Abstract
Desert locusts in the solitarious phase were repeatedly touched on various body regions to identify the site of mechanosensory input that elicits the transition to gregarious phase behavior. The phase state of individual insects was measured after a 4-h period of localized mechanical stimulation, by using a behavioral assay based on multiple logistic regression analysis. A significant switch from solitarious to gregarious behavior occurred when the outer face of a hind femur had been stimulated, but mechanical stimulation of 10 other body regions did not result in significant behavioral change. We conclude that a primary cause of the switch in behavior that seeds the formation of locust swarms is individuals regularly touching others on the hind legs within populations that have become concentrated by the environment.
In many animal species, crowding stimulates changes in physiology, behavior, and morphology. These changes can be interpreted as adaptations for high population density or migration (1). An extreme example is density-dependent phase polyphenism in locusts, where being kept in a crowd stimulates individuals to change from the shy, cryptically colored, “solitarious” phase into the conspicuously colored, swarm-forming, “gregarious” phase. Phase transition includes rapid behavioral change (occurring in a matter of hours), whereas color, shape, and reproductive physiology alter more gradually (2–5).
Changes in behavior resulting from crowding include becoming more active and being attracted rather than repelled by other locusts—responses that can initiate swarm formation under suitable environmental conditions (6). Phase change has a large impact on the small-scale distribution of locusts (6–10), their migration and large-scale distribution (11), birth and development rates (2–5), nutritional ecology (12), and predation (13). Migrating swarms of gregarious locusts provide a serious agricultural threat, and outbreaks remain difficult to predict and manage. Given the significance of phase change to the biology and economic significance of locusts, it is important to understand its underlying mechanisms.
Two key issues are the stimuli provided by other locusts that cause an individual to change from the solitarious to the gregarious behavioral state and the means by which these stimuli are detected. We have found recently that physical contact is the single most potent stimulus causing solitarious locusts to assume gregarious behavioral traits; visual and olfactory stimulation play lesser roles (14, 15). Most of a locust's integument is covered with touch-sensitive hairs and other mechanoreceptors (16, 17), and it remains to be discovered whether certain body regions are more important than others in inducing gregarization or whether being touched anywhere causes behavior to change.
The aim of the present study was to map the sites on the body surface where mechanical stimulation results in behavioral gregarization.
Materials and Methods
Insects.
Final-instar nymphs were used that had been reared in physical, visual, and olfactory isolation for three generations, i.e., they were the solitary-reared progeny of solitary-reared parents and grandparents. Rearing was performed as described in ref. 18.
Stimulation Protocol.
A total of 170 solitarious nymphs of Schistocerca gregaria (Forskål) had one body region mechanically stimulated for 5 s every 60 s over a 4-h period. Being kept in a crowd for 4 h is known to cause a solitarious locust to change into the gregarious behavioral state (5). Each insect was placed into a clear plastic cage (78 × 60 × 105 mm, width × height × length). The ends of each container were covered with wire mesh, through which a fine paintbrush (Synthetic Humbrol no. 2) was inserted and used to simulate the effect of mechanical contact with other locusts. The wire mesh allowed ready access to all body regions of the locust even when it was moving or had changed position in the chamber. The brush was stroked over a particular body region for 5 s every 60 s throughout the 4-h period, except in the control treatment, in which the paintbrush was moved near the animal but without contact. The following regions were stimulated on the left side of the body: antenna, face (eye, frons, and gena), mouth parts, pronotum and lateral thorax, wing pad, abdomen, foreleg, midleg (femur and tibia), hind femur (outer surface), hind tibia, and hind tarsus. There was an additional, control treatment.
Assaying Behavioral Phase State.
At the end of the 4-h treatment period, each locust was introduced via a modified syringe into the middle of a rectangular observation arena (41 cm long × 30 cm wide). Behind a perforated clear plastic partition at one end of the arena were 20 gregarious final-instar nymphs within a 7.5 × 30-cm, backlit chamber. The gregarious insects came from a crowd-reared culture (500–1,000 insects per 56 × 76 × 60-cm rearing bin). These insects served as the stimulus group. At the other end of the arena, there was a similar, but empty backlit chamber. The behavior of the test insect was recorded on an event recorder in real time for 500 s after introduction into the arena. Values for variables describing the final position of the insect in the arena, the tortuosity of its track during the assay, the frequency and duration of locomotory events, and the incidence of grooming and other small body movements were derived from the raw behavioral records for each test locust. Further details may be found in refs. 5 and 18.
Multiple logistic regression in spss (version 6.1.1.) provided a measure of behavioral phase state for each test insect. The initial step was to build a model with data from 96 gregarious and 96 solitarious final-instar nymphs that were individually observed in the arena but not otherwise used in the experiment. The binary variable (gregarious vs. solitarious phase state) was regressed against the series of behavioral variables by using a logistic algorithm that weighted and combined the variables to produce an optimally fitting model. The resulting model correctly classified 92.7% of the gregarious and 91.7% of the solitarious insects. Compared with solitarious locusts, the gregarious insects walked and groomed more frequently, spent less time resting, and moved toward rather than away from the stimulus group. The model algorithm was then used to calculate the behavioral phase state of the experimental insects. The algorithm yielded the probability that a particular locust, having performed as it did in the behavioral assay, belonged to the solitarious model group. Values for P(solitary) range from 1.0 (indistinguishable in behavior from the solitarious group) to 0.0 (behaving fully gregariously) and provided a linear predictor of behavioral phase state. Further details may be found in refs. 5 and 18.
Results
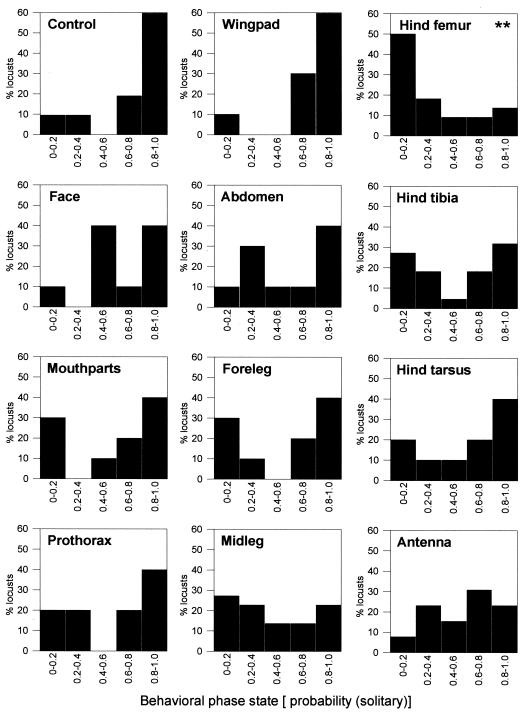
Frequency histograms showing the percentage of locusts falling into different categories of behavioral phase state after the 4-h treatment period are presented in Fig. 1. P values from two-tailed Dunnett's post hoc comparisons (a procedure that corrects robustly for the multiple comparisons) with the control group (n = 21), after ANOVA with normalized ranked data for P(solitary) (18), were as follows: face, 0.755 (n = 10); mouth parts, 0.199 (n = 10); prothorax, 0.999 (n = 10); wing pad, 0.999 (n = 10); abdomen, 0.999 (n = 10); foreleg, 0.858 (n = 10); midleg, 0.181 (n = 22); hind femur, 0.010 (n = 22); hind tibia, 0.606 (n = 22); hind tarsus, 0.973 (n = 10); antenna, 0.751 (n = 13). When one-tailed Dunnett's comparisons were made (the a priori assumption being that treatments gregarized rather than solitarized), P values were as follows: face, 0.412; mouth parts, 0.100; prothorax, 0.783; wing pad, 0.938; abdomen, 0.806; foreleg, 0.495; midleg, 0.090; hind femur, 0.006; hind tibia, 0.317; hind tarsus, 0.650; antenna, 0.409.
Figure 1.
Frequency histograms showing the percentage of test locusts falling into different categories of behavioral phase state [P(solitary)], after a 4-h treatment in which they were mechanically stimulated on one body region for 5 s every 60 s. Insects in the control group were not stimulated and provide the reference for testing the extent to which stimulation evoked a change into the gregarious behavioral state.
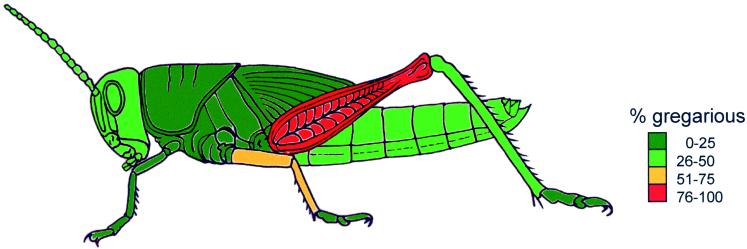
Hence, a statistically significant shift to the gregarious behavioral state was evoked by stimulating the hind femur but not by stroking any of the other body regions. Fig. 2 provides a color-coded map of the body surface based on median values for each treatment.
Figure 2.
Color-coded map of the body, showing the effect of 4-h mechanostimulation of a particular body region on the behavioral phase state of locust nymphs in the solitarious phase. Colors are based on median values for each treatment group. The map shows the extent to which locusts had changed into the gregarious behavioral state, with percentage of gregarious insects calculated as [1 − median P(solitary)] × 100.
Discussion
Two questions arise from these results. First, why are the hind femora so effective as sites of mechanosensory input for behavioral gregarization? Second, why are other body regions less effective, despite the fact that they are well endowed with mechanoreceptors?
It is perhaps to be expected that touching the mouth parts, face, antennae, tarsi, lateral thorax, and abdomen should not cause pronounced behavioral phase change, because these structures are regularly stimulated by the animal itself during feeding, grooming, and walking. The antennae and compound eyes mediate behavioral phase change in other ways, via reception of chemical and visual stimuli (5, 14, 15, 19, 20). In contrast, the outer surfaces of the hind femora are not consistently self-stimulated during normal behavior; also, because they project laterally and dorsally, the outer surfaces of the hind femora are well positioned to indicate the physical presence of other locusts. The hind legs demonstrate a range of reflex avoidance movements to localized touch, the underlying neural bases of which are well understood (17). The hind legs are also used to fend off other locusts; a response that is especially pronounced in solitarious phase insects (21). Given the level of understanding of the neural circuitry underlying hind leg movements, the current findings provide an opportunity to pursue the neural basis of behavioral phase change in the locust.
If locusts are to contact each other regularly and hence stimulate behavioral phase change, they must occur at high local densities. However, the predisposition of solitarious locusts to avoid each other must first be overcome. Our recent laboratory experiments, field experiments in Africa, and computer simulations have shown that crowding of solitarious locusts is highly dependent on small-scale characteristics of the habitat, such as the spatial distribution (6–10) and chemical composition (8) of food resources. Our results provide an explanation for field observations that a population of solitarious locusts is more likely to gregarize in vegetation consisting of compact clumps than where vegetation is spread evenly but sparsely (22, 23). Once brought together by characteristics of the habitat, there is an increased likelihood that locusts will make contact with each other. As they bump into others while moving within and between resource sites, the process of behavioral gregarization is initiated, leading to the formation of local aggregations, which may in turn seed large-scale swarms.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dingle H. Migration: The Biology of Life on the Move. Oxford: Oxford Univ. Press; 1995. [Google Scholar]
- 2.Uvarov B. Grasshoppers and Locusts. Vol. 1. Cambridge, U.K.: Cambridge Univ. Press; 1966. [Google Scholar]
- 3.Pener M P. Adv Insect Physiol. 1991;23:1–79. [Google Scholar]
- 4.Pener M P, Yerushalmi Y. J Insect Physiol. 1998;44:365–377. doi: 10.1016/s0022-1910(97)00169-8. [DOI] [PubMed] [Google Scholar]
- 5.Simpson S J, McCaffery A R, Hägele B F. Biol Rev. 1999;74:461–480. [Google Scholar]
- 6.Collett M, Despland E, Simpson S J, Krakauer D C. Proc Natl Acad Sci USA. 1998;95:13052–13055. doi: 10.1073/pnas.95.22.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouaïchi A, Simpson S J, Roessingh P. Physiol Entomol. 1996;21:247–256. [Google Scholar]
- 8.Despland E, Simpson S J. Animal Behav. 2000;59:643–652. doi: 10.1006/anbe.1999.1335. [DOI] [PubMed] [Google Scholar]
- 9.Despland E, Simpson S J. Physiol Entomol. 2000;25:74–81. [Google Scholar]
- 10.Despland E, Simpson S J, Collett M. Oikos. 2000;88:652–662. [Google Scholar]
- 11.Rainey R C. Migration and Meteorology. Oxford: Clarendon; 1989. [Google Scholar]
- 12.Simpson S J, Raubenheimer D. Proc Nutr Soc. 2000;58:779–789. doi: 10.1017/s0029665199001068. [DOI] [PubMed] [Google Scholar]
- 13.Sword G A, Simpson S J, El Hadi O T M, Wilps H. Proc R Soc London Ser B. 2000;267:63–68. doi: 10.1098/rspb.2000.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roessingh P, Bouaïchi A, Simpson S J. J Insect Physiol. 1998;44:883–893. doi: 10.1016/s0022-1910(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 15.Hägele B, Simpson S J. J Insect Physiol. 2000;46:1295–1301. doi: 10.1016/S0022-1910(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 16.Uvarov B. Grasshoppers and Locusts. Vol. 2. London: Centre for Overseas Pest Research; 1977. [Google Scholar]
- 17.Burrows M. The Neurobiology of an Insect Brain. Oxford: Oxford Univ. Press; 1996. [Google Scholar]
- 18.Roessingh P, Simpson S J, James S. Proc R Soc London Ser B. 1993;252:43–49. [Google Scholar]
- 19.Hassanali A, Torto B. In: Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants. Hardie J, Minks A K, editors. Wallingford, U.K.: CABI; 1999. pp. 305–328. [Google Scholar]
- 20.Heifetz Y, Boekhoff I, Breer H, Applebaum S W. Insect Biochem Mol Biol. 1997;27:563–568. [Google Scholar]
- 21.Ellis P E, Pearce A. Anim Behav. 1962;10:305–318. [Google Scholar]
- 22.Kennedy J S. Trans R Entomol Soc London. 1939;89:385–542. [Google Scholar]
- 23.Roffey J, Popov G. Nature (London) 1968;219:446–450. [Google Scholar]