activation (and standard error) associated with high- and low-confidence false memories for both age groups (see Table 4 for coordinates).
Additionally of interest, an age-related increase in FRA was also found in the fronto-polar cortex. Functional neuroimaging studies have associated fronto-polar activity with several processes, including the directing of one’s attention between current sensory input and internally generated thought processes (Gilbert, Spengler, Simons, Frith, & Burgess, 2006; Gilbert et al., 2005; Simons, Owen, Fletcher, & Burgess, 2005; Christoff, Ream, Geddes, & Gabrieli, 2003; Christoff & Gabrieli, 2000). In accord with this interpretation of fronto-polar function, older adults in the current study may be recruiting this area along with the middle temporal gyrus by internally generating associations between the critical lure and encoding items—with these associations leading to a false recognition response. More work is necessary in order to confirm this interpretation.
Conclusion
Consistent with our predictions, older compared to young adults exhibited a decrease in hippocampal activity during true retrieval but an increase in left temporal activity during false retrieval. The hippocampal reduction is consistent with previous evidence that older adults are impaired in the retrieval of item-specific details and that this form of memory depends on the hippocampus. Despite deficits in hippocampal activity, we found an age-related increase in retrosplenial activity during true retrieval. The significant negative correlation found in older adults between the two regions supports the idea that recruitment of the retrosplenial cortex may partly compensate for the deficit in hippocampal function. During false retrieval, older adults recruited the left middle temporal gyrus to a greater extent than young adults. This finding is consistent with the role of this region in semantic processing, and the idea that older adults’ false memories reflect their greater reliance on semantic gist.
Taken together, the age-related reduction in hippocampal activity and the age-related increase in left temporal activity fit very well with the fuzzy trace theory of false memory, as it pertains to aging. As noted in the Introduction, the fuzzy trace theory posits that older adults experience deficits in their memory for item-specific details as well as an increased reliance on gist memory. Within the framework of the fuzzy trace theory, our results suggest that age-related decreases in recollection, associated with true memories, and age-related increases in semantic gist, associated with false memories, each contribute to age deficits in memory retrieval.
Acknowledgments
We thank Amber Baptiste Tarter for help in preparation of this manuscript and Steve Prince, Sander Daselaar, and Florin Dolcos for helpful comments. This work was supported by NIH grant AG19731 awarded to R. C. and NIA grant T32 AG000029 awarded to N. A. D.
Footnotes
There is extensive evidence for separate, independent memory processes that support recollection (verbatim memory) versus gist memory. That evidence includes independent parameters in mathematical models required by the data for goodness of fit (e.g., the conjoint recognition model; Brainerd et al., 1999); selective responsiveness of these parameters to theoretically predicted factors; and a host of single and double dissociations in behavioral data. Therefore, memory for item-specific details associated with true memories is theoretically independent of memory for semantic gist associated with false memories.
REFERENCES
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal–anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–444. discussion 444–489. [PubMed] [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, McDermott KB, et al. Veridical and false memories in healthy older adults and in dementia of the Alzheimer’s type. Cognitive Neuropsychology. 1999;16:361–384. [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: A study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Battig WF, Montague WE. Category norms for verbal items in 56 categories: A replication and extension of the Connecticut norms. Journal of Experimental Psychology. 1969;80:1–46. [Google Scholar]
- Bayen UJ, Phelps MP, Spaniol J. Age-related differences in the use of contextual information in recognition memory: A global matching approach. Journal of Gerontology: Series B, Psychological Sciences and Social Sciences. 2000;55:P131–P141. doi: 10.1093/geronb/55.3.p131. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurologica Scandinavica Supplement. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. European Neurology. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF. Gist is the gist: The fuzzy-trace theory and new intuitionism. Developmental Review. 1990;10:3–47. [Google Scholar]
- Brainerd CJ, Reyna VF. Explaining developmental reversals in false memory. Psychological Science. 2007;18:442–448. doi: 10.1111/j.1467-9280.2007.01919.x. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Mojardin AH. Conjoint recognition. Psychological Review. 1999;106:160–179. doi: 10.1037/0033-295x.106.1.160. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Wright R, Reyna VF, Mojardin AH. Conjoint recognition and phantom recollection. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:307–327. doi: 10.1037/0278-7393.27.2.307. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Budson AE, Todman RW, Chong H, Adams EH, Kensinger EA, Krangel TS, et al. False recognition of emotional word lists in aging and Alzheimer disease. Cognitive and Behavioral Neurology. 2006;19:71–78. doi: 10.1097/01.wnn.0000213905.49525.d0. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Prefrontal and medial temporal lobe contributions to relational memory in young and older adults. In: Zimmer D, Mecklinger A, Lindenberger U, editors. Binding in human memory: A neurocognitive approach. New York: Oxford University Press; 2006. [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition: II. An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proceedings of the National Academy of Sciences, U.S.A. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behavioral Neuroscience. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza RE. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cerebral Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cognitive, Affective & Behavioral Neuroscience. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Salthouse TA, Craik FEM, editors. Handbook of aging and cognition. 3rd ed. 2008. pp. 1–54. [Google Scholar]
- Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiology of Aging. 2006;28:1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Kim HK, Cabeza R. Effects of aging on the neural correlates of true and false memory formation. Neuropsychologia. 2007;45:3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. Medial temporal lobes and recognition in memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. European Journal of Neuroscience. 2005;21:1423–1431. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Frith CD, Burgess PW. Differential functions of lateral and medial rostral prefrontal cortex (area 10) revealed by brain–behavior associations. Cerebral Cortex. 2006;16:1783–1789. doi: 10.1093/cercor/bhj113. [DOI] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: Multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Lehnertz K, Heinze HJ, Helmstaedter C, Elger CE. Verbal novelty detection within the human hippocampus proper. Proceedings of the National Academy of Sciences, U.S.A. 1998;95:3193–3197. doi: 10.1073/pnas.95.6.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, et al. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Kable JW, Lease-Spellmeyer J, Chatterjee A. Neural substrates of action event knowledge. Journal of Cognitive Neuroscience. 2002;14:795–805. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter D. When true memories suppress false memories: Effects of aging. Cognitive Neuropsychology. 1999;16:399–415. [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. Journal of Comparative Neurology. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Schacter DL. Gist-based false recognition of pictures in older and younger adults. Journal of Memory and Language. 1997;37:555–583. [Google Scholar]
- LaVoie DJ, Faulkner K. Age differences in false recognition using a forced choice paradigm. Experimental Aging Research. 2000;26:367–381. doi: 10.1080/036107300750015750. [DOI] [PubMed] [Google Scholar]
- Light LL. The organization of memory in old age. In: Salthouse TA, Craik FIM, editors. The handbook of aging and cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 111–165. [Google Scholar]
- Light LL, Burke DM. Patterns of language and memory in old age. In: Light LL, Burke DM, editors. Language, memory, and aging. New York: Cambridge University Press; 1988. pp. 244–271. [Google Scholar]
- Madden DJ, Langley LK, Denny LL, Turkington TG, Provenzale JM, Hawk TC, et al. Adult age differences in visual word identification: Functional neuroanatomy by positron emission tomography. Brain and Cognition. 2002;49:297–321. doi: 10.1006/brcg.2001.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. Functional neuroimaging of semantic memory. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging. Cambridge, MA: The MIT Press; 2001. [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: Exploring the characteristics of illusory memories. Memory & Cognition. 1997;25:838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Park DC, Puglisi JT. Older adults’ memory for the color of pictures and words. Journal of Gerontology. 1985;40:198–204. doi: 10.1093/geronj/40.2.198. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychology and Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Prull MW, Gabrieli JDE, Bunge SA. Age-related changes in memory: A cognitive neuroscience perspective. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: A study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:8033–8814. [Google Scholar]
- Salthouse TA. Adult cognition: An experimental psychology of human aging. New York: Springer-Verlag; 1982. [Google Scholar]
- Schacter D, Verfaellie M, Pradere D. The neuropsychology of memory illusions: False recall and recognition in amnesiac patients. Journal of Memory and Language. 1996;35:319–334. [Google Scholar]
- Simons JS, Owen AM, Fletcher PC, Burgess PW. Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia. 2005;43:1774–1783. doi: 10.1016/j.neuropsychologia.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Simons JS, Verfaellie M, Hodges JR, Lee AC, Graham KS, Koutstaal W, et al. Failing to get the gist: Reduced false recognition of semantic associates in semantic dementia. Neuropsychology. 2005;19:353–361. doi: 10.1037/0894-4105.19.3.353. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: A meta-analysis. Psychology and Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Kan IP, Oliver RT. Functional neuroimaging of semantic memory. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press; 2006. pp. 149–190. [Google Scholar]
- Tun PA, Wingfield A, Rosen MJ, Blanchard L. Response latencies for false memories: Gist-based processes in normal aging. Psychology and Aging. 1998;13:230–241. doi: 10.1037//0882-7974.13.2.230. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Dick E, Bright P, Fletcher P, Moss H. Objects and their actions: Evidence for a neurally distributed semantic system. Neuroimage. 2003;18:542–557. doi: 10.1016/s1053-8119(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Watson JM, McDermott KB, Balota DA. Attempting to avoid false memories in the Deese/Roediger–McDermott paradigm: Assessing the combined influence of practice and warnings in young and old adults. Memory & Cognition. 2004;32:135–141. doi: 10.3758/bf03195826. [DOI] [PubMed] [Google Scholar]
- Whiting WL, Madden DJ, Langley LK, Denny LL, Turkington TG, Provenzale JM, et al. Lexical and sublexical components of age-related changes in neural activation during visual word identification. Journal of Cognitive Neuroscience. 2003;15:475–487. doi: 10.1162/089892903321593171. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Price CJ. Functional imaging of language. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. 2nd ed. Cambridge, MA: MIT Press; 2006. pp. 191–228. [Google Scholar]
- Yonelinas AP. Components of episodic memory: The contribution of recollection and familiarity. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Feinberg F, Hu P, Gutchess AH, Hedden T, Chen HY, et al. Category norms as a function of culture and age: Comparisons of item responses to 105 categories by American and Chinese adults. Psychology of Aging. 2004;19:379–393. doi: 10.1037/0882-7974.19.3.379. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah, NJ: Erlbaum; 2000. pp. 293–358. [Google Scholar]


