Abstract
A series of α2–3-sialylated β1–3-linked galactosides, including sialyl T-antigens, 3′-sialyl galacto-N-biose, 3′-sialyl lacto-N-biose, and their derivatives containing natural and non-natural sialic acid forms have been synthesized from simple monosaccharides using an efficient sequential two-step multienzyme approach.
Introduction
α2–3-Sialylated β1–3-linked galactosides including 3′-sialyl type 1 (Siaα2–3Galβ1–3GlcNAcOR), 3′-sialyl type 3 or core 1 (Siaα2–3Galβ1–3GalNAcαOR), and 3′-sialyl type 4 (Siaα2–3Galβ1–3GalNAcβOR) glycan structures are important sialylated carbohydrate moieties on glycoproteins and glycolipids in nature. For example, α2–3-sialylated type 1 glycan (Siaα2–3Galβ1–3GlcNAcOR) is a part of important cancer-related antigens such as sialyl Lewisa antigen presented in the glycoproteins and glycolipids on cell surface.1 It is also a common structure presented in human milk oligosaccharides (HMOs).2α2–3-Linked sialyl type 3 glycans (Siaα2–3Galβ1–3GalNAcαOR) have been found in glycoproteins.3 Sialyl core 1 structure attached to a serine or a threonine residue on peptides or proteins is also called sialyl T-antigen (sialyl Thomsen-Friedenreich antigen or sialyl TF antigen, Siaα2–3Galβ1–3GalNAcα1-O-Ser/Thr). It has been found at an elevated level in O-linked glycoproteins such as mucins in certain cancers including breast cancer.4 Desialylation of sialyl T-antigen in erythrocytes may cause hemolytic-uremic syndrome (HUS), a disease predominantly affecting children.5 Sialyl T-antigens and derivatives have been used in diagnosis for early stages of HUS. They have also been considered as potential cancer vaccines.6 3′-Sialyl type 4 structures (Siaα2–3Galβ1–3GalNAcβOR) are presented on complex gangliosides, a family of sialic acid-containing glycosphingolipids, such as GM1b, GD1a, GT1a, GT1b, GQ1b, and c-series ganglioside GQ1c, GP1c, GH1c, etc.7
We recently reported an efficient one-pot two-enzyme approach for preparative-scale (50–100 mg scale) synthesis of β1–3-linked galactosides including T-antigens, galacto-N-biose (Galβ1–3GalNAc), lacto-N-biose (Galβ1–3GlcNAc), and their derivatives using a recombinant Escherichia coli K-12 galactokinase (GalK) and a novel D-galactosyl-β1–3-N-acetyl-D-hexosamine phosphorylase cloned from Bifidobacterium infantis (BiGalHexNAcP).8 Here we show that the one-pot two-enzyme β1–3-galactosylation system can be easily scaled up to prepare a larger amount of β1–3-linked galactosyl disaccharides as demonstrated here for the synthesis of Galβ1–3GalNAcαProN3. We also present data that the β1–3-linked galactodisaccharide products obtained from the one-pot two-enzyme reactions can be used as sialyltransferase acceptors for synthesizing α2–3-sialosides containing natural and non-natural sialic acid forms in a one-pot three-enzyme sialylation system9 containing a sialic acid aldolase, a CMP-sialic acid synthetase, and a recombinant Pasteurella multocida multifunctional α2–3-sialyltransferase (PmST1).10 Using this two-step multienzyme reaction process in sequential, α2–3-sialylated β1–3-linked galactoside trisaccharides containing the common sialic acid form N-acetylneuraminic acid (Neu5Ac), a non-human sialic acid form N-glycolylneuraminic acid (Neu5Gc), a less common sialic acid form 2-keto-3-deoxy-D-glycero-D-galacto-nonulosonic acid (Kdn), and their azido-derivatives have been successfully prepared. Overall, this sequential two-step multienzyme method allows facile access of diverse sialyltrisaccharides from simple monosaccharides, including N-acetyl-D-galactosamine (GalNAc), N-acetyl-D-glucosamine (GlcNAc), or their derivatives as simple acceptor substrates and D-galactose (Gal) as well as D-mannose, N-acetyl-D-mannosamine (ManNAc), or their derivatives as donor precursors of carbohydrate phosphorylases or glycosyltransferases.
Results and discussion
Sequential two-step multienzyme approach for the synthesis of sialyl galactosides
As shown in Fig. 1, α2–3-sialylated β1–3-linked galactoside trisaccharides containing different sialic acid forms and various underlying glycans can be readily obtained from simple monosaccharides using an efficient two-step multienzyme approach carried out in sequence.
Fig. 1.
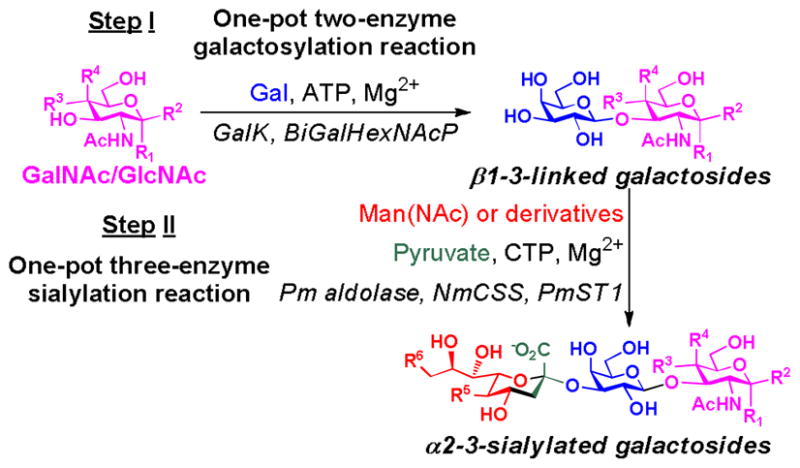
Sequential two-step multienzyme synthesis of α2–3-sialylated β1–3-linked galactoside trisaccharides containing different sialic acid forms and diverse underlying glycans. Enzymes: GalK, E. coli K-12 galactokinase; BiGalHexNAcP, B. infantis D-galactosyl-β1–3-N-acetyl-D-hexosamine phosphorylase; Pm aldolase, P. multocida sialic acid aldolase; NmCSS, N. meningitidis CMP-sialic acid synthetase; PmST1, P. multocida multifunctional α2–3-sialyltransferase. Compounds: Gal, D-galactose; GalNAc, N-acetyl-D-galactosamine; GlcNAc, N-acetyl-D-glucosamine; ATP, adenosine 5′-triphosphate; Man, D-mannose; ManNAc, N-acetyl-D-mannosamine; CTP, cytidine 5′-triphosphate; Sia, sialic acid.
Step I is a one-pot two-enzyme reaction involving a recombinant E. coli K-12 galactokinase (GalK) and a B. infantis phosphorylase (BiGalHexNAcP).8 GalK is responsible for synthesizing galactosyl-1-phosphate (Gal-1-P) from Gal and adenosine 5′-triphosphate (ATP). The Gal-1-P generated in situ can be directly used as a donor substrate by the BiGalHexNAcP which catalyzes the transfer of Gal to simple monosaccharides such as GalNAc, GlcNAc, and their derivatives for the synthesis of a library of β1–3-linked galactodisaccharides in excellent yields. Therefore, in this step, simple monosaccharides are readily linked together by a two-enzyme process to form disaccharides.
The disaccharides formed in Step I can be purified and used as sialyltransferase acceptors in Step II for the formation of α2–3-sialylated β1–3-linked galactoside trisaccharides. Step II is a one-pot three-enzyme reaction involving a sialic acid aldolase, a CMP-sialic acid synthetase, and an α2–3-sialyltransferase.9 The sialic acid aldolase is responsible for synthesizing different sialic acid forms from mannose, ManNAc, and their derivatives in the presence of pyruvate. The sialic acids generated in situ can then be activated by the CMP-sialic acid synthetase to form CMP-sialic acids, the donor substrates of the α2–3-sialyltransferase. The α2–3-sialyltransferase is then responsible for the formation of α2–3-linked sialylgalactosides. A Pasteurella multocida sialic acid aldolase,11 an Neisseria meningitidis CMP-sialic acid synthetase,12 and a Pasteurella multocida multifunctional α2–3-sialyltransferase10 were chosen for this reaction due to their superior expression levels in E. coli and promiscuous substrate specificities. In this step, different sialic acid forms are formed from mannose, ManNAc, or their derivatives, activated, and linked to various disaccharides formed in the Step I for the synthesis of trisaccharides with different terminal sialic acids by a three-enzyme process.
The application of the sequential two-step multienzyme approach has been explored for the synthesis of two libraries of sialylgalactosides. One library contains α2–3-linked sialylgalactosides with different terminal sialic acid forms and the same underlying β1–3-linked galactoside (Galβ1–3GalNAcαProN3) (Fig. 2). The other contains α2–3-linked sialylgalactosides with a common Neu5Ac form, and different underlying β1–3-linked galactosides (Table 1).
Fig. 2.
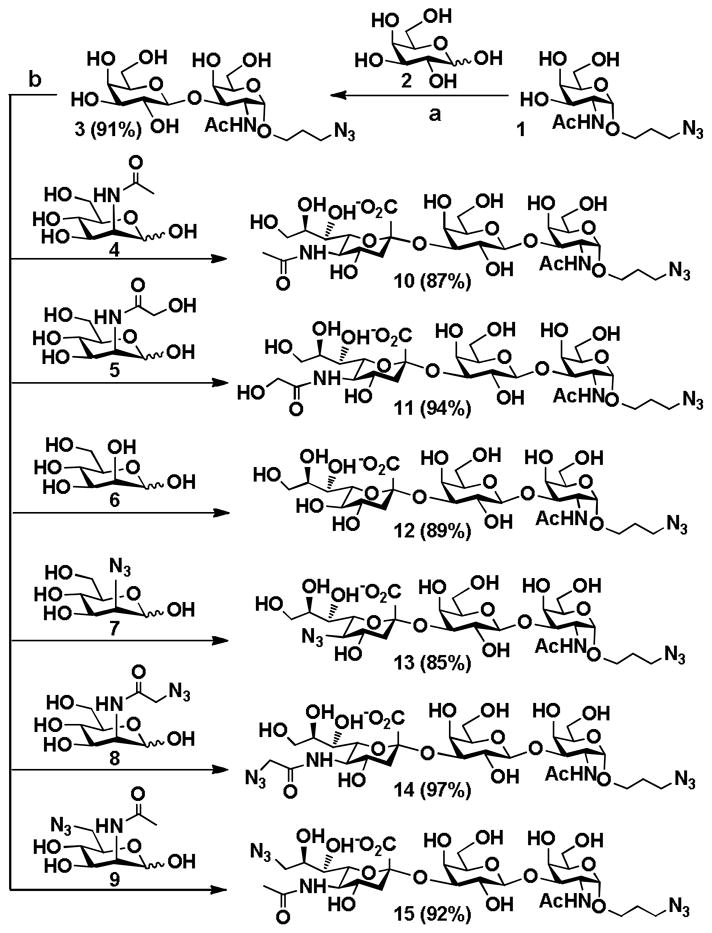
Synthesis of sialyl T-antigens Siaα2–3Galβ1–3GalNAcαProN3 containing different natural and non-natural sialic acid forms. Reagents and conditions: (a) ATP, Mg2+, Tris-HCl buffer (pH 6.5), GalK, BiGalHexNAcP; (b) pyruvate, CTP, Mg2+, Tris-HCl buffer (pH 8.5), Pm aldolase, NmCSS, and PmST1.
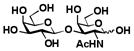
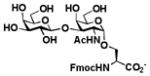
Table 1.
Enzymatic synthesis of α2–3-linked sialosides with terminal Neu5Ac and different underlying β1–3-linked galactosides.
| Acceptors | Products | Yields (%) |
|---|---|---|
 16 |
23 |
90 |
|
17 |
24 |
92 |
 18 |
25 |
86 |
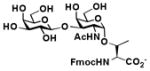 19 |
26 |
89 |
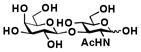 20 |
27 |
94 |
|
21 |
28 |
91 |
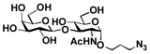 22 |
29 |
89 |
Preparation of sialyl T-antigens containing different sialic acid forms
For preparative-scale synthesis of sialyl T antigens containing different sialic acid forms, galactoside disaccharide with a propyl azide aglycon, Galβ1–3GalNAcαProN3 3, was synthesized at pH 6.5 from 3-azidopropyl α-N-acetyl-D-galactosamine (GalNAcαProN3) 113 and D-galactose 2 using the Step I one-pot two-enzyme galactosylation reaction (Fig. 1). As shown in Fig. 2, Compound 3 was readily obtained in an excellent 91% yield after purification by silica gel and gel filtration chromatography. The obtained Galβ1–3GalNAcαProN3 3 was then used as a sialyltransferase acceptor for the synthesis of sialyl T-antigens containing different terminal sialic acid forms using the Step II one-pot three-enzyme sialylation reaction at pH 8.5.
As shown in Fig. 2, PmST1 exhibited promiscuous donor substrate specificity and catalyzed the transfer of sialic acids from in situ generated CMP-sialic acids to Galβ1–3GalNAcαProN3 3 synthesized by the Step I to produce sialyltrisaccharides 10–15 containing different terminal sialic acid forms in good to excellent (85–97%) yields. The sialosides containing three natural occurring sialic acid forms, Neu5Acα2–3Galβ1–3GalNAcαProN3 10,15 Neu5Gcα2–3Galβ1–3GalNAcαProN3 11,15 and Kdnα2–3Galβ1–3GalNAcαProN3 12 were obtained in 87%, 94%, and 89% yields, respectively, from ManNAc 4, ManNGc 5, and mannose 6 as six-carbon sialic acid precursors. Neu5Gc is a non-human sialic acid which is overexpressed in certain cancer cells.5,14 Sialyl T-antigen trisaccharides containing non-natural sialic acids including Kdn5N3α2–3Galβ1–3GalNAcαProN3 13, Neu5AcN3α2–3Galβ1–3GalNAcαProN3 14, and Neu5Ac9N3α2–3Galβ1–3GalNAcαProN3 15, which contain an azido group at the C-5 or C-9 position of the terminal sialic acid residue, were also obtained in high yields (85–97%) from C2- or C6-modified ManNAc or mannose derivatives 2-azido-mannose (Man2N3) 7, N-azidoacetyl mannosamine (ManNAcN3) 8, and N-acetyl-6-azido-mannosamine (ManNAc6N3) 9, respectively. The structures of all purified sialyl T-antigen trisaccharide products were confirmed by nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HRMS).
Preparation of α2–3-linked sialosides with terminal Neu5Ac and different underlying β1–3-linked galactosides
Sialylgalactosides with the most common sialic acid form Neu5Ac and different underlying β1–3-linked galactosides (Table 1) were also synthesized using the sequential two-step multienzyme approach. To do this, β1–3-linked galactodisaccharides 16–22 were synthesized from D-galactose and the corresponding GalNAc, GlcNAc, or derivatives as the BiGalHexNAcP acceptors in the Step I one-pot two-enzyme galactosylation process (Fig. 1) as described previously.8 These β1–3-linked galactodisaccharides 16–22 were then used as sialyltransferase acceptors in the Step II one-pot three-enzyme sialylation process for the synthesis of α2–3-linked sialosides containing Neu5Ac formed from ManNAc 4 as a sialic acid precursor. As shown in Table 1, preparative-scale sialylation of galacto-N-biose (GNB) Galβ1–3GalNAc 16 and Galβ1–3GalNAcβProN3 17 by Neu5Ac formed from ManNAc and pyruvate successfully produced the sialylated products 3′-sialyl galacto-N-biose (GNB) Neu5Acα2–3Galβ1–3GalNAc 2316 and Neu5cα2–3Galβ1–3GalNAcβProN3 2415 in 90% and 92% yields, respectively. The one-pot three-enzyme system was also applied in sialylation of biologically important T antigens Galβ1–3GalNAcα1-O-Ser and Galβ1–3GalNAcα1-O-Thr to produce the desired trisaccharide products 25 and 26 in excellent 86% and 89% yields, respectively. Similarly, α2–3-sialylation of lacto-N-biose (LNB) Galβ1–3GlcNAc 20 with Neu5Ac produced 3′-sialyl lacto-N-biose (LNB) Neu5Acα2–3Galβ1–3GlcNAc 2717 in 94% yield. In addition, α2–3-sialylation of type 1 disaccharides 21 and 22 with Neu5NAc using the one-pot three-enzyme system produced sialylated products Neu5Acα2–3Galβ1–3GlcNAcβProN3 2815 and Neu5Acα2–3Galβ1–3GlcNAcαProN3 29 in 91% and 89% yields, respectively.
Conclusions
In conclusion, we have developed an efficient sequential two-step multienzyme approach for the synthesis of a series of α2–3-sialylated β1–3-galactosyl disaccharides containing different underlying glycans and various natural and non-natural terminal sialic acid residues. Both LNB (Galβ1–3GlcNAc) and GNB (Galβ1–3GalNAc) type disaccharides, including T-antigens are excellent acceptor substrates for the Pasteurella multodica multifunctional α2–3-sialyltransferse (PmST1). The sequential two-step multienzyme synthetic approach thus allows facile access of complex trisaccharides from simple monosaccharides and derivatives.
Experimental
General methods
Chemicals were purchased and used without further purification. 1H NMR (600 MHz) and 13C NMR (150 MHz) spectra were recorded on a Varian VNMRS 600 MHz spectrometer. High resolution electrospray ionization (ESI) mass spectra were obtained at the Mass Spectrometry Facility in the University of California-Davis. Optical rotation was recorded on an Autopol IV Automatic polarimeter at 589 nm wavelength. Infrared spectra were recorded on a PerkinElmer Spectrum 100 ATR-FTIR. Silica gel 60 A was used for flash column chromatography. Thin-layer chromatography (TLC) was performed on silica gel plates using p-anisaldehyde sugar stain or 5% sulfuric acid in ethanol stain for detection. Gel filtration chromatography was performed using a column (100 cm × 2.5 cm) packed with BioGel P-2 Fine resins.
General procedures for sequential two-step multienzyme synthesis of α2–3-sialylated β1–3-linked galactosides
Step I: One-pot two-enzyme synthesis of β1–3-linked galactoside Galβ1–3GalNAcαProN3 3
The process started with chemical synthesis of GalNAcαProN3 1 similar to that described previously.13 To obtain Galβ1–3GalNAcαProN3 3, GalNAcαProN3 1 (300 mg, 0.98 mmol), galactose (266 mg, 1.48 mmol), ATP (816 mg, 1.48 mmol) and MgCl2 (132 mg, 22 mM) were dissolved in a Tris-HCl buffer (30 mL, 100 mM, pH 6.5). After the addition of GalK (10.8 mg) and BiGalHexNAcP (9.6 mg), the reaction was carried out by incubating the solution in an incubator shaker for 24 h at 37 °C. The reaction was then stopped by adding cold EtOH (30 mL) and the mixture was centrifuged to remove the precipitates. The filtrate was concentrated by a rotary evaporator and purified by a BioGel P-2 filtration column (eluted with water) and a silica gel column (EtOAc:MeOH:H2O = 7:2:1, by volume) to produce Galβ1–3GalNAcαProN3 3 (418 mg, 91%). 1H NMR (600 MHz, D2O): δ 4.90 (d, 1H, J = 3.6 Hz), 4.48 (d, 1H, J = 7.8 Hz), 4.35 (dd, 1H, J = 10.8 and 3.6 Hz), 4.26 (d, 1H, J = 2.4 Hz), 4.04 (dd, 1H, J = 10.8 and 3.0 Hz), 4.00 (t, 1H, J = 6.0 Hz), 3.92 (d, 1H, J = 3.0 Hz), 3.83–3.74 (m, 5H), 3.68–3.63 (m, 2H), 3.58–3.45 (m, 4H), 2.04 (s, 3H), 1.92 (m, 2H); 13C NMR (150 MHz, D2O): δ 174.65, 104.89, 97.41, 77.40, 75.18, 72.71, 70.81, 68.92, 68.79, 65.13, 61.39, 61.19, 48.87, 48.41, 28.17, 22.22.
Compounds 16–22 were synthesized using the same procedures as reported before8 which were similar to that described above for preparing compound 3.
Step II: One-pot three-enzyme preparative-scale synthesis of α2–3-linked sialosides
β1–3-Linked galactosides (50–100 mg) produced from the Step I, a sialic acid precursor (mannose, ManNAc, or their derivatives, 1.5 equiv.), sodium pyruvate (7.5 equiv.), and CTP (1.5 equiv.) were dissolved in Tris-HCl buffer (10 mL, 100 mM, pH 8.5) containing MgCl2 (20 mM) and appropriate amounts of Pm aldolase (0.7–1.0 mg), NmCSS (0.5–0.8 mg), and PmST1 (0.2–0.4 mg). The reactions were carried out by incubating the reaction mixture in an incubator shaker at 37 °C for 1–2 h with agitation at 140 rpm. The product formation was monitored by TLC developed with EtOAc:MeOH:H2O:HOAc = 4:2:1:0.1 (by volume) and stained with p-anisaldehyde sugar stain. When an optimal yield was achieved, the reaction was stopped by adding the same volume (10 mL) of cold EtOH and incubation at 4 °C for 30 min. The mixture was then centrifuged and the precipitates were removed. The supernatant was concentrated, passed through a BioGel P-2 gel filtration column, and eluted with water to obtain partially purified product. A silica gel column was then used to obtain pure sialylated products using EtOAc:MeOH:H2O = 6:2:1 (by volume) as the mobile phase.
Neu5Acα2–3Galβ1–3GalNAcαProN3 (10)
54 mg, yield 87%. [α]24D = +4.16 (c = 1.0 H2O). Wavenumbermax (film)/cm−1 3292 (s, OH), 2944 (s, C–H alkene), 2101 (s, N3), 1621 (s, C=O, carboxylic acid), 1560 (m, C=O, amide), 1023 (s, C-N). 1H NMR (600 MHz, D2O): δ 4.87 (d, 1H, J = 3.6 Hz), 4.51 (d, 1H, J = 7.8 Hz), 4.29–4.27 (m, 1H), 4.21 (m, 1H), 4.05–3.31 (m, 21H), 2.72 (dd, 1H, J = 4.8 and 12.6 Hz), 1.99 (s, 6H), 1.87 (m, 2H), 1.75 (t, 1H, J = 12.3 Hz). 13C NMR (150 MHz, D2O): δ 175.10, 174.69, 174.03, 104.58, 99.83, 97.30, 77.52, 75.76, 74.90, 72.92, 71.95, 70.75, 69.21, 68.69, 68.51, 68.16, 67.51, 65.03, 62.62, 61.35, 61.10, 59.58, 51.78, 48.81, 48.30, 39.83, 28.09, 22.17. HRMS (ESI) m/z calcd for C28H47N5O19Na (M+Na) 780.2763, found 780.2750.
Neu5Gcα2–3Galβ1–3GalNAcαProN3 (11)
58 mg, yield 94%. [α]24D = +5.31 (c = 1.0 H2O). Wavenumbermax (film)/cm−1 3287 (s, OH), 3012 (s, C–H alkene), 2101 (s, N3), 1614 (s, C=O, carboxylic acid), 1562 (m, C=O, amide), 1026 (s, C-N). 1H NMR (600 MHz, D2O): δ 4.88 (d, 1H, J = 4.2 Hz), 4.52 (d, 1H, J = 7.8 Hz), 4.29 (dd, 1H, J = 3.6 and 11.4 Hz), 4.22 (d, 1H, J = 2.4 Hz), 4.09 (s, 2H), 4.07–3.67 (m, 15H), 3.63–3.40 (m, 6H), 2.75 (dd, 1H, J = 4.8 Hz and 12.6 Hz), 2.00 (s, 3H), 1.89 (m, 2H), 1.78 (t, 1H, J = 12.6 Hz). 13C NMR (150 MHz, D2O): δ 175.88, 174.71, 174.07, 104.60, 99.85, 97.32, 77.51, 75.77, 74.92, 72.65, 72.03, 70.77, 69.22, 68.71, 68.26, 68.10, 67.50, 65.05, 62.59, 61.36, 61.10, 51.49, 48.83, 48.31, 39.93, 28.10, 22.18. HRMS (ESI) m/z calcd for C28H48N5O20 (M+H) 774.2893, found 774.2898.
Kdnα2–3Galβ1–3GalNAcαProN3 (12)
55 mg, yield 89%. [α]24D = +1.57 (c = 1.1 H2O). Wavenumbermax (film)/cm−1 3291 (s, OH), 2932 (s, C–H alkene), 2102 (s, N3), 1611 (s C=O, carboxylic acid), 1560 (m, C=O, amide), 1029 (s, C-N).1H NMR (600 MHz, D2O): δ 4.86 (d, 1H, J = 3.6 Hz), 4.51 (d, 1H, J = 7.8 Hz), 4.30 (dd, 1H, J = 3.2 and 11.2 Hz), 4.22 (m, 1H), 4.04–4.02 (m, 2H), 3.97 (dd, 1H, J = 4.8 and 7.2 Hz), 3.90–3.66 (m, 10H), 3.62–3.45 (m, 8H), 2.69 (dd, 1H, J = 12.6 Hz and 4.8 Hz), 2.00 (s, 3H), 1.89 (m, 2H), 1.72 (t, 1H, J = 12.6 Hz). 13C NMR (150 MHz, D2O): δ 174.46, 173.94, 104.37, 99.51, 97.07, 77.18, 75.49, 74.69, 73.70, 72.03, 70.52, 70.14, 69.59, 68.95, 68.48, 67.56, 67.16, 64.80, 62.43, 61.10, 60.87, 48.59, 39.32, 27.85, 26.29, 21.93. HRMS (ESI) m/z calcd for C26H45N4O19 (M+H) 717.2678, found 717.2688.
Kdn5N3α2–3Galβ1–3GalNAcαProN3 (13)
51 mg, yield 85%. [α]24D = +6.56 (c = 1.0 in H2O). Wavenumbermax (film)/cm−1 3291 (s, OH), 2927 (s, C–H alkene), 2106 (s, N3), 1606 (s, C=O, carboxylic acid), 1555 (m, C=O, amide), 1027 (s, C-N). 1H NMR (600 MHz, D2O): δ 4.92 (d, 1H, J = 3.0 Hz), 4.55 (d, 1H, J = 7.8 Hz), 4.34 (dd, 1H, J = 3.0 and 10.8 Hz), 4.26 (m, 1H)), 4.07–3.89 (m, 6H), 3.85–3.65 (m, 9H), 3.57–3.45 (m, 6H), 2.76 (dd, 1H, J = 4.8 and 12.6 Hz), 2.05 (s, 3H), 1.92 (m, 2H), 1.80 (t, 1H, J = 12.6 Hz). 13C NMR (150 MHz, D2O): δ 174.73, 173.87, 104.62, 99.87, 97.38, 77.47, 75.82, 74.96, 72.97, 72.21, 70.82, 69.65, 69.24, 68.78, 68.55, 67.46, 65.14, 62.76, 62.65, 61.40, 61.16, 48.89, 48.39, 39.82, 28.15, 22.25. HRMS (ESI) m/z calcd for C26H44N7O18 (M+H) 742.2743, found 742.2753.
Neu5AcN3α2–3Galβ1–3GalNAcαProN3 (14)
58 mg, yield 97%. [α]24D = +7.81 (c = 1.0 in H2O). Wavenumbermax (film)/cm−1 3285 (s, OH), 2926 (s, C–H alkene), 2100 (s, N3), 1611 (s, C=O, carboxylic acid), 1563 (m, C=O, amide), 1029 (s, C-N). 1H NMR (600 MHz, D2O): δ 4.88 (d, 1H, J = 3.0 Hz), 4.52 (d, 1H, J = 7.8 Hz), 4.29 (dd, 1H, J = 3.0 and 10.8 Hz), 4.22 (m, 1H)), 4.06–4.02 (m, 4H), 3.97–3.69 (m, 12H), 3.62–3.42 (m, 7H), 2.74 (dd, 1H, J = 4.8 Hz and 12.6 Hz), 2.00 (s, 3H), 1.88 (m, 2H), 1.77 (t, 1H, J = 12.6 Hz). 13C NMR (150 MHz, D2O): δ 174.71, 174.04, 171.28, 104.60, 99.87, 97.33, 77.52, 75.79, 74.93, 72.61, 72.06, 70.78, 69.23, 68.72, 68.36, 68.13, 67.52, 65.07, 62.63, 61.37, 61.13, 52.05, 51.89, 48.83, 48.33, 39.91, 28.12, 22.21. HRMS (ESI) m/z calcd for C28H47N8O19 (M+H) 799.2957, found 799.2969.
Neu5Ac9N3α2–3Galβ1–3GalNAcαProN3 (15)
56 mg, yield 92%. [α]24D = +5.31 (c = 1.0 H2O). Wavenumbermax (film)/cm−1 3291 (s, OH), 2932 (s, C–H alkene), 2101 (s, N3), 1608 (s, C=O, carboxylic acid), 1560 (m, C=O, amide), 1030 (s, C-N). 1H NMR (600 MHz, D2O): δ 4.93 (d, 1H, J = 3.6 Hz), 4.56 (d, 1H, J = 7.8 Hz), 4.34 (dd, 1H, J = 3.6 and 10.8 Hz), 4.26 (d, 1H, J = 2.4 Hz), 4.21–4.00 (m, 4H), 3.95 (d, 1H, J = 3.0 Hz), 3.88–3.61 (m, 11H), 3.58–3.46 (m, 5H), 2.77 (dd, 1H, J = 4.8 and 12.6 Hz), 2.05 (s, 6H), 1.93 (m, 2H), 1.80 (t, 1H, J = 12.6 Hz). 13C NMR (150 MHz, D2O): δ 175.08, 174.68, 173.93, 104.55, 99.88, 97.32, 77.48, 75.82, 74.92, 72.74, 70.78, 70.67, 69.25, 68.86. 68.69, 68.51, 67.45, 65.09, 61.36, 61.12, 53.20, 51.79, 48.85, 48.35, 39.91, 28.11, 22.20. HRMS (ESI) m/z calcd for C28H47N8O18 (M+H) 783.3008, found 783.3022.
Neu5Acα2–3Galβ1–3GalNAc (23)
55 mg, yield 90%. [α]24D = +1.48 (c = 1.0 H2O). Wavenumbermax (film)/cm−1 3283 (s, OH), 2937 (s, C–H alkene), 1614 (s, C=O, carboxylic acid), 1560 (m, C=O, amide), 1043 (s, C-N). 1H NMR (600 MHz, D2O): δ 5.21 (d, 0.6H, J = 3.6 Hz, H-1′α), 4.67 (d, 0.4H, J = 8.4 Hz, H-1′β), 4.55 (d, 0.6H, J = 7.8 Hz), 4.49 (d, 0.4H, J = 7.8 Hz), 4.26–3.51 (m, 19H), 2.74–2.73 (m, 1H), 2.00 (s, 6H), 1.77 (t, 1H, J = 12.3 Hz). 13C NMR (150 MHz, D2O): δ 175.11, 174.81, 174.08, 174.04, 173.95, 104.74, 104.57, 99.83, 95.31, 91.29, 80.38, 77.36, 75.81, 75.73, 74.97, 74.89, 72.93, 71.96, 70.33, 69.22, 69.15, 68.70, 68.51, 68.18, 68.06, 67.51, 62.63, 61.33, 61.11, 61.08, 52.46, 51.80, 49.12, 39.86, 22.47, 22.23, 22.19. HRMS (ESI) m/z calcd for C25H42N2O19Na (M+Na) 697.2279, found 697.2270.
Neu5Acα2–3Galβ1–3GalNAcβProN3 (24)
60 mg, yield 92%. [α]24D = −0.83 (c = 1.0 H2O). Wavenumbermax (film)/cm−1 3280 (s, OH), 2936 (s, C–H alkene), 2103 (s, N3), 1636 (s, C=O, carboxylic acid), 1563 (m, C=O, amide), 1027 (s, C-N). 1H NMR (600 MHz, D2O): δ 4.53 (d, 1H, J = 7.8 Hz), 4.52 (d, 1H, J = 8.4 Hz), 4.19 (d, 1H, J = 3.0 Hz), 4.08 (dd, 1H, J = 3.0 and 10.2 Hz), 4.04–3.98 (m, 2H), 3.90 (d, 1H, J = 2.4 Hz), 3.87–3.54 (m, 16H), 3.40 (t, 2H, J = 6.6 Hz), 2.77 (dd, 1H, J = 4.5 and 12.3 Hz), 2.05 (s, 3H), 2.04 (s, 3H), 1.86 (m, 2H), 1.80 (t, 1H, J = 12.3 Hz). 13C NMR (150 MHz, D2O): δ 175.24, 174.95, 174.14, 104.80, 101.64, 99.95, 80.24, 75.82, 74.98, 73.04, 72.80, 72.04, 69.26, 68.59, 68.32, 68.12, 67.64, 67.27, 62.89, 62.77, 61.19, 51.93, 51.43, 48.07, 39.99, 28.36, 22.56, 22.31. HRMS (ESI) m/z calcd for C28H47N5O19Na (M+Na) 780.2763, found 780.2744.
Neu5Acα2–3Galβ1–3GalNAcα1-O-Ser (25)
49 mg, yield 86%. [α]24D = +3.63 (c = 0.9 H2O). Wavenumbermax (film)/cm−1 3286 (s, OH), 2942 (s, C–H alkene), 1636 (s, C=O, carboxylic acid), 1560 (m, C=O, amide), 1401 (m, ArC=C), 1029 (s, C-N). 1H NMR (600 MHz, D2O): δ 7.85–7.37 (m, 8H), 4.79 (d, 1H, J = 3.6 Hz), 4.56–3.97 (m, 8H), 3.89–3.42 (m, 17H), 2.70 (dd, 1H, J = 4.8 and 12.6 Hz), 1.98 (s, 3H), 1.86 (s, 3H), 1.74 (t, 1H, J = 12.3 Hz). 13C NMR (150 MHz, D2O): δ 176.42, 175.05, 174.66, 174.07, 157.74, 143.84, 141.72, 141.07, 140.75, 128.16, 127.64, 125.13, 120.30, 104.47, 99.79, 98.02, 83.77, 77.33, 75.68, 74.61, 74.02, 72.88, 71.88, 70.63, 69.29, 69.10, 68.47, 68.04, 67.38, 66.39, 62.50, 60.91, 60.47, 51.74, 48.51, 46.92, 39.78, 22.17, 22.13. HRMS (ESI) m/z calcd for C43H57N3O23Na (M+Na) 1006.3281, found 1006.3267.
Neu5Acα2–3Galβ1–3GalNAcα1-O-Thr (26)
52 mg, yield 89%. [α]24D = +3.55 (c = 1.0 H2O). Wavenumbermax (film)/cm−1 3275 (s, OH), 2932 (s, C–H alkene), 1600 (s, C=O, carboxylic acid), 1562 (m, C=O, amide), 1446 (m, ArC=C), 1032 (s, C-N). 1H NMR (600 MHz, D2O): δ 7.77–7.28 (m, 8H), 4.77 (d, 1H, J = 3.6 Hz), 4.71 (dd, 1H, J = 3.0 and 12.0 Hz), 4.48 (m, 1H), 4.36 (d, 1H, J = 7.8 Hz), 4.14–3.72 (m, 13H), 3.67–3.42 (m, 14H), 2.70 (dd, 1H, J = 4.8 and 12.6 Hz), 1.98 (s, 3H), 1.88 (s, 3H), 1.75 (t, 1H, J = 12.0 Hz), 0.96 (d, 3H, J = 6.6 Hz). 13C NMR (150 MHz, D2O): δ 176.70, 175.08, 174.62, 174.13, 158.23, 144.12, 143.69, 141.76, 141.12, 128.10, 127.64, 127.58, 125.05, 124.93, 120.23, 104.46, 99.92, 98.98, 77.45, 77.28, 75.67, 74.73, 73.77, 72.92, 71.88, 70.76, 69.40, 69.17, 68.72, 68.55, 68.13, 67.54, 65.88, 61.19, 61.03, 60.72, 60.60, 51.80, 48.60, 47.32, 39.68, 22.41, 22.16, 18.28. HRMS (ESI) m/z calcd for C44H59N3O23Na (M+Na) 1020.3437, found 1020.3421.
Neu5Acα2–3Galβ1–3GlcNAc (27)
69 mg, yield 94%. [α]24D =−0.34 (c = 1.0 in H2O). Wavenumbermax (film)/cm−1 3284 (s, OH), 2891 (s, C–H alkene), 1610 (s, C=O, carboxylic acid), 1565 (m, C=O, amide), 1046 (s, C-N). 1H NMR (600 MHz, D2O): δ 5.00 (d, 0.6H, J = 3.6 Hz, H-1′α), 4.57 (d, 0.4H, J = 8.4 Hz, H-1′β), 4.35 (d, 0.6H, J = 7.8 Hz), 4.31 (d, 0.4H, J = 7.8 Hz), 3.91–3.34 (m, 19H), 2.58 (dd, 0.6H, J = 4.8 and 12.6 Hz), 2.54 (dd, 0.4H, J = 4.8 and 12.6 Hz), 1.85 (s, 6H), 1.60 (t, 1H, J = 12.3 Hz). 13C NMR (150 MHz, D2O): δ 175.10, 175.05, 174.92, 174.66, 173.98, 103.63, 103.53, 99.78, 94.84. 91.17, 82.87, 80.41, 75.82, 75.78, 75.60, 75.22, 75.18, 72.93, 71.98, 71.39, 69.29, 69.22, 68.89, 68.82, 68.52, 68.17, 67.39, 62.59, 61.13, 60.87, 60.71, 59.45, 55.62, 52.98, 51.79, 39.90, 22.48, 22.22, 22.19. HRMS (ESI) m/z calcd for C25H42N2O19Na (M+Na) 697.2279, found 697.2252.
Neu5Acα2–3Galβ1–3GlcNAcβProN3 (28)
103 mg, yield 91%. [α]24D = −1.72 (c = 1.0 H2O). Wavenumbermax (film)/cm−1 3278 (s, OH), 2932 (s, C–H alkene), 2101 (s, N3), 1612 (s, C=O, carboxylic acid), 1560 (m, C=O, amide), 1030 (s, C-N). 1H NMR (600 MHz, D2O): δ 4.52 (d, 1H, J = 8.4 Hz), 4.46 (d, 1H, J = 8.4 Hz), 4.05 (dd, 1H, J = 3.0 and 9.6 Hz), 3.90–3.44 (m, 20H), 3.36 (t, 2H, J = 8.4 Hz), 2.72 (dd, 1H, J = 4.8 and 12.6 Hz), 2.00 (s, 3H), 1.99 (s, 3H), 1.80 (m, 2H), 1.75 (t, 1H, J = 12.3 Hz). 13C NMR (150 MHz, D2O): δ 175.08, 174.69, 174.04, 103.56, 101.02, 99.76, 82.63, 75.72, 75.49, 75.21, 72.91, 71.96, 69.19, 68.84, 68.51, 68.14, 67.36, 67.26, 62.55, 61.14, 60.84, 59.43, 51.77, 47.90, 39.88, 28.22, 22.42, 22.16. HRMS (ESI) m/z calcd for C28H47N5O19Na (M+Na) 780.2763, found 780.2746.
Neu5Acα2–3Galβ1–3GlcNAcαProN3 (29)
74 mg, yield 89%. [α]24D = +1.46 (c = 1.2 H2O). Wavenumbermax (film)/cm−1 3284 (s, OH), 2929 (s, C–H alkene), 2101 (s, N3), 1612 (s, C=O, carboxylic acid), 1560 (m, C=O, amide), 1029 (s, C-N). 1H NMR (600 MHz, D2O): δ 4.88 (d, 1H, J = 3.6 Hz), 4.53 (d, 1H, J = 7.8 Hz), 4.11 (dd, 1H, J = 3.6 and 10.8 Hz) 3.96–3.83 (m, 10H), 3.72–3.47 (m, 12H), 2.78 (dd, 1H, J = 4.8 and 12.6 Hz), 2.05 (s, 6H), 1.93 (m, 2H), 1.80 (t, 1H, J = 12.3 Hz). 13C NMR (150 MHz, D2O): δ 175.16, 174.60, 174.05, 103.51, 99.86, 97.26, 80.57, 75.85, 75.22, 72.98, 72.01, 71.80, 69.34, 68.87, 68.54, 68.22, 67.46, 65.15, 62.66, 61.44, 61.17, 60.72, 52.65, 51.85, 48.36, 39.94, 28.14, 22.24. HRMS (ESI) m/z calcd for C28H47N5O19Na (M+Na) 780.2763, found 780.2756.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01GM076360 and U01CA128442. X. Chen is a Camille Dreyfus Teacher-Scholar and a UC-Davis Chancellor’s Fellow. We thank Ryan Davis at the Department of Chemistry in the University of California-Davis for his help with the FTIR measurements.
Footnotes
Electronic Supplementary Information (ESI) available: NMR spectra of sialylated galactosides. See DOI: 10.1039/b000000x/
Notes and references
- 1.(a) Anderson B, Davis LE, Venegas M. Adv Exp Med Biol. 1988;228:601. doi: 10.1007/978-1-4613-1663-3_25. [DOI] [PubMed] [Google Scholar]; (b) Magnani JL. Arch Biochem Biophys. 2004;426:122. doi: 10.1016/j.abb.2004.04.008. [DOI] [PubMed] [Google Scholar]; (c) Ugorski M, Laskowska A. Acta Biochim Pol. 2002;49:303. [PubMed] [Google Scholar]; (d) Sanders DS, Kerr MA. Mol Pathol. 1999;52:174. doi: 10.1136/mp.52.4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Schnaar RL, Suzuki A, Stanley P. Glycosphingo-lipids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 129–141. [Google Scholar]
- 2.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Annu Rev Nutr. 2000;20:699. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 3.Maes E, Florea D, Coppin A, Strecker G. Eur J Biochem. 1999;264:301. doi: 10.1046/j.1432-1327.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Storr SJ, Royle L, Chapman CJ, Hamid UM, Robertson JF, Murray A, Dwek RA, Rudd PM. Glycobiology. 2008;18:456. doi: 10.1093/glycob/cwn022. [DOI] [PubMed] [Google Scholar]; (b) Brockhausen I. EMBO Rep. 2006;7:599. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki A. Trends Mol Med. 2008;14:351. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J. Science. 1993;262:841. doi: 10.1126/science.8235605. [DOI] [PubMed] [Google Scholar]
- 7.(a) Scarsdale JN, Prestegard JH, Yu RK. Biochemistry. 1990;29:9843. doi: 10.1021/bi00494a014. [DOI] [PubMed] [Google Scholar]; (b) Sabesan S, Duus JO, Fukunaga T, Bock K, Ludvigsen S. J Am Chem Soc. 1991;113:3236. [Google Scholar]; (c) Wiseman JM, Li JB. Anal Chem. 2010;82:8866. doi: 10.1021/ac1016453. [DOI] [PubMed] [Google Scholar]; (d) Yu RK, Ando S. Adv Exp Med Biol. 1980;125:33. doi: 10.1007/978-1-4684-7844-0_5. [DOI] [PubMed] [Google Scholar]; (e) Saito M, Kitamura H, Sugiyama K. J Neurochem. 2001;78:64. doi: 10.1046/j.1471-4159.2001.00365.x. [DOI] [PubMed] [Google Scholar]; (f) Saito M, Kitamura H, Sugiyama K. Biochim Biophys Acta. 2002;1571:18. doi: 10.1016/s0304-4165(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Thon V, Lau K, Cai L, Chen Y, Mu S, Li Y, Wang PG, Chen X. Chem Comm. 2010;46:7507. doi: 10.1039/c0cc02850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Chokhawala HA, Huang S, Chen X. Nat Protoc. 2006;1:2485. doi: 10.1038/nprot.2006.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. J Am Chem Soc. 2005;127:17618. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Yu H, Cao H, Lau K, Muthana S, Tiwari VK, Son B, Chen X. Appl Microbial Biotechnol. 2008;79:963. doi: 10.1007/s00253-008-1506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Yu H, Karpel R, Chen X. Bioorg Med Chem. 2004;12:6427. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Chokhawala HA, Varki A, Chen X. Org Biomol Chem. 2007;5:2458. doi: 10.1039/b706507h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padler-Karavani V, Yu H, Cao H, Chokhawala HA, Karp F, Varki N, Chen X, Varki A. Glycobiology. 2008;18:818. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Yu H, Chen X. Sci China Chem. 2011;54:117. doi: 10.1007/s11426-010-4175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Izumi M, Shen GJ, Wacowich-Sgarbi S, Nakatani T, Plettenburg O, Wong CH. J Am Chem Soc. 2001;123:10909. doi: 10.1021/ja011382r. [DOI] [PubMed] [Google Scholar]; (b) Kren V, Thiem J. Angew Chem Int Ed Engl. 1995;34:893. [Google Scholar]; (c) Paulsen H, Von Deessen U. Carbohydr Res. 1988;175:283. doi: 10.1016/0008-6215(88)84150-8. [DOI] [PubMed] [Google Scholar]
- 17.(a) Lee SG, Kim BG. Enzyme Microb Technol. 2001;28:161. doi: 10.1016/s0141-0229(00)00313-6. [DOI] [PubMed] [Google Scholar]; (b) Vetere A, Miletich M, Bosco M, Paoletti S. Eur J Biochem. 2000;267:942. doi: 10.1046/j.1432-1327.2000.01068.x. [DOI] [PubMed] [Google Scholar]; (c) Lubineau A, Auge C, Francois P. Carbohydr Res. 1992;228:137. doi: 10.1016/s0008-6215(00)90555-x. [DOI] [PubMed] [Google Scholar]; (d) Xia J, Alderfer JL, Piskorz CF, Locke RD, Matta KL. Carbohydr Res. 2000;328:47. doi: 10.1016/s0008-6215(00)00080-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


