Abstract
Purpose
To assess the genetic and epigenetic status of parthenogenetic human embryonic stem cells (phESCs).
Methods
Cytogenetics, X chromosome inactivation (XCI) and gene expression patterns were analyzed in one phESC line (FY-phES-018) that was derived from our laboratory.
Results
FY-phES-018 cells displayed the classical characteristics of normal hESCs. These cells had a 46, XX karyotype, and no inactive X chromosomes were observed before passage 20. After being cultured long term in vitro, some cells lost one X, and the proportion of cells with only one X gradually increased. At passage 35, almost all the cells displayed a 45, XO karyotype. Interestingly, at passage 45, the recovery of the X-chromosome was observed, and XCI became detectable; the mosaic ratio of 46, XX to 45, XO was 67:33. After passage 60, most cells displayed the 46, XX karyotype again with a mosaic ratio of 97:3. Some aberrant genomic imprinting was also observed in these cells.
Conclusions
The phESCs line FY-phES-018 is both genetically and epigenetically unstable; therefore, further research is needed before using these cells.
Keywords: Parthenogenetic, hESC, Genetic, Epigenetic, Instability
Introduction
Human embryonic stem cells (hESCs) have tremendous potential in clinical applications [1, 2]. However, hESC immunological rejection and genetic and epigenetic instability greatly limit the transplantation of these cells into patients.
Histocompatible hESCs would pose a lower risk of immune rejection, and parthenogenetic human embryonic stem cells (phESCs) represent one potential source for regenerative medicine that would be histocompatible with the oocyte donor [3]. Because the derivation of phESCs does not involve the destruction of viable human embryos, they are associated with many advantages compared to traditional hESCs. Recently, a few phESC lines were established [4–8]. These cells display self-renewal and multi-differentiation properties similar to those of normal hESCs derived from fertilized embryos [4–8]. Although the biological characterization of phESCs is documented, the genetic and epigenetic behaviors of these cells must still be investigated.
It has been demonstrated that clonal evolution and selection in hESCs cultures can cause genomic alterations and epigenetic fluidity [9, 10]. It is also known that genetic changes associated with neoplasia and epigenetic instability may affect cellular behavior and fate [11, 12]. In cultured hESCs, specific chromosomal abnormalities have been reported for the aneuploidy of chromosomes 12, 17 and X (reviewed from ref. [13]), and the relationships between genomic instability and carcinogenesis have been well investigated [12, 14]. Maintaining the correct epigenetic patterns is critically important to ensure the function and safety of hESCs in regenerative medicine. DNA methylation, X-chromosome inactivation (XCI), and genomic imprints are the most important epigenetic modifications. The appropriate initiation and maintenance of XCI is very important for embryogenesis and cell physiology [15]. Aberrant allele-specific imprinted gene expression can also cause genetic disorders, such as Prader Willi/Angelman syndrome [16]. In parthenogenetic mouse embryonic stem cells (pmESCs), epigenetic reprogramming and gene expression changes were reported [17, 18]. However, whether phESCs are genetically and epigenetically stable is still unclear. Although the long-term culturing of phESCs may have negative implications for therapeutic application, the genetic homozygosity of phESCs is still a major advantage for investigating genetic and epigenetic mechanisms. Therefore, in this study, we used a phESC line (named FY-phES-018) that was established and maintained in our laboratory, as well as several normal hESC lines derived from fertilized embryos, to investigate and compare the genetic and epigenetic status of the different cell lines.
Materials and methods
Informed consent and parthenogenetic activation of oocytes
The experiment was approved by the Ethical Committee of Guangzhou Medical College. Donors voluntarily donated oocytes with no financial compensation. The signed informed consent documents stated that all embryos would only be used for basic research and not for reproductive purposes. Metaphase II (MII) oocytes, after collecting and removing culumus cells, were used in this study. The activation of oocytes was performed in G1.3 medium (Vitrolife) supplemented with 5-μM calcium ionophore A23187 (Sigma, St. Louis, MO) for 5 min, followed by a 4 h incubation in 1-mM 6-dimethylaminopurine (6-DMAP, Sigma) and 5-nM Trichostatin A (TSA, Sigma). Female pronucleus formation was examined after the eggs were cultured for another 10 h in G1.3 medium with 5-nM TSA. The eggs with pronuclei were transferred into G1.3 medium for 72 h and then into G2.3 blastocyst medium (Vitrolife) for another 48 h. All blastocysts were cultured for additional 2 days in a blastocyst optimum culture medium in which 2,000 U/ml of human recombined leukemia inhibitory factor (hLIF; Chemicon, Temecula, CA) and 10 ng/ml of human basic fibroblast growth factor (bFGF; Vitrolife) were supplemented.
Isolation and culturing of parthenogenetic stem cells
The ICMs of the embryos were isolated by immunosurgery and then placed on mitomycin C-treated murine embryonic fibroblasts (MEF) feeder layers for further culturing. The derivation and culturing of undifferentiated hESCs and embryoid body formation were conducted as previously described [19].
Characterization of phESCs
Alkaline phosphatase (AP) activity was assessed by histochemical staining. The phESC colonies on the MEF feeder layer were fixed with 7.5% sucrose plus 1% paraformaldehyde for 10 min, and then the cells were stained with BCIP/NBT (Sigma) for 10 min prior to examination.
For immunoflourescence staining, the phESCs were fixed with 4% paraformaldehyde for 20 min and blocked with 4% goat serum for 1 h. The cells were then incubated with the primary antibodies overnight at 4°C. The primary antibodies included antibodies against stage-specific embryonic antigen (SSEA)-1, SSEA-4, tumor-rejection antigen (TRA)-1-60 and TRA-1-81 (1:50, Chemicon). The cells were then rinsed three times with PBS and incubated at 37°C for 30 min with the corresponding goat fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Santa Cruz). The stained colonies were examined using a confocal laser scanning microscope (EZ-C1; Nikon TE2000, Japan).
Gene expression profile analysis
Total RNA was extracted and purified using a Trizon Kit, and then, the RNA was reverse-transcribed (Invitrogen). The PCR amplification was carried out using primers for OCT4, NANOG, TDGF1, SOX 2, THY-1, FGF4, REX-1 [20]. The total RNA from the EB cells was extracted by the same method used for the analysis of the differentiated marker genes after 14 days of differentiation. For genome imprinting analysis, H19, insulin-like growth factor 2 (IGF2) and small nuclear ribonucleoprotein polypeptide N (SNRPN) were analyzed (Table 1). FY-hES-7, a female hESC line was used as a control. The PCR amplified products were analyzed on a 1.5% agarose gel, visualized by ethidium bromide (Invitrogen) staining and imaged using the BioImaging system (UVP, Upland, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as a ubiquitously expressed control.
Table 1.
Primers for RT-PCR and MSP
| Gene primer | primer forward 5′-3′ | primer reverse5′-3′ | size (bp) |
|---|---|---|---|
| OCT4 | cttgctgcagaagtgggtggaggaa | ctgcagtgtgggtttcgggca | 169 |
| NANOG | cagaaggcctcagcacctac | attgttccaggtctggttgc | 110 |
| SOX2 | aaccccaagatgcacaactc | gcttagcctcgtcgatgaac | 100 |
| TDGF1 | gcctcttttccccctaattg | ggcagcaggttctgtttagc | 279 |
| THY1 | ggactgagatcccagaacca | acgaaggctctggtccacta | 124 |
| FGF4 | gatgagtgcacgttcaagga | gaggaagtgggtgaccttca | 152 |
| REX1 | gcgtacgcaaattaaagtccaga | atcctaaacagctcgcagaat | 303 |
| AFP | agaacctgtcacaagctgtg | gacagcaagctgaggatgtc | 676 |
| NEUROD1 | aagccatgaacgcagaggaggact | gctgtccatggtaccgtaa | 579 |
| HBZ | ctgaccaagactgagaggac | atgtcgtcgatgctcttcac | 224 |
| H19 | ccggacacaaaaccctctagct | tgttccgatggtgtctttgatg | 142 |
| IGF2 | tcccctgattgctctaccca | gcagttttgctcacttccgatt | 86 |
| SNRPN | tggcacctttaaggcttttg | ccgcttttcttcacgctct | 112 |
| XIST | agctcctcggacagctgtaa | ctccagatagctggcaacc | 242 |
| XIST-M | gttggagcgtagtggtattatttc | aaaccaacctaaccaacataacg | 176 |
| XIST-U | gggttggagtgtagtggtattatttt | aaaccaacctaaccaacataacaaa | 174 |
| SNRPN-M | taaataagtacgtttgcgcggtc | aaccttacccgctccatcgcg | 174 |
| SNRPN-P | gtaggttggtgtgtatgtttaggt | acatcaaacatctccaacaacca | 100 |
| HUMARA-M | gcgagcgtagtatttttcggc | aaccaaataacctataaaacctctacg | 177–221a |
| HUMARA-U | gttgtgagtgtagtattttttggt | caaataacctataaaacctctaca | 177–221a |
| GAPDH | ggagtcaacggatttggtcg | tcctggaagatggtgatggg | 218 |
aIndicates polymorphism patterns of the HUMARA gene
HLA typing and DNA fingerprinting
HLA typing was performed by PCR with sequence-specific primers (Biotest HLA SSP Kit, Biotest, Dreieich Germany, http://www.biotest.de). The products were identified using agarose gel electrophoresis. DNA bands were visualized under UV light with the aid of the Biotest SSP typing software.
For short tandem repeat (STR) analysis, genomic DNA was extracted from the oocyte donor and FY-phES-018 at two different passages using a Qiagen DNeasy Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The extracted DNA was amplified for 16 different genetic loci using the Promega PowerPlex 16 System Kit (Promega, USA) or the AmpF/STR® Identifiler® PCR Amplification Kit (Applied Biosystems, USA), and capillary electrophoresis was carried out on an ABI 3100 Genetic Analyzer (Applied Biosystems, CA, USA).
Karyotype analysis and fluorescent in-situ hybridization (FISH)
For karyotype analysis, cells were collected at every 10 passage, treated with 0.25-μg/ml colcemid (Gibco, Invitrogen, USA) for 4 h and then incubated in a solution containing 0.4% sodium citrate and 0.4% potassium chloride (1:1, v/v) at 37°C for 5 min. The cells were fixed in methanol:acetic acid (3:1, v/v) three times. The cells were dropped on slides for Giemsa stained, and at least 100 cells were examined.
For FISH analysis, cells were dropped onto wet slides and dried at 63°C overnight and dehydrated with ethanol in sequential concentrations of 70%, 85%, and 100% prior to hybridization. The Vysis MultiVysion® PGT Multi-color Probe set (Vysis Inc., USA) with five probes for chromosomes 13, 18, 21, X and Y was used to visualize specific chromosomes. At least 20 cells were examined.
X chromosome inactivation (XCI) status
For XCI analysis, XIST expression and the DNA methylation status of the HUMARA gene were analyzed. Total RNA of FY-phES-018 at two different passages was extracted and reverse-transcribed using a Trizon Kit (Invitrogen). The genomic DNA from the undifferentiated cells was extracted using a Qiagen DNeasy Tissue Kit and bisulfite-treated using the EpiTect Bisulfite Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Two sets of PCR were prepared: one for methylated X and the other for unmethylated X. The PCR amplification was performed as previously described [21]. Bisulfite-converted DNA from normal female hESCs was used as a control. Samples were loaded and analyzed on an ABI 3100 Genetic Analyzer.
Genome methylation assay
The methylation of SNRPN and XIST was studied using a methylation-specific PCR (MSP) Assay [22]. Genomic DNA was extracted and bisulfite-treated according to the Qiagen DNeasy Tissue Kit and the EpiTect Bisulfite Kit instructions (Qiagen). PCR amplification and gel analysis were performed as described [22].
Microarray analysis
Total RNA was isolated using Trizol reagent (Invitrogen) and analyzed using the CapitalBio 22 K Human Genome Oligo Array from CapitalBio Corporation (Beijing, China). Briefly, a Human Genome Oligo Set Version 2.1 consisting of 5′-amo-modified 70-mer probes and representing 21,522 well-characterized Homo sapiens genes were analyzed on amo-silaned glass slides. A space and intensity-dependent normalization based on the LOWESS program was employed in the R language package (http://www.R-project.org/) [23]. To assess differences in the expression levels of imprinted genes between FY-phES-018 and the calibrator hESC line, two hybridizations were performed according to a reversal fluorescent strategy. The threshold ratio for the up-regulation and down-regulation of gene expression was 1.5 and 0.5, respectively.
Results
Derivation and characteristics of FY-phES-018 cells
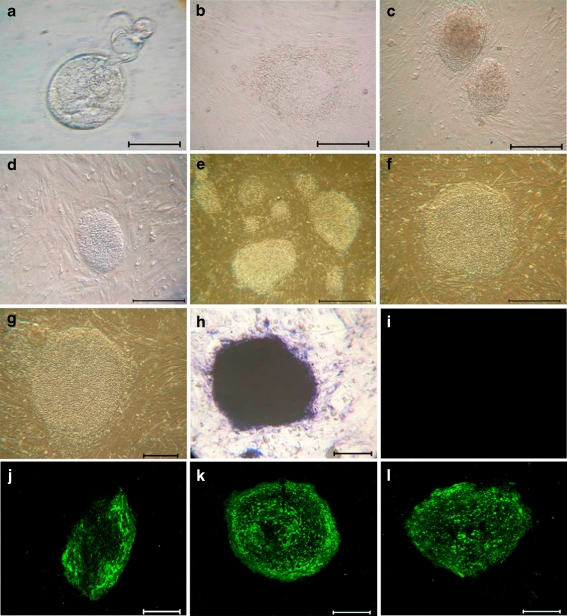
After oocyte collection and parthenogenetic activation, four of the 18 eggs developed into early blastocysts on Day 5, and three developed to expanded blastocysts on Day 7 (Fig. 1a). ICMs isolated by immunosurgery were cultured on MEF feeder layers and showed dome-like colonies after 8–9 days of culturing (Fig. 1b). One phESC line (FY-phES-018) was established and has been cultured for more than 70 passages (Fig. 1c–g). Undifferentiated FY-phES-018 cells showed high levels of alkaline phosphatase activity and strongly expressed TRA-1-60, TRA-1-81, and SSEA-4, but not SSEA-1 (Fig. 1h–l). Pluripotency-related genes such as OCT-4, SOX2, NANOG, THY-1, FGF4, and REX-1 were all expressed in FY-phES-018 (Fig. 2a). The investigation of the differentiation potential of FY-phES-018 cells demonstrated that they can differentiate into all three germ layers in vitro: endoderm (AFP), ectoderm (NEUROD1), and mesoderm (HBZ) (Fig. 2b).
Fig. 1.
Morphology of parthenogenesis in human embryos and phESC. Isolation and characterization of FY-phES-018: a Day 7 expanded blastocyst; b primary colony of phESCs. c–g FY-phES-018 colonies at: c passage 10; d passage 20; e passage 30; f passage 40 and g passage 60. h–l Expression of molecular markers in undifferentiated FY-phES-018 cells: h undifferentiated cells stain positive for AP; i negative expression of SSEA-1; j positive expression of SSEA-4; k TRA-1-60 and l TRA-1-81. Scale bar = 100 μm
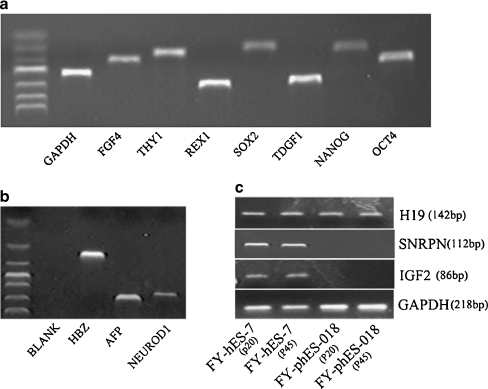
Fig. 2.
RT-PCR analysis of gene expression patterns. a Expression of pluripotency related genes: OCT4 (169 bp), NANOG (110 bp) TDGF1 (279 bp), SOX2 (100 bp), REX1 (303 bp), THY1 (124 bp) and FGF4 (152 bp) in undifferentiated FY-phES-018 cells. b Three embryonic germ layer marker genes, HBZ (mesoderm), AFP (endoderm), and NEUROD1 (ectoderm), were all expressed in Day 14 differentiated EBs of FY-phES-018 cells. c Imprinted gene expression was analyzed in FY-phES-018 at passages 20 and 45. FY-hES-7, a normal hESC line, was used as a control. The paternally expressed genes IGF2 and SNRPN were not expressed in FY-phES-018 at passages 20 or 45 but were expressed in biparental FY-hES-7 cells. The maternally expressed gene H19 was expressed in all cells
Confirmation of parthenogenetic origin
Because all of the genetic material in phESCs derive from the maternal genome, and there is no paternal genome, the lack of paternal imprinting and methylation can be used to identify cells of parthenogenetic origin. In this study, we observed the expression of maternally expressed H19 but not paternally expressed IGF2 or SNRPN in FY-phES-018 cells at passage 20 and passage 45. All of the above imprinted genes were expressed in the FY-hES-7 control hESCs (Fig. 2c).
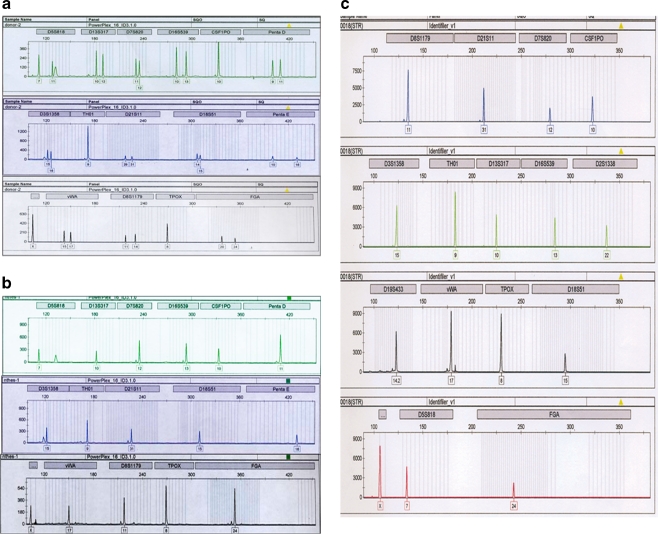
HLA-A, -B, and DRB loci analyses showed that FY-phES-018 cells were hemizygously matched to the oocyte donor (Table 2). The 16 STR loci at passage 20 and passage 60 were scattered in different regions of 12 different chromosomes, and they also hemizygously matched to the donor loci, indicating that FY-phES-018 was derived from the parthenogenetic activation of an oocyte (Fig. 3a–c).
Table 2.
HLA typing of oocyte donor and FY-phES-018
| HLA type | A | B | DRB |
|---|---|---|---|
| Donor | A11/A11 | B62/B54 | DR4/DR8 |
| FY-phES-018 | A11/A11 | B54/B54 | DR8/DR8 |
Fig. 3.
STR analysis of FY-phES-018. a STR analysis of the oocyte donor indicated that most of the 16 STR loci (except for TH01, CSF1P0 and TPOX) of the donor were heterozygous; two obvious peaks for those locus markers can be seen. STR analysis of FY-phES-018 at passage 20 (b) and passage 60 (c) shows only one peak in all of the 16 STR loci, indicating that FY-phES-018 is homozygous, and that all of the loci are hemizygously matched to the donor loci
X chromosome instability
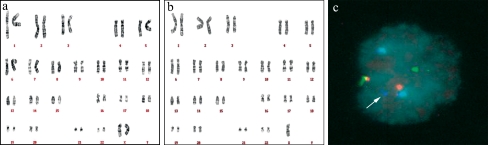
The karyotype of FY-phES-018 at passage 20 was 46, XX in all examined cells (Fig. 4a). However, after being cultured long term in vitro, some cells lost one X, and the proportion of cells with only one X increased. At passage 35, we found that almost all of the cells had a 45, XO karyotype and that no 46, XX cells were observed among 100 examined cells (Fig. 4b). To confirm this result, we used FISH analysis. FISH examination at passage 35 showed the existence of only one X-chromosome among 20 investigated cells (Fig. 4c). Interestingly, the recovery of the X chromosome in FY-phES-018 cells was observed at passage 45. The proportion of cells with a 46, XX karyotype increased, and the ratio of 46, XX to 45, XO cells was 67:33. After 60 passages, most of the cells had a normal 46, XX karyotype, and the mosaic ratio was 97:3. FY-hES-7 and FY-hES-11, two female hESC lines that were derived in our laboratory, were also examined under the same culture conditions and with the same medium. However, we did not observe any loss or instability of the X-chromosome or of autosomes in any of those cells at any passage number (data not shown).
Fig. 4.
Cytogenetic analysis and FISH result of FY-phES-018. a At passage 20, the karyotype of FY-phES-018 was 46, XX. b At passage 35, almost all of FY-phES-018 cells had a 45, XO karyotype. c FISH showed only one X chromosome in all investigated FY-phES-018 cells at passage 35 (arrow image). Red, green and aqua spots indicate chromosomes 13, 21 and 18, respectively
Variable X chromosome inactivation
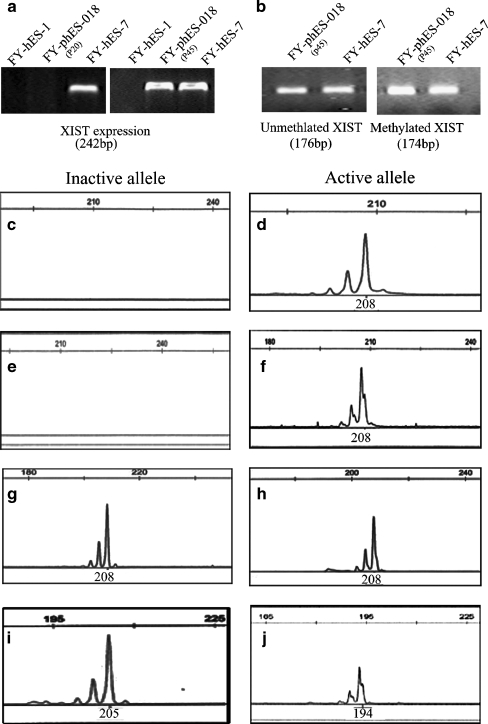
An examination of XIST expression at passage 20 indicated that this gene was not expressed in FY-phES-018 cells, which was similar to the results from FY-hES-1 male control cells (Fig. 5a). At passage 45, after the cells were cultured extensively in vitro, the expression of XIST in FY-phES-018 cells was observed (Fig. 5a). To confirm this observation, we further detected the methylation pattern of XIST at passage 45 in FY-phES-018 cells, and both methylated and unmethylated alleles were detected (Fig. 5b). The above results indicate that XCI in FY-phES-018 cells was variable after long-term culturing.
Fig. 5.
XCI variability in FY-phES-018. a The XIST gene was expressed in FY-hES-7 (46, XX) but not in FY-hES-1(46, XY) or FY-phES-018 (46, XX, passage 20). At passage 45, the expression of XIST in FY-phES-018 became detectable. b The methylation status of XIST was analyzed in FY-phES-018 and FY-hES-7 at passage 45. The observation of both methylated and unmethylated XIST alleles combined with the XIST expression in FY-phES-018 cells at passage 45 suggest that XCI was initiated in those cells. c–j The methylation patterns of the HUMARA gene indicate the XCI status of FY-phES-018. The left column shows inactive X alleles and the right column shows active X alleles. At passage 20 (46, XX) (c, d) and passage 35 (45, XO) (e, f), FY-phES-018 showed only the active 208-bp X allele and no inactive X allele. However, at passage 45, when the cells with two Xs had increased, both active and inactive X alleles could be detected (g, h). In the female control cell line FY-hES-11 (46,XX), the presence of only the 194-bp active allele and the 205-bp inactive allele indicated skewed XCI (i, j)
MS-PCR of the HUMARA gene is a standard method for analyzing XCI status [21], so we next used this classical method to confirm the XCI variability in FY-phES-018 cells. At passage 20 (46, XX) (Fig. 5c, d) and passage 35 (45, XO or 45, XO/46, XX), we could not find any inactivated X-chromosome alleles in FY-phES-018 (Fig. 5e, f). However, at passage 45, inactive X alleles of FY-phES-018 were detectable and 46, XX cell numbers had increased, indicating that XCI was initiated in these cells (Fig. 5g, h). One female hESC line (FY-hES-11) that was derived in our laboratory was also examined. FY-hES-11 had both active and inactive X-chromosomes in the undifferentiated stage at early passages and maintained a stable skewed XCI pattern after long-term culturing (Fig. 5i, j).
DNA methylation and gene chip analysis
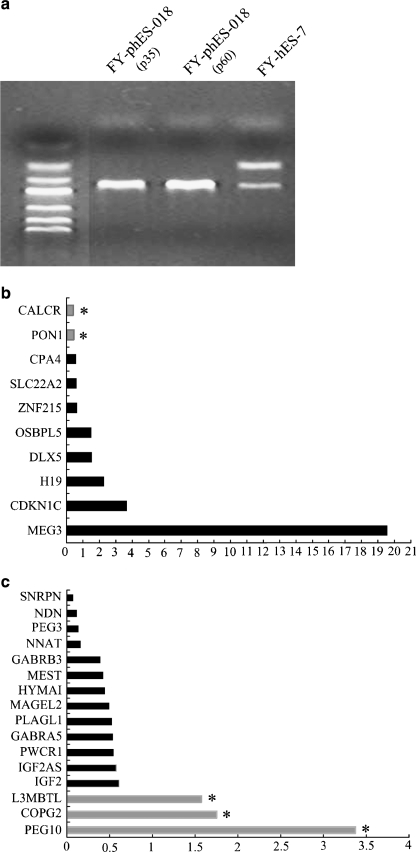
MSP results showed that FY-phES-018 cells had only methylated allele of the SNRPN gene and no unmethylated alleles at passages 35 and 60 (Fig. 6a). In contrast, both unmethylated and methylated alleles of the SNRPN gene were detected in FY-hES-7 (Fig. 6a). These results further indicated that FY-phES-018 was derived from the parthenogenetic activation of an oocyte.
Fig. 6.
DNA methylation and gene chip analysis in FY-phES-018. a Detection of the methylation status of SNRPN revealed that FY-phES-018 at passage 35 and 60 showed only a methylated pattern (174 bp), and no unmethylated pattern could be detected (100 bp). Both methylated maternal SNRPN and unmethylated paternal SNRPN were detected in FY-hES-7. Relative expression analysis of maternally expressed genes (b) and paternally expressed genes (c) by gene chip analysis in FY-phES-018 cells with normal hESCs used as a calibrator. Black bars and gray bars are the relative gene expression levels between FY-phES-018 and the calibrator. The gray bars with an asterisk (*) in (b) indicate maternally expressed genes down-regulated in FY-phES-018 with a ratio of less than 0.5. In addition, the gray bars with an asterisk (*) in (c) indicate paternally expressed genes up-regulated in FY-phES-018 with a ratio of more than 1.5
A gene chip was also used to compare the expression patterns of imprinted genes in phESCs and hESCs. It revealed that most paternally expressed genes were down-regulated while maternally expressed genes were up-regulated in FY-phES-018 cells, indicating that most of the imprinted genes maintained their correct expression patterns (Fig. 6b, c). However, we also observed some aberrant imprinted gene expression in FY-phES-018. For example, the expression levels of the maternally expressed imprinted genes CALCR and PON1 were abnormally down-regulated, and the expression of paternally expressed levels imprinted genes, such as PEG10, COPG2 and L3MBTL, were up-regulated (Fig. 6b, c).
Discussion
In the present study, 18 donated metaphase II (MII) oocytes were used for parthenogenetic activation. Four of the 18 oocytes developed into early blastocysts after being cultured for 5 days, and three developed into expanded blastocysts on Day 7, one pluripotent homozygous phESC line was derived. The derivation efficiency was 5.6% (1/18) when calculated from all collected oocytes or 25% (1/4) when calculated from Day 5 blastocysts. The derivation efficiency was similar to those reported in previous reports [6, 7]. These cells displayed morphologies and characteristics similar to those of normal hESCs. HLA and STR typing showed that FY-phES-018 was homozygous and hemizygously matched to the oocyte donor. The lack of expression of the paternally imprinted gene IGF2 and the methylation pattern of SNRPN further demonstrated that these cells were of parthenogenetic origin.
The phESCs provide a new strategy for potential cell therapies. Because these cells have a homozygous genome with minimal crossover-associated heterozygosity [24], and the tissue derived from these cells express only one of the two sets of parental histocompatible antigens, phESCs seem more suitable for patients with less risk of tissue rejection [25]. However, whether the loss of heterozygosity of critical genomic regions might disrupt the function of phESCs is still unknown. Therefore, the investigation of the genetic and epigenetic properties of phESCs is desirable prior to considering their use in vivo.
In this study, we monitored the genetic and epigenetic status of X-chromosome variability in FY-phES-018 cells. Genetic and epigenetic stability is an important factor in determining whether cells can be used for therapies [9, 26]. From previous reports, phESCs appear to be less stable than hESCs derived from fertilized embryos. It has been found that two of four HLA homozygous phESC lines have karyotype anomalies [7], and one of the six HLA heterozygous phESC lines is aneuploid [5]. Kim et al. [27] also found that SCNT-hES-1 (the first derived phESC line), which was initially thought to be generated from somatic nuclear transfer, lost one of its two X chromosomes and duplicated chromosome 7 after long-term culturing. The failure to silence one of the X chromosome and unstable XX karyotype diploid pmESC lines were also frequently observed [28]. The instability of mouse XX ES cells suggests that XX is an unfavorable situation that leads to hypomethylation. This hypomethylation results in the loss of an X chromosome [29]. However, in hESCs, unlike their rodent counterparts, most of those cells already have an inactive X in an undifferentiated state and can stably maintain the XaXi status [10, 30, 31]. Therefore, the aneuploidy and genetic instability of hESCs may be a natural result of embryo cleavage mosaics [32], or it may be caused by long-term maintenance leading to genetic defects [33].
In the present study, we found X chromosome instability with two active Xs in FY-phES-018, which behaved more like mouse ES cells. Cytogenetic analysis at early passages showed that FY-phES-018 cells had a normal 46, XX karyotype, so a mosaic originating from embryo cleavage could be ruled out. Considering that the whole genomes were duplicated from the same maternal genome and that the cells lacked a paternal genome, we hypothesize that the genetic and epigenetic instability of the X chromosome in FY-phES-018 cells may be explained by the combined effects of culture selection and global hypomethylation. Hypomethylation due to X-chromosome duplication may have led to two active Xs in phESCs, which causes competition between the XaXa cells and ultimately provides selective pressure favoring the deletion of sequences from one of the two X chromosomes.
Another interesting finding in this study was that FY-phES-018 cells gradually recovered a normal karyotype after most of cells lost an X chromosome. STR examination at passage 20 and passage 60 demonstrated that the recovered XX cells were the same line as the one X cells. This phenomenon could be due to two possibilities. One possibility is that in the XO cells, the remaining X initiated a duplication process. A similar phenomenon has been observed in SCNT-hES-1 cells and some other cell lines [27, 34]. Then, those duplicated cells ultimately adapted to selective pressures and acquired a growth advantage. The second and more likely possibility is that despite the fact that at passage 35, the cytogenetic and FISH analyses revealed that the cell karyotype was 45, XO, some 46, XX cells might have been present but at an undetectable level. These few remaining XX cells may have gradually adapted to selection pressures, initiated XCI, acquired a growth advantage, and gradually displaced the 45, XO cells. At passage 65, the XX to XO reached 97:3, indicating that the selection was still ongoing and that this process seems to be almost complete.
To date, the data available for XCI in hESCs is diverse, and the research on XCI in phESCs has been relatively limited. In mouse ES cells, the initiation of XCI correlates with low levels of pluripotency, and XCI is recapitulated only when the cells are induced to differentiate ex vivo. In hESCs, the XCI status is unstable, prone to change during culturing and can be categorized into three epigenetic classes [10, 30, 31]. Class I hESCs show pre-XCI with the capacity to recapitulate XCI upon differentiation, which means that the undifferentiated hESCs possess two active X chromosomes. Class II hESCs show XCI markers in both undifferentiated and differentiated states, and Class III hESCs lose the ability to express XIST but undergo XCI [10]. A recently study demonstrated that the derivation of hESCs at a physiological oxygen concentration (5%) caused them to possess two active X chromosomes in undifferentiated states, and they initiated XCI when cultured in atmospheric oxygen concentration (20%) conditions [35]. In the present study, XX phESCs did not initiate XCI at early passages, indicating that both of the two X chromosomes were active. However, after being cultured under atmospheric oxygen concentration conditions, XCI became detectable, and cells with two X chromosomes gradually adapted to the selection pressure. To our knowledge, this is the first time XCI variation has been detected together with X chromosome loss and recovery. In our previous report, we demonstrated that secondarily selected XCI patterns may occur under stressful culture conditions in a triploid cell line, suggesting that selective pressure may lead to XCI instability [36]. Therefore, the X chromosome alterations and unstable XCI in FY-phES-018 cells suggest that XaXi cells are more prone to culture selection during passage in atmospheric oxygen concentration conditions.
Genomic imprinting is also an important criterion for examining epigenetic mechanisms. In this study, several imprinted genes were assessed, including H19, IGF2 and SNRPN. Mutation in these genes leads to many human diseases [37, 38]. In phESCs, all of the genetic material is derived from the maternal genome, thus paternally expressed imprinted genes should not be expressed in phESCs. However, in this study, we found aberrant expression of some imprinted genes in FY-phES-018 cells. Although altered expression of paternally expressed imprinted genes has been reported recently in pmESC lines [17, 18, 39] and in a differentiated phESC line (chHES-32) [4], the mechanism of imprinting changes in phESCs is not fully understood. Thus, phESCs could be a useful model for studying the mechanisms of epigenetic regulation and imprinting.
Considering the genetic and epigenetic instability of FY-phES-018 cells, further research is needed before using them for cell therapies. We do not know whether other phESC lines also display aneuploidy or are genetically and epigenetically unstable. Therefore, more genetic and epigenetic investigations should be conducted in additional phESC lines to demonstrate the safety and efficiency of these cells for therapeutic applications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30871378).
Author Disclosure Statement No competing financial interests exist.
Footnotes
Capsule
X chromosome loss and recovery accompanied by variations in X chromosome inactivation were found in one parthenogenetic human embryonic stem cell line.
Weiqiang Liu, Yifei Yin, and Yonghua Jiang contributed equally to this work.
Contributor Information
Shaorong Gao, Phone: +86-10-80728967, FAX: +86-10-80727535, Email: gaoshaorong@nibs.ac.cn.
Xiaofang Sun, Phone: +86-20-81292202, FAX: +86-20-81292013, Email: xiaofangsun@hotmail.com.
References
- 1.Oh SK, Kim HS, Ahn HJ, Seol HW, Kim YY, Park YB, et al. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells. 2005;23:211–219. doi: 10.1634/stemcells.2004-0122. [DOI] [PubMed] [Google Scholar]
- 2.Peura TT, Bosman A, Stojanov T. Derivation of human embryonic stem cell lines. Theriogenology. 2007;67:32–42. doi: 10.1016/j.theriogenology.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Cibelli JB, Cunniff K, Vrana KE. Embryonic stem cells from parthenotes. Methods Enzymol. 2006;418:117–135. doi: 10.1016/S0076-6879(06)18008-8. [DOI] [PubMed] [Google Scholar]
- 4.Lin G, OuYang Q, Zhou X, Gu Y, Yuan D, Li W, et al. A highly homozygous and parthenogenetic human embryonic stem cell line derived from a one-pronuclear oocyte following in vitro fertilization procedure. Cell Res. 2007;17:999–1007. doi: 10.1038/cr.2007.97. [DOI] [PubMed] [Google Scholar]
- 5.Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, et al. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432–449. doi: 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- 6.Mai Q, Yu Y, Li T, Wang L, Chen MJ, Huang SZ, et al. Derivation of human embryonic stem cell lines from parthenogenetic blastocysts. Cell Res. 2007;17:1008–1019. doi: 10.1038/cr.2007.102. [DOI] [PubMed] [Google Scholar]
- 7.Revazova ES, Turovets NA, Kochetkova OD, Agapova LS, Sebastian JL, Pryzhkova MV, et al. HLA homozygous stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2008;10:11–24. doi: 10.1089/clo.2007.0063. [DOI] [PubMed] [Google Scholar]
- 8.Lu Z, Zhu W, Yu Y, Jin D, Guan Y, Yao R, et al. Derivation and long-term culture of human parthenogenetic embryonic stem cells using human foreskin feeders. J Assist Reprod Genet. 2010;27:285–291. doi: 10.1007/s10815-010-9408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, et al. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- 10.Silva SS, Rowntree RK, Mekhoubad S, Lee JT. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:4820–4825. doi: 10.1073/pnas.0712136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allegrucci C, Thurston A, Lucas E, Young L. Epigenetics and the germline. Reproduction. 2005;129:137–149. doi: 10.1530/rep.1.00360. [DOI] [PubMed] [Google Scholar]
- 12.Allegrucci C, Denning C, Priddle H, Young L. Stem-cell consequences of embryo epigenetic defects. Lancet. 2004;364:206–208. doi: 10.1016/S0140-6736(04)16636-1. [DOI] [PubMed] [Google Scholar]
- 13.Allegrucci C, Young LE. Differences between human embryonic stem cell lines. Hum Reprod Update. 2007;13:103–120. doi: 10.1093/humupd/dml041. [DOI] [PubMed] [Google Scholar]
- 14.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 15.Tomkins DJ, McDonald HL, Farrell SA, Brown CJ. Lack of expression of XIST from a small ring X chromosome containing the XIST locus in a girl with short stature, facial dysmorphism and developmental delay. Eur J Hum Genet. 2002;10:44–51. doi: 10.1038/sj.ejhg.5200757. [DOI] [PubMed] [Google Scholar]
- 16.Kantor B, Kaufman Y, Makedonski K, Razin A, Shemer R. Establishing the epigenetic status of the Prader-Willi/Angelman imprinting center in the gametes and embryo. Hum Mol Genet. 2004;13:2767–2779. doi: 10.1093/hmg/ddh290. [DOI] [PubMed] [Google Scholar]
- 17.Gong SP, Kim H, Lee EJ, Lee ST, Moon S, Lee HJ, et al. Change in gene expression of mouse embryonic stem cells derived from parthenogenetic activation. Hum Reprod. 2009;24:805–814. doi: 10.1093/humrep/den388. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Chen Z, Liu Z, Huang J, Zhang W, Zhou L, et al. Correlation of expression and methylation of imprinted genes with pluripotency of parthenogenetic embryonic stem cells. Hum Mol Genet. 2009;18:2177–2187. doi: 10.1093/hmg/ddp150. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Long X, Yin Y, Jiang Y, Chen X, Liu W, et al. Similar biological characteristics of human embryonic stem cell lines with normal and abnormal karyotypes. Hum Reprod. 2008;23:2185–2193. doi: 10.1093/humrep/den137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brivanlou AH, Gage FH, Jaenisch R, Jessell T, Melton D, Rossant J. Stem cells. Setting standards for human embryonic stem cells. Science. 2003;300:913–916. doi: 10.1126/science.1082940. [DOI] [PubMed] [Google Scholar]
- 21.Kubota T, Nonoyama S, Tonoki H, Masuno M, Imaizumi K, Kojima M, et al. A new assay for the analysis of X-chromosome inactivation based on methylation-specific PCR. Hum Genet. 1999;104:49–55. doi: 10.1007/s004390050909. [DOI] [PubMed] [Google Scholar]
- 22.Kubota T, Das S, Christian SL, Baylin SB, Herman JG, Ledbetter DH. Methylation-specific PCR simplifies imprinting analysis. Nat Genet. 1997;16:16–17. doi: 10.1038/ng0597-15. [DOI] [PubMed] [Google Scholar]
- 23.Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K, Lerou P, Yabuuchi A, Lengerke C, Ng K, West J, et al. Histocompatible embryonic stem cells by parthenogenesis. Science. 2007;315:482–486. doi: 10.1126/science.1133542. [DOI] [PubMed] [Google Scholar]
- 25.Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 26.Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25:207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 27.Kim K, Ng K, Rugg-Gunn PJ, Shieh JH, Kirak O, Jaenisch R, et al. Recombination signatures distinguish embryonic stem cells derived by parthenogenesis and somatic cell nuclear transfer. Cell Stem Cell. 2007;1:346–352. doi: 10.1016/j.stem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Robertson EJ, Evans MJ, Kaufman MH. X-chromosome instability in pluripotential stem cell lines derived from parthenogenetic embryos. J Embryol Exp Morphol. 1983;74:297–309. [PubMed] [Google Scholar]
- 29.Zvetkova I, Apedaile A, Ramsahoye B, Mermoud JE, Crompton LA, John R, et al. Global hypomethylation of the genome in XX embryonic stem cells. Nat Genet. 2005;37:1274–1279. doi: 10.1038/ng1663. [DOI] [PubMed] [Google Scholar]
- 30.Hall LL, Byron M, Butler J, Becker KA, Nelson A, Amit M, et al. X-inactivation reveals epigenetic anomalies in most hESC but identifies sublines that initiate as expected. J Cell Physiol. 2008;216:445–452. doi: 10.1002/jcp.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Y, Matsuno Y, Fouse SD, Rao N, Root S, Xu R, et al. X-inactivation in female human embryonic stem cells is in a nonrandom pattern and prone to epigenetic alterations. Proc Natl Acad Sci USA. 2008;105:4709–4714. doi: 10.1073/pnas.0712018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bielanska M, Tan SL, Ao A. Chromosomal mosaicism throughout human preimplantation development in vitro: incidence, type, and relevance to embryo outcome. Hum Reprod. 2002;17:413–419. doi: 10.1093/humrep/17.2.413. [DOI] [PubMed] [Google Scholar]
- 33.Allegrucci C, Young LE. Differences between human embryonic stem cell lines. Hum Reprod Update. 2007;13:103–120. doi: 10.1093/humupd/dml041. [DOI] [PubMed] [Google Scholar]
- 34.Donahue SL, Lin Q, Cao S, Ruley HE. Carcinogens induce genome-wide loss of heterozygosity in normal stem cells without persistent chromosomal instability. Proc Natl Acad Sci USA. 2006;103:11642–11646. doi: 10.1073/pnas.0510741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lengner CJ, Gimelbrant AA, Erwin JA, Cheng AW, Guenther MG, Welstead GG, et al. Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell. 2010;141:872–883. doi: 10.1016/j.cell.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Sun X. Skewed X chromosome inactivation in diploid and triploid female human embryonic stem cells. Hum Reprod. 2009;24:1834–1843. doi: 10.1093/humrep/dep126. [DOI] [PubMed] [Google Scholar]
- 37.Rocha ST, Ferguson-Smith AC. Genomic imprinting. Curr Biol. 2004;14:R646–R649. doi: 10.1016/j.cub.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Lopes S, Lewis A, Hajkova P, Dean W, Oswald J, Forne T, et al. Epigenetic modifications in an imprinting cluster are controlled by a hierarchy of DMRs suggesting long-range chromatin interactions. Hum Mol Genet. 2003;12:295–305. doi: 10.1093/hmg/ddg022. [DOI] [PubMed] [Google Scholar]
- 39.Jiang H, Sun B, Wang W, Zhang Z, Gao F, Shi G, et al. Activation of paternally expressed imprinted genes in newly derived germline-competent mouse parthenogenetic embryonic stem cell lines. Cell Res. 2007;17:792–803. doi: 10.1038/cr.2007.70. [DOI] [PubMed] [Google Scholar]