Abstract
Purpose
During laboratory manipulations, oocytes and embryos are inevitably exposed to suboptimal conditions that interfere with the normal development of embryos.
Materials and methods
In this study, we examined the effects of antioxidants, feeder cells and a conditioned medium on embryo development and cleavage rate following exposure of the embryos to suboptimal conditions. We exposed mouse two-cell embryos to visible light and divided them into four groups: control (E-ctr), co-culture (Co-c), conditioned medium (Cndm) and antioxidant-plus medium (Aopm). We used human umbilical cord matrix-derived mesenchymal cells for co-culture. A group of embryos was not exposed to visible light and served as the non-exposed control (NE-ctr) group.
Results
The developmental rate was higher in NE-ctr embryos than in the E-ctr group. Exposed embryos in the various groups showed a comparable developmental rate at different stages. Blastomere number significantly increased (P < 0.05) in the Co-c and Aopm groups compared with the E-ctr and Cndm groups. No significant difference was observed between the Co-c and Aopm groups.
Conclusions
Our data indicate that in suboptimal conditions, antioxidants could improve the embryo cleavage rate in the same way as feeder cells. Antioxidants probably improve embryo quality through their ability to scavenge reactive oxygen species.
Keywords: Visible light, Mouse embryos, Conditioned media, Antioxidants, Co-culture
Introduction
Early mammalian development occurs inside the changeable, lightless environment of the female reproductive tract. Several studies have shown that the exposure of mammalian embryos to various amounts of visible light disrupts normal embryo development. In a previous study, exposure of mouse two-cell embryos to 1600 l× visible light for 30 min to 60 min dramatically decreased the developmental rate [1, 2]. This indicates the absence of mechanisms that protect against visible light in mammalian embryos. In Assisted Reproduction Technology (ART) procedures embryos are inevitably exposed to environmental stress including visible light. The dissimilarity between in vivo embryo developmental rates and in vitro embryo developmental rates has suggested that environmental conditions including visible light could induce the production of critical concentrations of reactive oxygen species (ROS) that are able to modify normal cell function, endanger cell survival and generate DNA damage in embryos [3]. Undoubtedly, the extent of cell damage after irradiation depends on the duration of exposure, the intensity and the wavelengths emitted. In experiments carried out to understand the mechanisms underlying mammalian cell damage caused by visible light, cardiomyocytes that were exposed to low energy visible light (3.6j/cm2, 1623 l×/min. for 1.5 min) showed a slow and transient increase in [Ca2+]i, whereas exposure of cardiomyocytes to visible light at 12j/cm2 (1623 l×/min. for 5 min) induced a linear rise in [Ca2+]i and damaged the cells [4]. Various mechanisms exist in in vivo systems that contribute to the detoxification and protection of mammalian cells against ROS. These include enzymes such as superoxide dismutase, which generates hydrogen peroxide from superoxide radicals, and glutathione peroxidase and catalase, which convert hydrogen peroxide to water [5, 6]. There are also low molecular weight antioxidants that function as ROS scavengers such as glutathione, vitamin C (ascorbic acid), alpha-tocopherols, and β-mercaptoethanol [7, 8].
The mammalian reproductive tract is rich in oxygen scavengers that protect embryos from oxidative damage while the embryo travels from the uterine tube towards the uterine cavity [9, 10]. Vitamin C is a water-soluble vitamin and is the major antioxidant component in extracellular fluids [11, 12]. Supplementation of bovine culture medium with vitamin C improved the porcine denuded oocyte maturation and blastocyst rate after parthenogenetic activation [13]. β-mercaptoethanol (β-ME) is also known as a low molecular weight antioxidant that is usually used in culture media, especially embryonic stem cell culture media [14, 15]. In an effort to refine culture media for vitrified-warmed embryos, supplementation of culture media with β-ME resulted in lower DNA fragmentation in vitrified-warmed bovine blastocysts [16]. Studies have shown that somatic cells possess the capacity to remove deleterious components including ROS from culture media to enhance embryo development following the co-culture of embryos with various somatic cells [17, 18].
In the present study, mouse embryos were exposed to 1600 l× visible light as a source of in vitro ROS production [4]. Some embryos were not exposed to visible light and served as the NE-ctr group. Embryo development was then assessed in the presence of ascorbic acid and β-ME as antioxidants. This culture system was compared with a somatic cell co-culture system composed of human umbilical cord matrix-derived mesenchymal cells (hUCM), and a conditioned medium in which hUCM cells were cultivated and the supernatant was removed as conditioned medium. The embryo developmental rates and the number of blastomeres were compared among these groups and the control group.
Materials and methods
Kerman University of Medical Sciences ethics committee approved these experiments. All chemicals were purchased from Sigma-Aldrich Chemical Company (Saint Louis, MO, USA); culture flasks, dishes and tubes were purchased from Falcon (BD Biosciences, San Jose, CA) unless stated otherwise.
Isolation of human umbilical cord matrix mesenchymal cells
The local ethics committee at Kerman University of Medical Sciences, Kerman, Iran approved the procedures for collecting human umbilical cords. Human umbilical cord matrix mesenchymal cells were cultivated as described elsewhere [19]. Umbilical cords were obtained from healthy mothers after written consent was obtained. Amniotic membrane, umbilical arteries and veins were carefully removed and the remaining tissues were diced into 3–5 mm fragments. The explants were seeded onto the surface of a culture flask in complete medium consisting of MEM-α supplemented with 10% (V/V) fetal bovine serum (FBS; Gibco, Invitrogen, Australia), penicillin (100 units/ml), and streptomycin (60 μg/ml). Explants were incubated at 37°C in a humid atmosphere with 5% CO2. Once the cells reached 80% confluence, the umbilical cord fragments were removed and the cells were trypsinized and seeded for further expansion.
Preparation of feeder cells and conditioned medium
hUCM cells at passage 2–7 were used as feeder cells. Forty-eight h before the experiments were carried out, hUCM cells at a density of 1 × 105 cells/ml were prepared in 50 μl drops of complete medium in 60 × 15 culture dishes, overlaid with seven ml light paraffin oil. The culture plates were incubated at 37°C in a humid atmosphere with 5% CO2. The medium was refreshed 24 h later with complete medium. For the preparation of conditioned medium (Cndm), 1 × 106 viable hUCM cells were seeded into 25 cm2 culture flasks with complete medium. After 72 h incubation, non-adherent cells were removed by two washes with PBS and adherent cells at a confluence of 90% were cultured in seven ml complete medium. Twenty-four h later the supernatant was removed, centrifuged at 500 g for 10 min, and used as 50 μl drops under light paraffin oil for further experiments.
Preparation of antioxidant-plus medium
One mM β-ME and 50 μg/ml vitamin C were added to complete medium to prepare antioxidant-plus medium (Aopm) [13, 16]. Fifty μl drops were loaded onto 60 × 15 mm culture dishes, overlaid by seven ml mineral oil, and incubated at 37°C in a humid atmosphere with 5% CO2 for 24 h.
Embryo collection
Four to 8-week-old female NMRI (Razi Institute, Karaj, Iran) mice were superovulated with an intra-peritoneal injection of 10 IU PMSG (Intervet, Denmark), followed by 10 IU hCG (Organon, Holland) 48 h later. Female mice were caged overnight with male mice with proven fertility from the same strain. Female mice with a vaginal plug were considered pregnant. Forty-eight h after hCG injection, two-cell mouse embryos were flushed from the uterine tube into drops of Hepes-buffered HTF containing 10% FBS (Gibco, USA). After two washes in the same medium, morphologically normal embryos were collected and pooled for further experiments.
Exposure of embryos to visible light
Shortly after the collection of embryos, morphologically normal embryos were transferred into 50 μl drops of complete medium under seven ml light paraffin oil and were exposed to visible light for 30 min. Light was emitted from a 30 W halogen lamp and was conducted via optic fibers, inside a CO2 incubator. The distance between the free end of the optic fibers and the culture dish (Falcon®) was adjusted so that 1600 l× illumination was applied to the embryos.
Differential staining of blastocysts
Expanded blastocysts were randomly selected for cell counting analysis as described elsewhere [20] with a few modifications. Embryos were placed in HTF medium supplemented with 1% Triton X-100 and 100 μg/ml propidium iodide (PI) for approximately 10 s. Once the color of the trophoectoderm had visibly changed to red and shrunk slightly due to the treatment, blastocysts were incubated in 500 μl absolute alcohol as a fixative, plus 25 μg/ml bisbenzimide (Hoechst 33342), overnight at 4°C in a dark chamber. Thereafter, samples were placed in a glycerol droplet on a glass slide and covered with a coverslip. Samples were monitored under a fluorescence microscope (IX71, Olympus, Japan) equipped with a UV filter. Inner cell mass (ICM) nuclei labeled with bisbenzamide appeared blue and trophectoderm (TE) nuclei labeled with both bisbenzamide and PI appeared pink to red. ICM, TE and total cell numbers were counted.
Experimental design and evaluation of embryo development
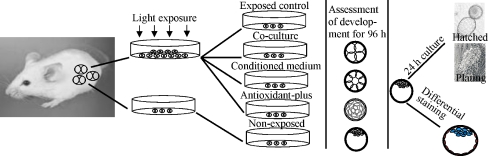
Morphologically normal two-cell embryos were pooled and divided into two unequal groups of non-exposed control (NE-ctr) embryos and exposed embryos. Exposed embryos were exposed to 1600 l× visible light for 30 min, and were randomly allocated into the exposed control (E-ctr), co-culture (Co-c), conditioned medium (Cndm) and antioxidant-plus medium (Aopm) groups. Embryos were monitored every 24 h for 96 h under an inverted microscope (Nikon TS100, Japan) and the developmental rate was recorded. Expanded blastocysts in each group were then randomly divided into two groups: some were removed for differential staining and the remaining blastocysts were cultured for another 24 h to assess their hatching and plating competence (Fig. 1). At 24, 48, 72, 96, and 120 h after embryo collection, the embryos were scored for stage of development using an inverted microscope. At each time point the embryos had to be at the following minimum developmental stage to be scored as developing: four-cell at 24 h, eight-cell at 48 h, morulae at 72 h, expanded blastocysts at 96 h, and hatched and plated embryos at 120 h. Experiments were replicated at least six times for developmental rate determination and differential staining.
Fig. 1.
Two-cell stage embryos were obtained from superovulated mice. Morphologically normal embryos were divided into two unequal groups of non-exposed embryos and exposed embryos. The exposed embryos received 1600 l× visible light and were then allocated to Exposed control, Co-culture, Conditioned medium and Antioxidant-plus groups. The embryos in light exposed groups and the embryos in non-exposed group were cultured for 96 h. Every 24 h developmental rate was evaluated under an inverted microscope. Expanded blastocysts in each group were then randomly divided into two groups: some were removed for differential staining and the remaining blastocysts were cultured for another 24 h to assess their hatching and plating competence
Data collection and statistical analysis
The difference between developmental rates in NE-ctr embryos and E-ctr embryos was compared by χ2 test. The developmental rates of exposed embryos in various groups were also statistically analyzed by χ2 test. The number of ICM, TE and total cell number were expressed as mean ± SEM. Differences in blastomere numbers between the NE-ctr and E-ctr groups were analyzed by t-test. Blastomere number was also analyzed by Kruskal-Wallis and Mann-Whitney U tests in various groups of exposed embryos. A difference with p < 0.05 was considered statistically significant.
Results
In vitro development of embryos at days 2–4
Non-exposed embryos showed a significantly (p < 0.05) higher developmental rate compared with E-ctr at every stage of development. Exposed embryos in each group including Co-c, Cndm, Aopm, and E-ctr were cultivated for 96 h. The developmental rates in various groups are shown in Table 1. No significant difference (p > 0.05) was observed regarding developmental rate at each stage within the 172, 195, 198 and 188 embryos in the E-ctr, Co-c, Cndm, and Aopm groups respectively (Table 1).
Table 1.
Development of 2-cell embryos following exposure to visible light and 96 h cultivation in various groups
| Groups | No. of embryos | At 48 h | At 72 h | At 96 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eight-cell | Morulae | Total | Morulae | Early blastocyst | Total | Expanded blastocyst | Hatching blastocyst | Total | ||
| NE-ctr | 104 | 48(46.1) | 24(23.1) | 72(69.2)** | 18(17.3) | 47(45.2) | 65(62.5)* | 41(39.4) | 20(19.2) | 61(58.6)* |
| E-ctr | 172 | 52(30.2) | 11(6.3) | 63(36.6) | 37(21.5) | 22(12.8) | 59(34.3) | 54(31.4) | 19(11.0) | 73(42.4) |
| Co-c | 195 | 55(28.2) | 14(7.2) | 69(35.4) | 37(19.0) | 41(21.0) | 78(40.0) | 64(32.8) | 29(14.9) | 93(47.7) |
| Cndm | 198 | 70(35.3) | 11(5.5) | 81(40.1) | 28(14.1) | 47(23.8) | 75(37.9) | 58(29.3) | 38(19.2) | 96(48.5) |
| Aopm | 188 | 57(30.3) | 7(3.7) | 64(34.0) | 45(23.9) | 25(13.3) | 70(37.2) | 47(25.0) | 27(14.4) | 74(39.4) |
Data are pooled from at least six independent experiments. Values in parenthesis are percentage. In each column, values with * and ** are significantly different from E-ctr group; *, P < 0.05; **, P < 0.001. NE-ctr = Non-exposed control, E-ctr = Exposed control, Co-c = Co-culture, Cndm = Conditioned medium, Aopm = Antioxidant plus medium.
Hatching and plating efficacy of the embryos
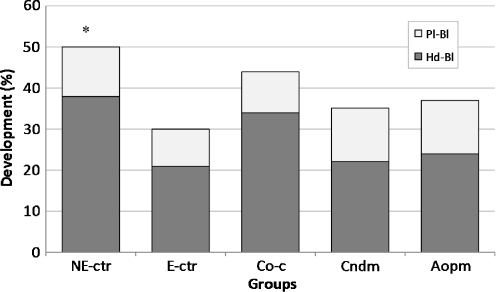
Two hundred and fifty-six expanded blastocysts in various groups (31, 45, 63, 65 and 49 blastocysts in the NE-ctr, E-ctr, Co-c, Cndm and Aopm groups respectively) were cultivated for 24 h in different treatment protocols to assess the hatching and plating ability of embryos in different groups. Embryos in the NE-ctr group (53%) had significantly (P < 0.05) greater hatching and plating capacity than those in the E-ctr group (30%). The hatching and plating capacity was also higher in the Co-C (43%), Aopm (37%) and Cndm (35%) groups than the E-ctr (30%) group, but the differences were not significant (Fig. 2).
Fig. 2.
In-vitro-produced early blastocysts were cultured for 24 h in different conditions. Development (%) to hatched blastocyst and plated blastocyst was evaluated. NE-ctr = non-exposed control, E-ctr = Exposed control, Co-c = Co-culture, Cndm = Conditioned medium, Aopm = Antioxidant-plus medium, Hd-bl = Hatched blastocyst, Pl-bl = plated blastocyst. *, significantly (P < 0.05) different from the E-ctr group
Differential cell count
Expanded blastocysts were stained with PI and Hoechst. The inner cell mass, trophectoderm and total cell numbers were counted under a fluorescent microscope. Thirty, 28, 29, 31 and 25 expanded blastocysts were analyzed in the NE-crt, E-ctr, Co-c, Cndm and Aopm groups, respectively. The ICM and total cell numbers were significantly (p < 0.05) higher in the Co-c and Aopm groups compared with the E-ctr group. There were no significant differences between the Co-c and Aopm groups. The number of TE cells was also significantly (p < 0.05) higher in the Co-c group compared with the E-ctr group (Fig. 3). The ICM/total cells ratio was 34.7, 31.5, 33.4, 32.5 and 33.9 in the NE-crt, E-ctr, Co-c, Cndm and Aopm groups, respectively. The ICM/total cells ratio, TE/total cells ratio and ICM/TE ratio were comparable among the groups.
Fig. 3.
Embryos were cultured in different groups to achieve expanded blastocysts. Groups of expanded blastocysts were stained with PI (red) and Hoechst (blue) fluorescent dyes, and the inner cells, the trophectoderm and total cell number were evaluated. Bars are representative of mean ± SD. NE-ctr = non-exposed control, E-ctr = Exposed control, Co-c = Co-culture, Cndm = Conditioned medium, Aopm = Antioxidant-plus medium. Bars with an * above them are significantly (P < 0.05) different from the E-ctr group
Discussion
The present study was undertaken to compare the effect of antioxidant supplementation, co-culture with human umbilical cord mesenchymal cells, and the use of a conditioned medium on embryo development and blastomere number following exposure to visible light. Exposure of two-cell mouse embryos to 1600 l× visible light for 30 min significantly decreased embryo development at each time point compared with non-exposed embryos. These findings are consistent with previous studies that have shown a reduction in embryo and zygote development and oocyte survival following exposure to visible light emitted from different sources [1, 2]. However, exposure of embryos to visible light did not significantly change the cleavage rate in comparison with non-exposed embryos. Also, the proportions of ICM, TE and total blastomeres were comparable between the various groups of exposed embryos. However, the cleavage rate was higher in co-cultured embryos followed by the antioxidant-treated group and the conditioned medium group. The cleavage rate has been proposed as a useful indicator of the rate of embryo survival and pregnancy [21, 22]. Although the ICM/total cell number and ICM/TE ratio were comparable among treatments, plating efficacy and hatching capacity were non-significantly higher in the Co-c group followed by the Aopm and Cndm groups. These results are consistent with the results of blastomere numbers in the different groups that were exposed to visible light. Cheng et al. showed that hatching mouse blastocysts have a greater implantation capacity than expanded blastocysts [22].The transfer of embryos after treatment in various conditions will optimize the ability of embryos to implant in in vivo conditions.
The present results demonstrated that antioxidant supplementation is comparable with co-culture of embryos with hUCM feeder cells and also with conditioned medium in terms of the developmental rate, and is as efficient as co-culturing with hUCMs when blastomere number is considered. Reactive oxygen species are a normal product of aerobic cellular metabolism but environmental factors including visible light can produce additional ROS in living cells. The production of excess ROS in living organisms leads to DNA mutations [23] and apoptosis via oxidative stress [24]. Under normal metabolic conditions cells are exposed to ROS but endogenous antioxidants, either enzymatic (e.g. catalase, glutathione peroxidase) or non-enzymatic (e.g. vitamins E, C), scavenge the ROS produced before they damage biological molecules. A balance between ROS and the amount of antioxidants is essential for the normal development of embryos especially in the laboratory. Numerous studies have demonstrated the antioxidant effects of vitamin C on living cells [25]. Vitamin C was also used to prevent cytogenetic damage to the embryos that were produced by breeding irradiated male and female mice [26]. Some reports have confirmed that the addition of antioxidants during cryopreservation also improved subsequent embryo development. They demonstrated that ascorbate as a water-soluble antioxidant can reduce the level of hydrogen peroxide [27], which is commonly elevated during the process of cryopreservation. The addition of ascorbate to the in vitro maturation medium of porcine oocytes protected them against oxidative stress and improved the in vitro fertilization rate [28]. In our study, vitamin C and β-ME protected mouse embryos against ROS produced by the exposure of embryos to visible light. Our results showed that the addition of antioxidants to the embryo culture media increased the number of ICM and TE compared with the control. However, supplementation of culture medium with a single dose of vitamin C did not affect blastocyst formation or blastomere number, while a divided supplement of vitamin C yielded a higher number of blastocysts [29]. In the present study we used 50 mM vitamin C because higher concentrations (<200 mM) of vitamin C produced toxicity in the mouse embryo [30]. Species variations and the nutritional requirements of different embryos may have led to the observed differences. The effect of β-ME on living cells is associated with the biosynthesis of intracellular glutathione (GSH), which protects cells against deleterious effects that are followed by oxidative injuries [15]. The simultaneous use of these two antioxidants in our experiments resulted in a higher number of blastomeres in treated embryos compared to the control (E-ctr group). Whether the application of vitamin C or β-ME alone could rescue embryos from the harmful effects of visible light requires further investigation. Mechanisms other than the protection of embryos against excessive ROS may also be involved in the higher blastomere number in the Aopm group compared with the control.
The results of the present study demonstrate that the use of human umbilical cord mesenchymal cells as feeder cells could enhance embryo quality following exposure to visible light by increasing the number of blastomeres. The accumulation of superoxide radicals is involved in apoptotic cell death and a decrease in embryo blastomere number. The developmental rate of embryos in the Co-c group was non-significantly higher than that of other exposed groups, but it was comparable with developmental rates in the Aopm and Cndm groups. It seems that by removing free radicals from the culture medium and/or secreting embryotrophic components into the culture medium hUCMs prepare appropriate conditions for embryo development. hUCM cells are among the myofibroblast family of cells. These cells propagate rapidly in the laboratory and have some similarities with embryonic and adult stem cells [31], and also contribute to cell and tissue regulation through the secretion of growth factors, chemokines, and cytokines [32]. In the Cndm group in which the supernatant of hUCMs was used as a conditioned medium, the developmental rate was higher than in the control (E-ctr) but the blastomere number was lower than in the Co-c and Aopm groups. This indicates that secreted or added growth factors may show a lower ability to protect embryos against ROS, and that trying to scavenge ROS from embryo culture media by means of radical scavengers such as chelators or antioxidants is more useful. Some investigators claim that co-culture systems may remove toxic components such as heavy metal divalent cations and metabolic inhibitors from the culture medium [33], and some reported somatic cells contain transcripts encoding for the main antioxidant enzymes, and allow the equilibrium between antioxidants and ROS to be maintained [17]. Feeder cells may improve the developmental competence of embryos during culture in the laboratory but the disadvantages of using somatic cells have limited their application especially in human culture media [18, 34]. On the other hand, supplementation of culture media with antioxidants increased the cleavage rate of in vitro cultured embryos at a rate that was comparable with the utilization of hUCMs as feeder cells. Since the blastomere number of early embryos has a great impact on the implantation and pregnancy rate [35], supplementation of embryo culture media with antioxidants may lead to a higher pregnancy rate, especially when embryos encounter suboptimal conditions. The optimum concentration of antioxidants, and the appropriate time and sequence for supplementation of culture media with antioxidants and their impact on the implantation rate requires further research.
Conclusion
Our results demonstrate the beneficial effects of antioxidants on embryo survival, development and the cleavage rate under suboptimal conditions. Because ROS are produced during the exposure of embryos to visible light, we can also conclude that antioxidants protect the embryos against cellular damage that commonly results from excessive ROS. The same effect was achieved by co-culture of embryos with human umbilical cord mesenchymal cells.
Acknowledgments
The authors thank members of the Parasitology Department at the Afzalipour School of Medicine, Kerman University of Medical Sciences and the following people: P. Salehinejad from the School of Nursing for her laboratory assistance and F. Moshkdanian for help in editing the manuscript.
Footnotes
Capsule Antioxidants improved the cleavage rate of mouse embryos that were exposed to suboptimal conditions, at a comparable rate to the co-culture system.
References
- 1.Nematollahi-mahani SN, Pahang H, Moshkdanian G, Nematollahi-mahani A. Effect of embryonic fibroblast cell co-culture on development of mouse embryos following exposure to visible light. J Assist Reprod Genet. 2009;26(2–3):129–35. doi: 10.1007/s10815-008-9290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proc Natl Acad Sci USA. 2007;104(36):14289–93. doi: 10.1073/pnas.0706687104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkind MM. Sedimentation of DNA released from Chinese hamster cells. Biophys J. 1971;11(6):502–20. doi: 10.1016/S0006-3495(71)86231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavi R, Shainberg A, Friedmann H, Shneyvays V, Rickover O, Eichler M, Kaplan D, Lubart R. Low energy visible light induces reactive oxygen species generation and stimulates an increase of intracellular calcium concentration in cardiac cells. J Biol Chem. 2003;278(42):40917–22. doi: 10.1074/jbc.M303034200. [DOI] [PubMed] [Google Scholar]
- 5.Guerin P, Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7(2):175–89. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 6.Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003;18(11):2270–4. doi: 10.1093/humrep/deg450. [DOI] [PubMed] [Google Scholar]
- 7.McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil. 2000;118(1):163–70. doi: 10.1530/reprod/118.1.163. [DOI] [PubMed] [Google Scholar]
- 8.Sturmey RG, Leese HJ. Energy metabolism in pig oocytes and early embryos. Reproduction. 2003;126(2):197–204. doi: 10.1530/rep.0.1260197. [DOI] [PubMed] [Google Scholar]
- 9.Gardiner CS, Reed DJ. Glutathione redox cycle-driven recovery of reduced glutathione after oxidation by tertiary-butyl hydroperoxide in preimplantation mouse embryos. Arch Biochem Biophys. 1995;321(1):6–12. doi: 10.1006/abbi.1995.1361. [DOI] [PubMed] [Google Scholar]
- 10.Lapointe S, Sullivan R, Sirard MA. Binding of a bovine oviductal fluid catalase to mammalian spermatozoa. Biol Reprod. 1998;58(3):747–53. doi: 10.1095/biolreprod58.3.747. [DOI] [PubMed] [Google Scholar]
- 11.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300(2):535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 12.Rose RC, Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J. 1993;7(12):1135–42. [PubMed] [Google Scholar]
- 13.Tao Y, Chen H, Tian NN, Huo DT, Li G, Zhang YH, Liu Y, Fang FG, Ding JP, and Zhang XR. Effects of L-ascorbic acid, alpha-tocopherol and co-culture on in vitro developmental potential of porcine cumulus cells free oocytes. Reprod Domest Anim. 45(1):19–25. [DOI] [PubMed]
- 14.Otoi T, Koyama N, Yamamoto K, Horikita N, Tachikawa S, Suzuki T. Developmental competence of frozen-thawed blastocysts from fair-quality bovine embryos cultured with beta-mercaptoethanol. Vet J. 2000;159(3):282–6. doi: 10.1053/tvjl.1999.0408. [DOI] [PubMed] [Google Scholar]
- 15.Caamano JN, Ryoo ZY, Thomas JA, Youngs CR. beta-mercaptoethanol enhances blastocyst formation rate of bovine in vitro-matured/in vitro-fertilized embryos. Biol Reprod. 1996;55(5):1179–84. doi: 10.1095/biolreprod55.5.1179. [DOI] [PubMed] [Google Scholar]
- 16.Hosseini SM, Forouzanfar M, Hajian M, Asgari V, Abedi P, Hosseini L, Ostadhosseini S, Moulavi F, Safahani Langrroodi M, Sadeghi H, Bahramian H, Eghbalsaied S, Nasr-Esfahani MH. Antioxidant supplementation of culture medium during embryo development and/or after vitrification-warming; which is the most important? J Assist Reprod Genet. 2009;26(6):355–64. doi: 10.1007/s10815-009-9317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouatassim S, Guerin P, Menezo Y. Mammalian oviduct and protection against free oxygen radicals: expression of genes encoding antioxidant enzymes in human and mouse. Eur J Obstet Gynecol Reprod Biol. 2000;89(1):1–6. doi: 10.1016/S0301-2115(99)00169-4. [DOI] [PubMed] [Google Scholar]
- 18.Nematollahi-Mahani SN, Nematollahi-Mahani A, Moshkdanian G, Shahidzadehyazdi Z, Labibi F. The role of co-culture systems on developmental competence of preimplantation mouse embryos against pH fluctuations. J Assist Reprod Genet. 2009;26:597–604. doi: 10.1007/s10815-009-9363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghaee-afshar M, Rezazadehkermani M, Asadi A, Malekpour-afshar R, Shahesmaeili A, Nematollahi-mahani SN. Potential of human umbilical cord matrix and rabbit bone marrow— derived mesenchymal stem cells in repair of surgically incised rabbit external Anal sphincter. Dis Colon Rectum. 2009;52(10):1753–61. doi: 10.1007/DCR.0b013e3181b55112. [DOI] [PubMed] [Google Scholar]
- 20.Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online. 2001;3(1):25–9. doi: 10.1016/S1472-6483(10)61960-8. [DOI] [PubMed] [Google Scholar]
- 21.Lane M, Gardner DK. Selection of viable mouse blastocysts prior to transfer using a metabolic criterion. Hum Reprod. 1996;11(9):1975–8. doi: 10.1093/oxfordjournals.humrep.a019527. [DOI] [PubMed] [Google Scholar]
- 22.Cheng TC, Huang CC, Huang LS, Chen CI, Lee MS, Liu JY. Evaluation of mouse blastocyst implantation rate by morphology grading. Chin J Physiol. 2004;47(1):43–7. [PubMed] [Google Scholar]
- 23.Lutsenko EA, Carcamo JM, Golde DW. Vitamin C prevents DNA mutation induced by oxidative stress. J Biol Chem. 2002;277(19):16895–9. doi: 10.1074/jbc.M201151200. [DOI] [PubMed] [Google Scholar]
- 24.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–63. doi: 10.1016/S0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 25.Schneider M, Diemer K, Engelhart K, Zankl H, Trommer WE, Biesalski HK. Protective effects of vitamins C and E on the number of micronuclei in lymphocytes in smokers and their role in ascorbate free radical formation in plasma. Free Radic Res. 2001;34(3):209–19. doi: 10.1080/10715760100300201. [DOI] [PubMed] [Google Scholar]
- 26.Mozdarani H, Nazari E. Cytogenetic damage in preimplantation mouse embryos generated after paternal and parental gamma-irradiation and the influence of vitamin C. Reproduction. 2009;137(1):35–43. doi: 10.1530/REP-08-0073. [DOI] [PubMed] [Google Scholar]
- 27.Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod. 2002;17(10):2686–93. doi: 10.1093/humrep/17.10.2686. [DOI] [PubMed] [Google Scholar]
- 28.Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-alpha-glucoside during in vitro maturation. Biol Reprod. 2001;65(6):1800–6. doi: 10.1095/biolreprod65.6.1800. [DOI] [PubMed] [Google Scholar]
- 29.Hossein MS, Hashem MA, Jeong YW, Lee MS, Kim S, Kim JH, Koo OJ, Park SM, Lee EG, Park SW, Kang SK, Lee BC, Hwang WS. Temporal effects of alpha-tocopherol and L-ascorbic acid on in vitro fertilized porcine embryo development. Anim Reprod Sci. 2007;100(1–2):107–17. doi: 10.1016/j.anireprosci.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Falcone T, Attaran M, Goldberg JM, Agarwal A, Sharma RK. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocyst development rate. Fertil Steril. 2002;78(6):1272–7. doi: 10.1016/S0015-0282(02)04236-X. [DOI] [PubMed] [Google Scholar]
- 31.Nematollahi-mahani SN, Rezazadeh-kermani M, Latifpour M, Salehinejad P. Biological and biochemical characteristics of human umbilical cord mesenchymal cells. J Reprod Infertil. 2009;10(1):8. [Google Scholar]
- 32.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277(1 Pt 1):C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 33.Tsai FC, Gardner DK. Nicotinamide, a component of complex culture media, inhibits mouse embryo development in vitro and reduces subsequent developmental potential after transfer. Fertil Steril. 1994;61(2):376–82. doi: 10.1016/s0015-0282(16)56534-0. [DOI] [PubMed] [Google Scholar]
- 34.Kattal N, Cohen J, Barmat LI. Role of coculture in human in vitro fertilization: a meta-analysis. Fertil Steril. 2008;90(4):1069–76. doi: 10.1016/j.fertnstert.2007.07.1349. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Lu C, Lin G, Gong F, Lu G. The number of blastomeres in post-thawing embryos affects the rates of pregnancy and delivery in freeze-embryo-transfer cycles. J Assist Reprod Genet. 2009;26(11–12):569–73. doi: 10.1007/s10815-009-9360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]