Abstract
Objective
To increase participation in cervical cancer screening of under-served women living in the Mississippi Delta, a U.S. population at high risk for cervical cancer
Methods
We conducted a door-to-door feasibility study of women living in the Mississippi Delta to increase participation in cervical cancer screening in 2009-10. Women (n=119) aged 26-65 years who had not been screened in last 3 years or more, were not pregnant, and had a cervix were offered a choice: clinic-based Pap testing or home self-collection with HPV DNA testing.
Results
Seventy-seven women (64.7%) chose self-collection with HPV testing, of which 62 (80.5%) returned their self-collected specimen. By comparison, 42 women (35.3%) chose Pap testing, of which 17 (40.5%) attended their clinic appointment. Thus there was an almost 4-fold greater participation of under-screened women in self-collection with HPV testing than in free Pap testing (78.4% vs. 21.5%).
Conclusions
We found that offering self-collection will increase participation in cervical cancer screening among under-screened populations living in the Mississippi Delta. Based on these preliminary results, we suggest that self-collection with HPV DNA testing might complement current Pap testing programs to reach under-screened populations of women, such as those living in the Mississippi Delta.
Keywords: Pap, cervical intraepithelial neoplasia (CIN), cervical cancer, human papillomavirus (HPV), atypical squamous cells of undetermined significance (ASC-US), Hybrid Capture 2 (HC2), health disparities, cervical cancer screening
Introduction
More than half of all cervical cancer occurring in the U.S. is found in medically underserved populations (http://www.cdc.gov/cancer/cervical/). Cervical cancer occurs mainly in these populations as part of a complex of diseases linked to poverty and/or racial/ethnic disparities (Freeman and Wingrove 2007). Although targeting the necessary cause of cervical cancer, human papillomavirus (HPV), by vaccination shows tremendous promise for prevention (2007;Garland et al. 2007;Paavonen et al. 2009), HPV vaccination does not prevent 30% of the cervical cancer caused by the untargeted HPV types nor does it treat the pre-existing HPV infections and related precursor conditions in the population (2007;Hildesheim et al. 2007). Given that only ¼ of U.S. adolescent women have received all three doses of their immunization against HPV vaccination (2010), who will glean the greatest benefit from HPV vaccination, and current HPV vaccines provide only 70% protection against cervical cancer, it seems likely that cervical cancer screening will be needed for decades to come, especially in under-served populations.
African-American women are more likely to get cervical cancer and twice as likely to die from cervical cancer than their Caucasian counterparts (Freeman and Wingrove 2007). The disparities in cervical cancer incidence and mortality (Figure 1) are still greater in the Mississippi Delta region (Freeman and Wingrove 2007) than in other African-American populations. In our previous work in the Mississippi Delta region (Scarinci et al. 2010), we found that door-to-door recruitment was more effective means to engage and recruit under-screened women in participating in cervical cancer screening. In that study, we found that under-screened women were willing to come to the clinic to participate, take a kit home for self-collection of a cervicovaginal specimen (for HPV testing), and return it to the clinic (unpublished data). However, that study was clinic based and women were offered a financial incentive to participate.
Figure 1. County-Specific Age-Standardized Cervical Cancer Rates in MS and AL.
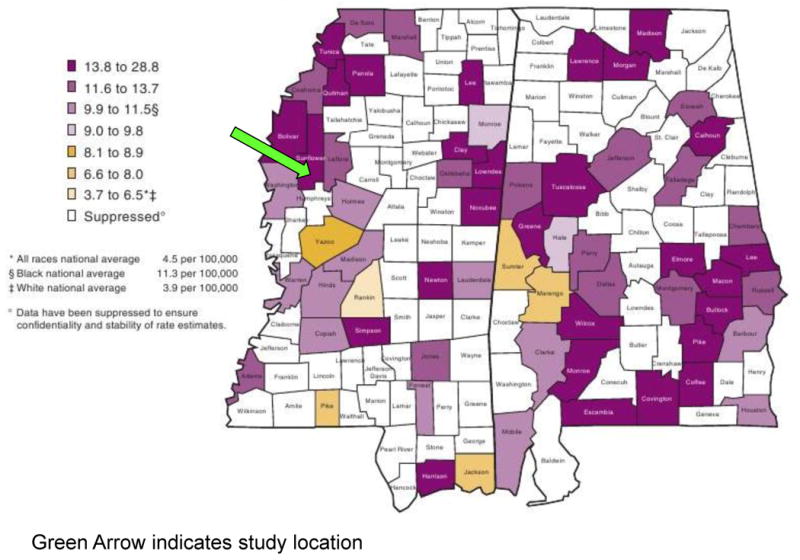
County-level mortality (per 100,000) due to cervical cancer in Mississippi (USA)(Freeman and Wingrove 2007) (courtesy of the National Cancer Institute, NIH). The green arrow indicates where the study was conducted in 2009-10.
In a follow-up feasibility study, we conducted a door-to-door recruitment without incentive to determine what type of intervention under-screened women living in the Mississippi Delta might choose and, given that choice, complete: clinic-based, physician-collected cervical specimens for Pap testing versus home-based, self-collected cervicovaginal specimen for HPV testing.
Methods
We conducted a door-to-door recruitment in 4 towns (Sunflower, Moorhead, Inverness, and Doddsville) in Sunflower County, a high-risk population living in rural northwest Mississippi (Figure 1), to identify women aged 26-65 years who might be eligible for the study in 2009-10 (Figure 2). Among the 543 women identified in this age group, 95.0% (n = 516) were available to participate. We then excluded 394 (76.4%) women for the following a priori reasons (not mutually exclusive): 345 (66.9%) had a Pap within the last 3 years; 91 (17.6%) had previous hysterectomy; 6 (1.1%) had a self-reported history of cervical cancer; and 4 (0.8%) were pregnant or 6 (1.1%) had given birth in the last 8 weeks.
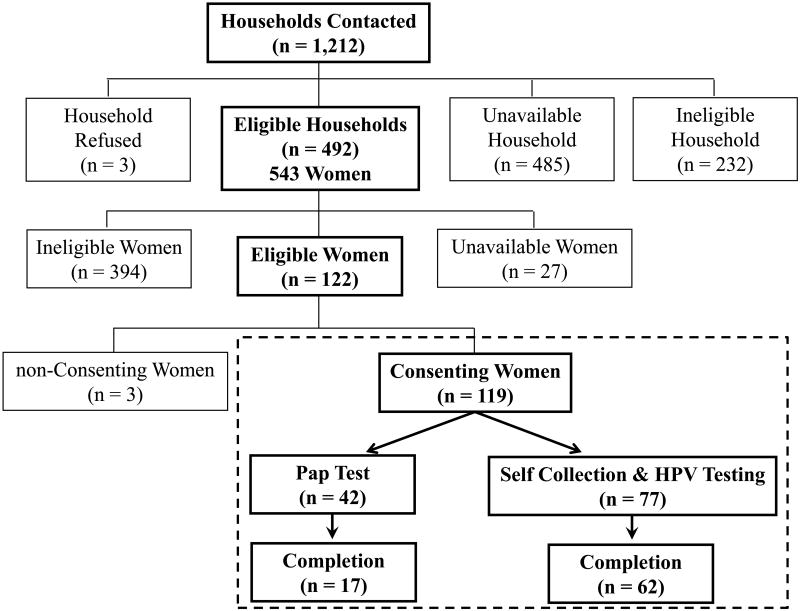
Figure 2. Consort Diagram for Participation.
The flowchart shows the outcomes of the study conducted in 2009-10 to increase cervical cancer screening participation in Sunflower County, Mississippi. Definitions are as follows: ineligible households had no women in the correct age range; unavailable households were those that we did not get a response; ineligible women = did not meet inclusion criteria (see text); unavailable women = women in eligible households who we could not assess for eligibility in the study (e.g., working, too busy, etc.).
In their homes, 122 of 543 (22.5%) eligible women were asked to provide written, informed consent, and 3 (2.5%) refused to participate. The study was approved by the institutional review boards of NCI, University of Alabama at Birmingham, Mississippi State Department of Health, and Westat, Inc. (Rockville, MD).
After consenting, 119 women (117 African-Americans and 2 Caucasians) were asked to complete a questionnaire and choose their preferred method of free screening; at this stage of the study, no woman refused to chose a method of screening, which would made them ineligible to participate. To explain the screening options, study personnel followed a script along with an educational flipchart on cervical cancer, risk factors, and Pap smear as the current recommendation for cervical cancer screening. Following this presentation/education, study personnel acknowledged that some women may not come to the clinic for their Pap smear, and, therefore, there was a second option to self-collect for HPV testing at home. Then, study personnel proceeded with an explanation of how to pursue either option. Women who selected Pap testing were given a voucher for free Pap testing and asked to make (or to get help from the study staff to make) an appointment at the local public health clinic.
Women who chose self-collection and HPV testing were given a kit that included a collection device (Castle et al. 2006), a vial of mouthwash as a safe transport medium for the cervicovaginal specimen (Castle et al. 2007), and written instructions on how to self-collect. The women were also given verbal instructions by the study staff on how to self-collect. Women could self-collect immediately and return the specimen to the study personnel or return the specimen by via mail. Self-collected specimens were tested for HPV using Hybrid Capture 2 (Qiagen, Gaithersburg, MD).
Women who did not make or complete their appointments within 30 days were re-contacted as a reminder, and offered the same choice between screening methods. Women who tested positive by their screening method of choice were referred to colposcopy for diagnostic procedures.
Results
Of the 119 participants (Figure 2), 77 (64.7%) chose self-collection with HPV testing and 42 (35.3%) women selected clinic-based Pap testing; three women who had originally chose to get Pap testing later chose self-collection while one woman chose to switch from self-collection to Pap testing. All but 2 participants (117 of 119, 98.3%) were African American. Women who chose Pap testing were significantly older than those chose self-collection with HPV testing (median ages: 49 years vs. 39 years, respectively, p = 0.005, Kruskal Wallis). Women who chose self-collection were better educated than those who chose Pap testing (p = 0.009) (Supplementary Table 1). There were no differences between the two groups in number of women who ever smoked, marital status, number of people living in household, number of children, and time since last Pap. In our small sample of 424 non-hysterectomized women contacted who were aged 26-65 years, 123 (29.0%, 95%CI = 24.7%-33.6%) (That includes 4 who were excluded from the study for other reasons) had not been screened in the last three years.
More women (62 of 77; 80.5%) completed their self-collection than women (17 of 42; 40.5%)1 completed their Pap testing (p = 0.0001, Fisher's exact). Twelve of 62 (19.4%) women who chose to self-collect collected their sample at the study visit (n.b., 4 specimen return dates were not recorded).
Discussion
In this feasibility study, we showed that offering free cervical cancer screening through direct contact could increase participation of under-screened, primarily African-American women living in the high-risk region of the Mississippi Delta. Importantly, we found that when offered, self-collection was more acceptable and effective intervention than offering free Pap testing at the local clinic.
We also noted with interest that it was women in their 30's and early 40's who chose self-collection, which is ideal since the cervical cancer incidence tends to peak in the mid- to late-40's (Wang et al. 2004) and precancer found in the late 30's has significant invasive potential (McCredie et al. 2008). Importantly, this was a high-risk population, with the women performing self-collection having a high-risk HPV prevalence (as detected by Hybrid Capture 2) of 14.5% (95%CI=6.9%-25.8%), which is approximately 3-fold greater than in low-risk populations at the same age (Castle et al. 2009).
Parenthetically, of the 9 women whose self-collected specimen tested high-risk HPV positive, 6 were notified of their results before the close of the study and 3 returned for colposcopy (the other 3 were notified after the study closed and data on whether they returned for colposcopy is not available. We did not have data on the number of positive Pap tests and the number of the Pap-positive that returned for colposcopy.). In conjunction with novel approaches to increasing screening coverage, strategies such patient navigation (Freeman 2006) should be implemented to ensure that screen-positive (higher risk) women return for the necessary clinical management.
There is now substantial evidence that self-collection with HPV testing has similar clinical sensitivity, albeit less specificity, for cervical precancerous lesions as physician-collected Pap tests (Belinson et al. 2003;Belinson et al. 1999;Wright, Jr. et al. 2000). Self-collection with HPV testing has been shown to be effective in reaching other hard-to-reach sub-populations in developed countries (Ogilvie et al. 2007;Gok et al. 2010;Sowjanya et al. 2009). Given the evidence, self-collection with HPV testing should be considered as a complementary method for cervical cancer screening to reach the underserved populations in the U.S. and thereby reduce the unequal burden of a largely preventive cancer in these populations. Large culturally-appropriate community outreach projects and trials on self-collection should now be initiated in these pockets of underserved populations to identify and overcome the barriers to adoption and implementation (Scarinci et al. 2010).
Supplementary Material
Footnotes
Acknowledgements: This research was supported by the Intramural Research Program of the NIH, NCI and by the NCI Center for Reducing Cancer Health Disparities (CRCHD). The authors acknowledge former members of CRCHD, Dr. Harold Freeman and Ms. Jane Daye for their unwavering support of this effort. The authors have no conflict of interest to report.
It was unknown whether two women who chose clinic-based cytology attended the clinic; these women were considered to have not completed their intervention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- National, state, and local area vaccination coverage among adolescents aged 13-17 years --- United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1018–1023. [PubMed] [Google Scholar]
- Belinson J, Qiao Y, Pretorius R, Zhang W, Keaton K, Elson P, Fischer C, Lorincz A, Zahniser D, Wilbur D, Pan Q, Li L, Biscotti C, Dawson A, Li A, Wu L, Ling Y, Ma CP, Yang XP. Prevalence of cervical cancer and feasibility of screening in rural China: a pilot study for the Shanxi Province Cervical Cancer Screening Study. Int J Gynecol Cancer. 1999;9:411–417. doi: 10.1046/j.1525-1438.1999.99055.x. [DOI] [PubMed] [Google Scholar]
- Belinson JL, Qiao YL, Pretorius RG, Zhang WH, Rong SD, Huang MN, Zhao FH, Wu LY, Ren SD, Huang RD, Washington MF, Pan QJ, Li L, Fife D. Shanxi Province cervical cancer screening study II: self-sampling for high-risk human papillomavirus compared to direct sampling for human papillomavirus and liquid based cervical cytology. Int J Gynecol Cancer. 2003;13:819–826. doi: 10.1111/j.1525-1438.2003.13611.x. [DOI] [PubMed] [Google Scholar]
- Castle PE, Aftab A, Saint-Jean G, Mendez L. Detection of carcinogenic human papillomavirus in specimens collected with a novel self-sampling device. J Clin Microbiol. 2006;44:2158–2159. doi: 10.1128/JCM.02358-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Five-year experience of human papillomavirus DNA and papanicolaou test cotesting. Obstet Gynecol. 2009;113:595–600. doi: 10.1097/AOG.0b013e3181996ffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle PE, Sadorra M, Garcia FA, Cullen AP, Lorincz AT, Mitchell AL, Whitby D, Chuke R, Kornegay JR. Mouthwash as a low-cost and safe specimen transport medium for human papillomavirus DNA testing of cervicovaginal specimens. Cancer Epidemiol Biomarkers Prev. 2007;16:840–843. doi: 10.1158/1055-9965.EPI-06-0909. [DOI] [PubMed] [Google Scholar]
- Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83:139–141. doi: 10.1007/s11524-006-9030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman HP, Wingrove BK. Excess cervical cancer mortality: A marker for low access to health care in poor communities. 05-5282. National Institutes of Health; 2007. [Google Scholar]
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- Gok M, Heideman DA, van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JW, Voorhorst F, Belien JA, Babovic M, Snijders PJ, Meijer CJ. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ. 2010;340:c1040. doi: 10.1136/bmj.c1040. c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, Skegg DC. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- Ogilvie G, Krajden M, Maginley J, Isaac-Renton J, Hislop G, Elwood-Martin R, Sherlock C, Taylor D, Rekart M. Feasibility of self-collection of specimens for human papillomavirus testing in hard-to-reach women. CMAJ. 2007;177:480–483. doi: 10.1503/cmaj.070013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, Greenacre M. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- Scarinci IC, Garcia FA, Kobetz E, Partridge EE, Brandt HM, Bell MC, Dignan M, Ma GX, Daye JL, Castle PE. Cervical cancer prevention: new tools and old barriers. Cancer. 2010 doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowjanya AP, Paul P, Vedantham H, Ramakrishna G, Vidyadhari D, Vijayaraghavan K, Laksmi S, Sudula M, Ronnett BM, Das M, Shah KV, Gravitt PE. Suitability of self-collected vaginal samples for cervical cancer screening in periurban villages in Andhra Pradesh, India. Cancer Epidemiol Biomarkers Prev. 2009;18:1373–1378. doi: 10.1158/1055-9965.EPI-08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer. 2004;100:1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- Wright TC, Jr, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283:81–86. doi: 10.1001/jama.283.1.81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.