Abstract
In India, more than 104 species of Myxobolus are recorded infecting freshwater and marine fishes. During our study on the myxozoan parasites of fishes of Punjab wetlands, India, two new myxosporean species, Myxobolus ropari sp. nov. and Myxobolus kanjali sp. nov. were recorded from mucous membrane around gill lamellae and scales of Cirrhina mrigala (Ham.), respectively. Spores of the first species, M. ropari sp. nov. measure 12.58 × 4.5 μm in size, elongately pyriform, slender in shape with a pointed anterior end and a rounded posterior end. Polar capsules are two, equal, elongately pyriform, measuring 4.96 × 1.50 μm in size, placed posteriorly from the tip of the spore running parallel to each other. Spores of the second species, M. kanjali sp. nov. measure 9.5 × 7.7 μm in size, spherical in shape with rounded anterior and posterior extremities. Polar capsules are two, equal, broadly pyriform with a blunt anterior and a rounded posterior end measuring 4.8 × 1.8 μm in size. A prominent tubular structure originate from the anterior end of one of the polar capsule and extend backward beyond the margin of the spore body and run upwards to join the posterior end of the other polar capsule.
Keywords: Freshwater fishes, Kanjali wetland, Myxobolus, Ropar wetland
Introduction
Classification of the phylum Myxozoa have a long history since the discovery of Myxosporea by Jurine (1825) and subsequent observations by Muller (1841). This actually began when Butschli (1882) classified Myxosporida (along with Sarcosporida) as a subclass of the then class Sporozoa. Smothers et al. (1994) made phylogenetic analysis of the first myxozoan based on SSU rDNA confirming the marginalized suppositions of earlier authors (Stolc 1899; Weill 1938) that myxozoa are multicellular organisms and placed myxozoans within the metazoan.
Myxosporeans are the abundant and diversified group of parasites infecting freshwater and marine fishes. They cause production loss and death, and some fish have to be discarded because they are unsightly and are not considered to be fit for human consumption. Up till now, phylum Myxozoa include four malacosporean and 2,180 myxosporean species to a total of 62 genera (Lom and Dykova 2006). Recently, a new genus Thelohanelloid bengalensis gen. nov. sp. nov. from gall bladder of Arius sagor (a marine fish in Bay of Bengal) has been described by Sarkar (2009). The genus, Myxobolus is predominant having more than 744 species reported (Eiras et al. 2005). Kalavati and Nandi (2007) have reported the existence of 104 myxobolid species from Indian species. Recently, Kaur and Singh (2008; 2009a,b; 2010a,b; 2010/2011; and 2011) reported ten new species infecting Indian major carps in wetlands of Punjab. During the present study on the fishes of Ropar and Kanjali wetlands of Punjab (India), a total number of 85 fishes belonging to Cirrhina mrigala were examined. The present communication describes two new species M. ropari sp. nov. and M. kanjali sp. nov. collected from gill lamellae and scales, respectively. The description has been prepared in accordance with the guidelines of Lom and Arthur (1989).
Materials and methods
Plasmodia from gills and scales of infected Cirrhina mrigala (Ham.) were smeared on clean slides in a drop of 0.98% Nacl solution covered with cover slip and were examined for the presence of spores. Fresh spores were treated with 8% KOH solution for the extrusion of polar filaments. For permanent preparation, air-dried smears fixed in Bouins fixative were stained with Ziehl-Neelsen and Iron-haematoxylin. Drawings were made from stained material with the aid of camera lucida. Measurements (based on 15–20 fresh spores treated with Lugol’s iodine solution) of spores were done under oil immersion with the aid of a calibrated ocular micrometer. All measurements are in microns (μm) as range values followed by mean ±SD in parentheses. The abbreviations used in the paper are as follows: LS length of spore; WS width of spore; LPC length of polar capsule; WPC width of polar capsule; ICP intercapsular process; TS thickness of shell valves; NC number of coils of polar filaments; SD standard deviation.
Results and discussion
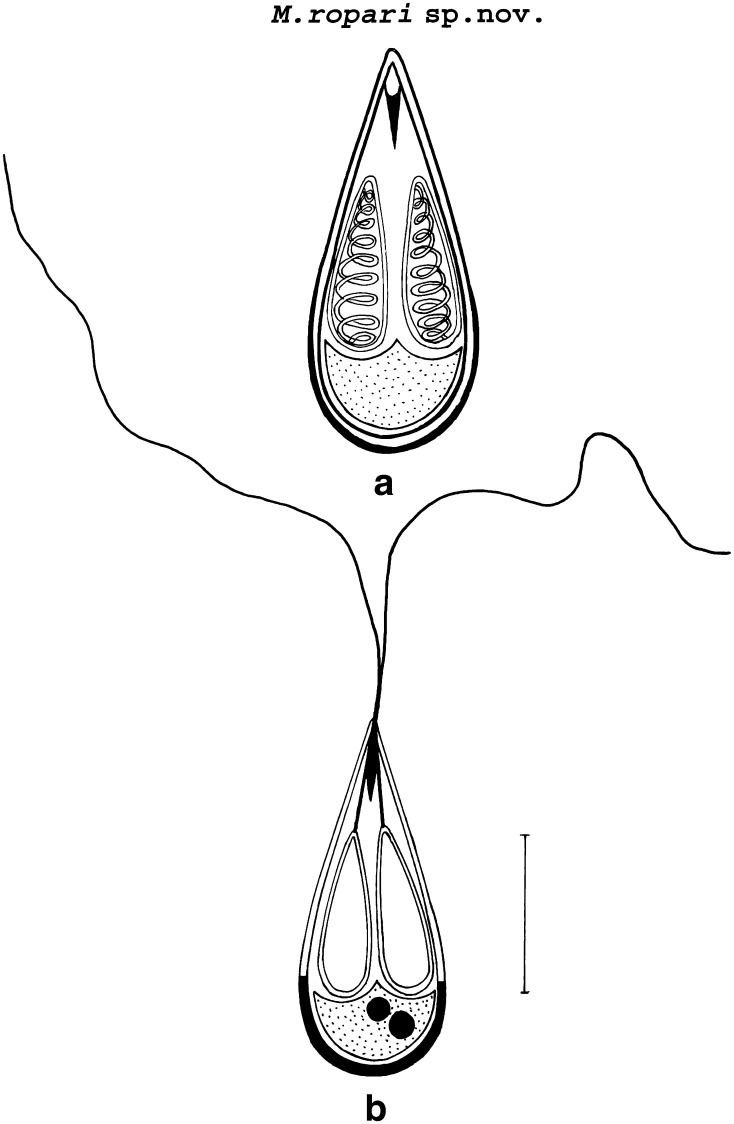
Myxobolus ropari sp. nov. (Figs. 1, 3, 5b, Table 1).
Fig. 1.
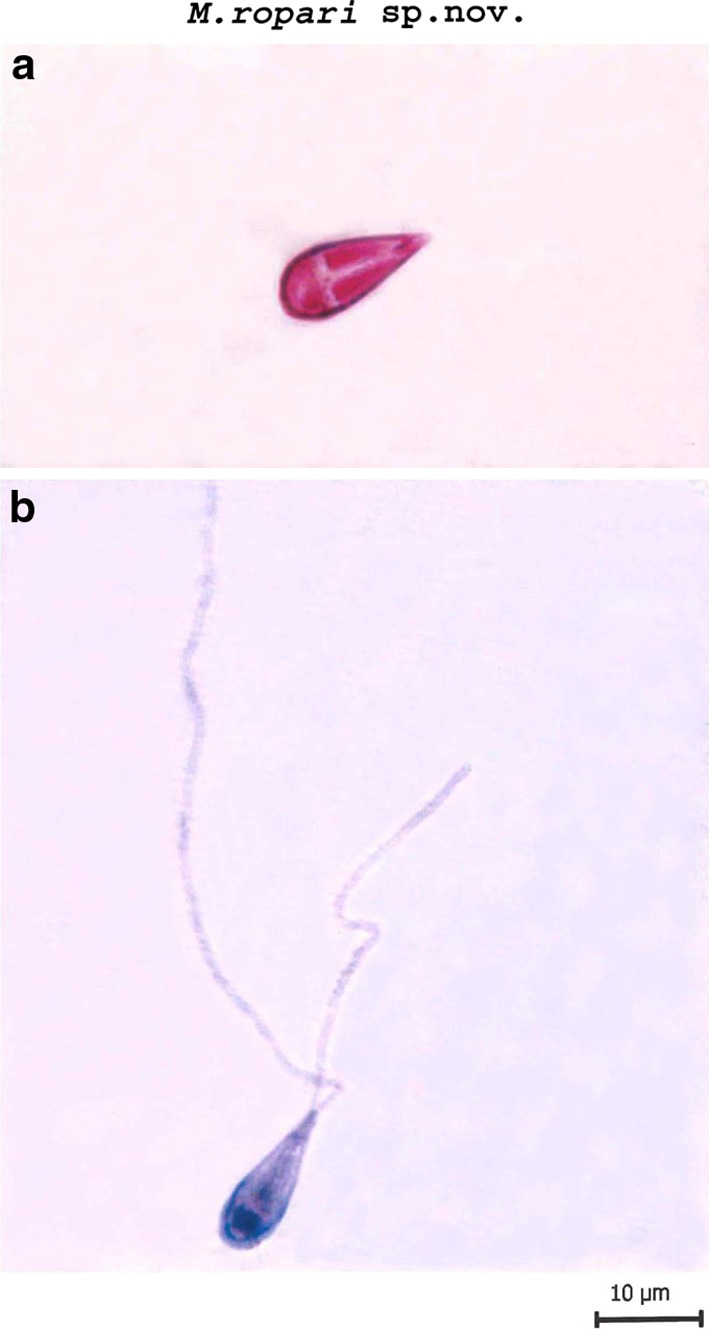
a Spore stained in Zieh1-Neelsen b Spore stained in Iron-haematoxylin (extruded polar filaments)
Fig. 3.
a Spore stained in Zieh1-Neelsen (valvular view) b Spore stained in Iron-haematoxylin (extruded polar filaments) scale bar 0.005 mm
Fig. 5.
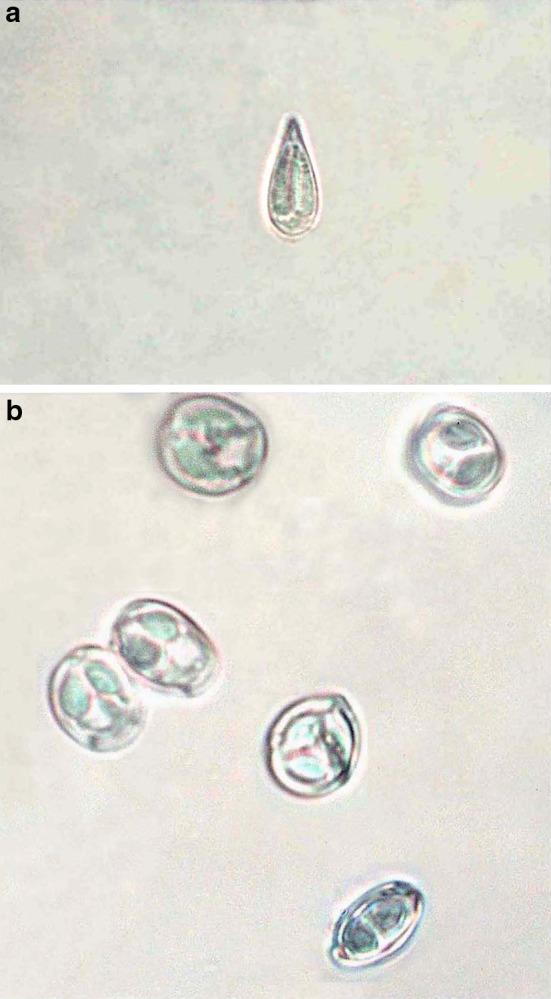
a Fresh spore of M. ropari sp. nov. b Fres spores of M. kanjali sp. nov.
Table 1.
Comparative description of M. ropari sp. nov. with morphologically similar species (measurements are in micrometers)
| Species | Host | Site of infection | Locality | Spore | Polar capsule |
|---|---|---|---|---|---|
| M. ropari sp. nov. (present study) | Cirrhina mrigala | Gill filaments | Ropar wetland, Punjab (India) | 12.58 × 4.5 | 4.96 × 1.50 |
| M. macrocapsularis Reuss 1906 | Blicca bjoerkna | Gills | Russia | 9.0–14.5 × 6.0–9.9 | 5.0–8.6 × 2.4–3.6 |
| M. aureatus Ward 1919 | Notropis anogenus | Fin | USA | 12.4–13.5 × 6.5–7.5 | 6.0–7.5 |
| M. angustus Kudo 1934 | Cliola vigilax | Gills | USA | 14.0–15.0 × 7.0–8.0 | 8.0–9.5 × 2.5–3 |
| M. calbasui Chakravarty 1939 | C. mrigala | Gall bladder | India | 12.4–15.0 × 8.2–10.0 | 6.18 × 4.12 and 4.12 × 3.09 |
| M. mrigalae Chakravarty 1939 | C. mrigala | Scales | India | 7.2–8.2 | 5.15 × 3.09 and 3.09 × 2.06 |
| M. catlae Chakravarty 1943 | C. mrigala | Gills | India | 14.5–16.5 × 6.18 | 10.3–12.36 × 2.06–3.01 |
| M. indicum Tripathi 1952 | C. mrigala | Muscles, liver, intestinal wall | India | 9.5–10.8 × 7.5–8.2 | 2.7–3.6 and 1.8 |
| M. rewensis Srivastava 1979 | C. mrigala | Scales | India | 9.66 × 8.05 | 4.8 × 3.2 |
| M. carnaticus Seenappa and Manohar 1980 | C. mrigala | Gills | India | 8.6 × 6.8 | 3.8 × 2.0 and 2.1 × 1.5 |
| M. vanivilasae Seenappa and Manohar 1980 | C. mrigala | Beneath muscles, scales integument | India | 8.0–10.0 × 7.0–9.0 | 3.57 × 2.57 |
| M. hosadurgensis Seenappa and Manohar 1981 | C. mrigala | Gills, muscles | India | 10.5 × 6.25 | 5.37 × 2.3 and 3.3 × 1.43 |
| M. shetti Seenappa and Manohar 1981 | C. mrigala | Gills | India | 8.8 × 7.4 | 3.4 × 2.3 |
| M. vedavatiensis Seenappa and Manohar 1981 | C. mrigala | Gills | India | 13.8 × 9.2 | 6.2 × 3.4 and 3.9 × 2.6 |
| M. venkateshi Seenappa and Manohar 1981 | C. mrigala | Gills | India | 9.75 × 7.15 | 5.25 × 2.0 |
| M. mathuri Jayasri et al. 1981 | Puntius saranae | Gills | India | 8.7–23.5 × 5.1–10.1 | 2.7–11.9 × 1.8–4.6 and 2.7–7.8 and 1.8–4.6 |
| M. pseudokoi Li and Desser 1985 | Notropis cornutus | Gills, skin | Canada | 13.5 × 6.5 | 6.5 × 2.5 |
| M. indirae (Kundu 1985) Gupta and Khera 1988 | C. mrigala | Scales of tail fin | India | 12.6 × 9.6 | 4.7 × 2.2 |
| M. trichogasteri (Sarkar 1985) Gupta and Khera 1988 | Trichogaster fasciatus | Gall bladder | India | 15.5 × 9.4 | 10.1 × 3.3 |
| M. haldari Gupta and Khera 1989 | C. mrigala | Fin, gills | India | 9.31 × 7.95 | 4.31 × 2.97 and 2.95 × 1.98 |
| M. yogindrai (Tripathi 1952) Landsberg and Lom 1991 | C. mrigala | Inner side of scales | India | 9.0–9.5 × 7.2 | 2.8 × 3.6 |
| M. longisporus Nie and Li 1992 | Cyprinus carpio | Gills | China | 16.0–17.5 × 6.5–7.0 | 7.5–8.2 × 2.0 |
| M. kribiensis Fomena and Bouix 1994 | Brycinus longispinnis | Skin, eye-sclera | Cameroon | 21.2 × 9.5 | 16.1 × 15.4 |
| M. orissae Haldar et al. 1997 | C. mrigala | Gills | India | 15.71 × 6.8 | 8.8 × 1.78 and 7.58 × 2.57 |
| M. maculates Casal et al.2002 | Metynnis maculates | Kidney | Brazil | 21.0 × 8.9 | 12.7 × 3.2 |
| M. ophthalmasculata Basu and Haldar 2002 | C. mrigala | Eye-muscles | India | 13.13 × 8.04 | 5.47 × 3.06 and 3.03 × 1.99 |
| M. rocatlae Basu and Haldar 2002 | Catla catla x L. rohita | Gills, gut | India | 18.5 × 5.9 | 12.9 × 2.8 |
| M. catmriglae Basu and Haldar 2004 | Catla catla x C. migala | Gill lamellae | India | 18.8 × 5.9 | 11.9 × 2.5 |
| M. bilobus Cone et al.2005 | Notemigonus crysoleucas | Gill filaments | Canada | 21.0 × 8.4 | 10.8 × 2.7 and 10.1 × 2.3 |
| M. shuleensis Eiras et al. 2005 | Pseudorasbora parva | Gills | China | 16.1 × 9.0 | 7.1 × 3.0 |
| M. naini Kaur and Singh 2008 | C. mrigala | Gills | India | 12.9 × 8.2 | 4.9 × 3.1 and 3.33 × 1.63 |
| M. eirasi Kaur and Singh 2009a | C. mrigala | Gills | India | 8.6 × 6.7 | 3.2 × 1.57 |
| M. leptobarbi Szekely et al.2009 | Leptobarbus hoevenii | Muscles | Malaysia | 16.0 × 8.9 | 10.5 × 3.0 and 9.9 × 3.0 |
| M. slendrii Kaur and Singh 2009b | C. mrigala | Gills | India | 14.87 × 3.4 | 5.74 × 1.48 |
| M. mehlhorni Kaur and Singh 2011 | C. mrigala | Gill lamellae (mucous membrane) | India | 8.9 × 6.8 | 3.7 × 2.5 and 2.6 × 1.5 |
Plasmodia
Small, microscopic and present in the mucous membrane around gill lamellae. 5–7 spores were present per plasmodium.
Description
LS: 12.58 ± 0.56 (12.18–12.98)
WS: 4.5 ± 0.70 (4.0–5.0)
LPC: 4.96 ± 0.14 (4.86–5.06)
WPC: 1.50 ± 0.56 (1.10–1.90)
Ratio: LS/WS = 2.7
ICP: Medium-sized
NC: 9–10
Parietal folds: absent
TS: 0.58
Spores
Histozoic, elongately pyriform and slender in shape having a pointed anterior end and a rounded posterior end. Shell valves are smooth and symmetrically thin but posterior end of the spore appear thicker (which stains dark blue with Heidenhains Iron-haematoxylin) than the rest on the spore body. Parietal folds are absent. Two equal polar capsules, elongately pyriform in shape, placed posteriorly from tip of the spore and run parallel to each other. Polar capsules are pointed anteriorly and rounded posteriorly. A medium-sized intercapsular process is present at the anterior end of spore. Sporoplasm is agranular and homogenous occupying entire extracapsular space behind the polar capsules. An iodinophilous vacuole is absent. Sporoplasmic nuclei two, each measuring 1.5–1.6 μm in diameter.
Taxonomic summary of M. ropari sp. nov.
| Type host | Cirrhina mrigala (Ham.) vern mrigal |
| Type locality | Ropar wetland, Punjab (India) |
| Type specimen | Paratypes are spores stained in Ziehl-Neelsen and Iron-haematoxylin, slide no.CM/H/ZN/23.05.2009 and CM/H/IH 23.05.2009 deposited in the museum of Department of Zoology, Punjabi University, Patiala (Punjab), India. Pin code-147002 |
| Site of infection | Gills |
| Prevalence of infection | 30/55(54.5%) |
| Etymology | The species epithet ropari is named after the name of type locality “Ropar wetland” |
Discussion
The present species was compared with M. macrocapsularis Reuss 1906 from gills of Blicca bjoerkna; M. aureatus Ward 1919 from fin of Notropis anogenus; M. angustus Kudo 1934 from gills of Cliola vigilax; M. calbasui Chakravarty 1939 from gall bladder of C. mrigala; M. mrigalae Chakravarty 1939 from scales of C. mrigala; M. catlae Chakravarty 1943 from gills of C. mrigala; M. indicum Tripathi 1952 from muscles, liver, intestinal wall of C. mrigala; M. rewensis Srivastava 1979 from scales of C. mrigala; M. carnaticus Seenappa and Manohar 1980 from gills of C. mrigala; M. vanivilasae Seenappa and Manohar 1980 from beneath muscles, scales integument of C. mrigala; M. hosadurgensis Seenappa and Manohar 1981 from gills, muscles of C. mrigala; M. shetti Seenappa and Manohar 1981 from gills of C. mrigala; M. vedavatiensis Seenappa and Manohar 1981 from gills of C. mrigala; M. venkateshi Seenappa and Manohar 1981 from gills of C. mrigala; M. pseudokoi Li and Desser 1985 from gills, skin of Notropis cornutus; M. indirae (Kundu 1985) Gupta and Khera 1988 from scales of tail fin of C. mrigala; M. trichogasteri (Sarkar 1985) Gupta and Khera 1988 from gall bladder of Trichogaster fasciatus; M. haldari Gupta and Khera 1989 from fin, gills of C. mrigala; M. yogindrai (Tripathi 1952) Landsberg and Lom 1991 from inner side of scales of C. mrigala; M. longisporus Nie and Li 1992 from gills of Cyprinus carpio; M. kribiensis Fomena and Bouix 1994 from skin, eye-sclera of Brycinus longispinnis; M. orissae Haldar et al. 1997 from gills of C. mrigala; M. maculates Casal et al. 2002 from kidney of Metynnis maculates; M. ophthalmasculata Basu and Haldar 2002 from eye-muscles of C. mrigala; M. rocatlae Basu and Haldar 2002 from gills, gut of Catla catla x L. rohita; M. catmriglae Basu and Haldar 2004 from gill lamellae of Catla catla x C. migala; M. bilobus Cone et al. 2005 from gill filaments of Notemigonus crysoleucas; M. shuleensis Eiras et al. 2005 from gills of Pseudorasbora parva; M. naini Kaur and Singh 2008 from gills of C. mrigala; M. eirasi Kaur and Singh 2009a from gills of C. mrigala; M. leptobarbi Szekely et al.2009 from muscles of Leptobarbus hoevenii; M. slendrii Kaur and Singh 2009b from gills of C. mrigala; and M. mehlhorni Kaur and Singh 2011 from gill lamellae (mucous membrane) of C. mrigala but differ from all of the above species in morphometric characteristics.
The present species show much similarity in having slender (LS/WS = 2.7) shape with M. longisporus (LS/WS = 2.4), M. maculates (LS/WS = 2.3), M. bilobus (LS/WS = 2.5), M. kribiensis (LS/WS = 2.2), M. rocatlae (LS/WS = 3.1), M. trichogasteri (LS/WS = 1.6), M. catmriglae (3.18), and M. catlae (LS/WS = 2.5) but differ in having a medium-sized intercapsular process at the anterior end and in having elongately pyriform polar capsules placed parallel in the middle of the spore cavity.
Furthermore, the present species resemble M. macrocapsularis (LS/WS: 1.4) in having slender shape and an intercapsular process, however, differ in having much wider spores as indicated above by comparing LS/WS ratios (indicated in parenthesis). In addition, the shell valves in the present species appear thicker at the posterior end of spore than the rest on the spore body. However, similar thickened posterior shell valves has also been reported in M. mathurii Jayasri et al. 1981 and M. slendrii Kaur and Singh (2009b).
Based on above differences cited, we hereby propose species under study as new to the science and named it as M. ropari sp. nov. through this communication.
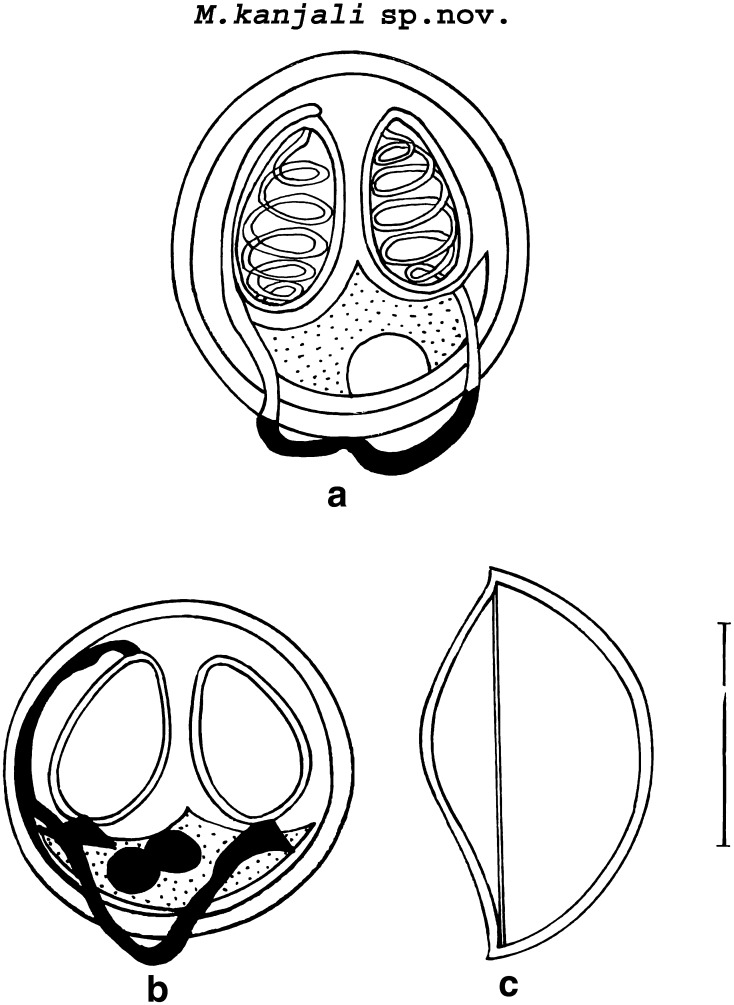
M. kanjali sp. nov. (Figs. 2, 4, 5b; Table 2).
Fig. 2.
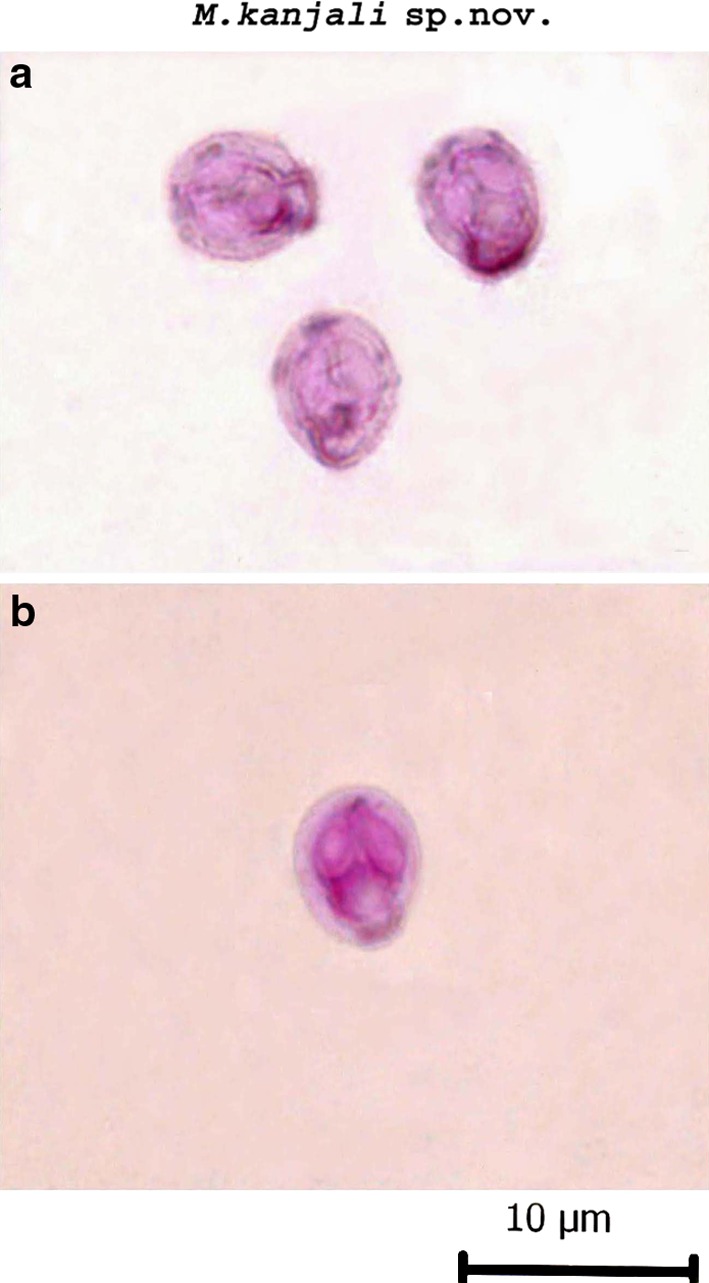
a, b Spore stained in Zieh1-Neelsen (showing tubular structure)
Fig. 4.
a Spore stained in Zieh1-Neelsen (valvular view) b Spore stained in Iron-haematoxylin c Spore in side view scale bar 0.005 mm
Table 2.
Comparative description of M. kanjali sp. nov. with morphologically similar species (measurements are in micrometers)
| Species | Host | Site of infection | Locality | Spore | Polar capsule |
|---|---|---|---|---|---|
| M. kanjali sp. nov. (present study) | Cirrhina mrigala | Scales | Kanjali wetland, Punjab (India) | 9.5 × 7.7 | 4.8 × 1.8 |
| M. pfeifferi Thelohan 1895 | Barbus barbus | Muscles, gills, kidney, spleen, body cavity | Basin of Amur rivers | 10.0–13.0 × 9.0–12.2 | 5.0–5.7 |
| M. calbasui Chakravarty 1939 | C. mrigala | Gall bladder | India | 12.4–15.0 × 8.2–10.0 | 6.18 × 4.12 and 4.12 × 3.09 |
| M. mrigalae Chakravarty 1939 | C. mrigala | Scales | India | 7.2–8.2 | 5.15 × 3.09 and 3.09 × 2.06 |
| M. catlae Chakravarty 1943 | C. mrigala | Gills | India | 14.5–16.5 × 6.18 | 10.3–12.36 × 2.06–3.01 |
| M. nemacheili Weiser 1949 | Nemacheilus barbatulus | Head connective tissue | Czech Republic | 9.0–11.0 × 8.0–9.0 | 5.0 × 2.0 |
| M. indicum Tripathi 1952 | C. mrigala | Muscles, liver, intestinal wall | India | 9.5–10.8 × 7.5–8.2 | 2.7–3.6 and 1.8 |
| M. amurensis Akhmerov 1960 | Cyprinus carpio haematopterus | Fin, gut | Amur basin | 9.0–13.5 × 9.0–12.5 | 4.5–7.0 × 3.8–4.2 |
| M. sprostoni Shulman 1962 | Silurus europius | Gut serosa | Amur basin | 11.0–13.0 × 10.0–11.7 | 5.5–7.5 × 3.5–4.0 |
| M. musajevi Kandilov 1963 | Varicorhinus capoeta | Gills | Caucasus | 11.5–14.0 × 10.0–11.0 | 6.0–7.0 × 3.3–5.0 |
| M. rewensis Srivastava 1979 | C. mrigala | Scales | India | 9.66 × 8.05 | 4.8 × 3.2 |
| M. carnaticus Seenappa and Manohar 1980 | C. mrigala | Gills | India | 8.6 × 6.8 | 3.8 × 2.0 and 2.1 × 1.5 |
| M. exsulatus Pugachev 1980 | Abramis brama, Catostomus catostomus | Gills | Siberia | 9.7–9.9 × 9.0–9.1 | 5.4–5.6 × 3.0 |
| M. vanivilasae Seenappa and Manohar 1980 | C. mrigala | Beneath muscles, scales integument | India | 8.0–10.0 × 7.0–9.0 | 3.57 × 2.57 |
| M. hosadurgensis Seenappa and Manohar 1981 | C. mrigala | Gills, muscles | India | 10.5 × 6.25 | 5.37 × 2.3 and 3.3 × 1.43 |
| M. shetti Seenappa and Manohar 1981 | C. mrigala | Gills | India | 8.8 × 7.4 | 3.4 × 2.3 |
| M. vedavatiensis Seenappa and Manohar 1981 | C. mrigala | Gills | India | 13.8 × 9.2 | 6.2 × 3.4 and 3.9 × 2.6 |
| M. venkateshi Seenappa and Manohar 1981 | C. mrigala | Gills | India | 9.75 × 7.15 | 5.25 × 2.0 |
| M. kuleminae Donec in Shulman 1984 | Aspius aspius, Leuciscus leucisucs | Muscles, heart | Ukraine | 15.0–19.5 × 12.0–15.0 | 7.0–9.0 × 4.0–5.0 |
| M. indirae (Kundu 1985) Gupta and Khera 1988 | C. mrigala | Scales of tail fin | India | 12.6 × 9.6 | 4.7 × 2.2 |
| M. haldari Gupta and Khera 1989 | C. mrigala | Fin, gills | India | 9.31 × 7.95 | 4.31 × 2.97 and 2.95 × 1.98 |
| M. crucifilus (synonyms Gyrosporacrucifilus Qadri 1962) Landsberg and Lom 1991 | Labeo fimbriatus | Gills | India | 9.0–10.0 × 8–8.5 | 4.0–4.5 |
| M. salmonis (Hoshina 1949) Landsberg and Lom 1991 | Oncorhynchus keta | Lower side of scales | Russia | 8.2–10.4 × 7.4–9.5 | 3.6–5.8 × 2.1–3.4 |
| M. yogindrai (Tripathi 1952) Landsberg and Lom 1991 | C. mrigala | Inner side of scales | India | 9.0–9.5 × 7.2 | 2.8 × 3.6 |
| M. orissae Haldar et al. 1997 | C. mrigala | Gills | India | 15.71 × 6.8 | 8.8 × 1.78 and 7.58 × 2.57 |
| M. ophthalmasculata Basu and Haldar 2002 | C. mrigala | Eye muscle | India | 13.13 × 8.04 | 5.47 × 3.06 and 3.03 × 1.99 |
| M. balantiocheili Levsen et al. 2004 | Balantiocheilos melanopterus | Central nervous system | Thailand | 12.3 × 10.0 | 5.7 × 3.6 |
| M. naini Kaur and Singh 2008 | C. mrigala | Gills | India | 12.9 × 8.2 | 4.9 × 3.1 and 3.33 × 1.63 |
| M. eirasi Kaur and Singh 2009a | C. mrigala | Gills | India | 8.6 × 6.7 | 3.2 × 1.57 |
| M. slendrii Kaur and Singh 2009b | C. mrigala | Gills | India | 14.87 × 3.4 | 5.74 × 1.48 |
| M. mehlhorni Kaur and Singh 2011 | C. mrigala | Gill lamellae (mucous membrane) | India | 8.9 × 6.8 | 3.7 × 2.5 and 2.6 × 1.5 |
Plasmodia
Large, white colored, 4–5 in number and present all over the scales. They measure 1–2 mm in size. 10–15 spores were present per plasmodium.
Description
LS: 9.5 ± 0.28 (9.3–9.7)
WS: 7.7 ± 0.42 (7.4–8.0)
LPC: 4.8 ± 0.56 (4.4–5.2)
WPC: 1.8 ± 0.28 (1.6–2.0)
Ratio: LS/WS = 1.2
ICP: absent
NC: 6–7
Parietal folds: absent
TS: 0.4–0.5
Spores
Histozoic, spherical in shape with rounded anterior and posterior extremities. Shell valves are smooth and symmetrically thin. Parietal folds are absent. Polar capsules are two, equal, broadly pyriform with blunt anterior and rounded posterior ends. An intercapsular process is absent. Both polar capsule converge towards anterior end and are placed at a distance posteriorly occupying nearly half of the spore body cavity. Sporoplasm is agranular, half-moon shaped and homogenous occupying entire extracapsular space behind the polar capsules. Two capsulogenic nuclei measuring 1.17 μm in diameter are present. Sporoplasm contain two nuclei and an iodinophilous vacuole measuring 1.175–1.181 μm and 2.1–4.1(3.1 ± 1.37) μm in diameter, respectively. A prominent tubular structure originate from anterior end of one of the polar capsule and extend backward beyond the margin of the spore body and run upwards to join the posterior end of the other polar capsule.
Taxonomic summary of M. kanjali sp. nov.
| Type host | Cirrhina mrigala (Ham.) vern mrigal |
| Type locality | Kanjali wetland, Punjab (India) |
| Type specimen | Paratypes are spores stained in Ziehl-Neelsen and Iron-haematoxylin, slide no.CM/l/ZN/24.05.2009 and CM/l/IH 24.05.2009 deposited in the museum of Department of Zoology, Punjabi University, Patiala (Punjab), India. Pin code-147002 |
| Site of infection | Scales |
| Prevalence of infection | 22/30(73.3%) |
| Etymology | The species epithet kanjali has been given after the name of type locality “Kanjali wetland” |
Discussion
The present species was compared with M. pfeifferi Thelohan 1895 from muscles, gills, kidney, spleen, body cavity of Barbus barbus; M. calbasui Chakravarty 1939 from gall bladder of C. mrigala; M. mrigalae Chakravarty 1939 from scales of C. mrigala; M. catlae Chakravarty 1943 from gills of C. mrigala; M. nemacheili Weiser 1949 from head connective tissue of Nemacheilus barbatulus; M. indicum Tripathi 1952 from muscles, liver, intestinal wall of C. mrigala; M. amurensis Akhmerov 1960 from fin, gut of Cyprinus carpio haematopterus; M. sprostoni Shulman 1962 from gut serosa of Silurus europius; M. musajevi Kandilov 1963 from gills of Varicorhinus capoeta; M. rewensis Srivastava 1979 from scales of C. mrigala; M. carnaticus Seenappa and Manohar 1980 from gills of C. mrigala; M. exsulatus Pugachev 1980 from gills of Abramis brama, Catostomus catostomus; M. vanivilasae Seenappa and Manohar 1980 from beneath muscles, scales integument of C. mrigala; M. hosadurgensis Seenappa and Manohar 1981 from gills, muscles of C. mrigala; M. shetti Seenappa and Manohar 1981 from gills of C. mrigala; M. vedavatiensis Seenappa and Manohar 1981 from gills of C. mrigala; M. venkateshi Seenappa and Manohar 1981 from gills of C. mrigala; M. kuleminae Donec in Shulman 1984 from muscles, heart of Aspius aspius, Leuciscus leucisucs; M. indirae (Kundu 1985) Gupta and Khera 1988 from scales of tail fin of C. mrigala; M. haldari Gupta and Khera 1989 from fin, gills of C. mrigala; M. crucifilus (synonyms Gyrosporacrucifilus Qadri 1962) Landsberg and Lom 1991 from gills of Labeo fimbriatus; M. salmonis (Hoshina 1949) Landsberg and Lom 1991 from lower side of scales of Oncorhynchus keta; M. yogindrai (Tripathi 1952) Landsberg and Lom 1991 from inner side of scales of C. mrigala; M. orissae Haldar et al. 1997 from gills of C. mrigala; M. ophthalmasculata Basu and Haldar 2002 from eye muscle of C. mrigala; M. balantiocheili Levsen et al. 2004 from central nervous system of Balantiocheilos melanopterus; M. naini Kaur and Singh 2008 from gills of C. mrigala; M. eirasi Kaur and Singh 2009a from gills of C. mrigala; M. slendrii Kaur and Singh 2009b from gills of C. mrigala and M. mehlhorni Kaur and Singh 2011 from gill lamellae (mucous membrane) of C. mrigala, however, differ from above species in morphometric characteristics.
In the present species, spores are spherical having rounded anterior and posterior ends without parietal folds. An intercapsular process is absent. A prominent tubular structure originate from the anterior end of one of the polar capsule and extend backwards beyond the margin of the spore body and run upwards to join the posterior end of the other polar capsule. In this respect, the present species is comparable with M. crucifilus in which a thread-like structure has also been reported in the sporoplasm which join the posterior ends of each polar capsule directly (without any prominent bodies) and to M. eirasi having a band-like structure running in its sporoplasm originating from one rounded body to join the second rounded body present just beneath the other polar capsule (laterally). However, the spores of M. crucifilus and M. eirasi are pyriform, with more narrower and bluntly curved posterior end in the former and anterior end broader with posterior end bluntly rounded in the later. The thread-like structure/band in both the above mentioned species are confined to sporoplasm only unlike in the present species in which the tubular structure extend backwards beyond the margin of the spore body and runs upwards to join the posterior end of the other polar capsule.
On the basis these difference, we propose the species under study as new to the science and named it as M. kanjali sp. nov. through this communication.
References
- Akhmerov AK. Myxosporidia of fishes of the Amur River basin. Rybn Khoz Vnutr Vodoemov Latv SSSR. 1960;5:239–308. [Google Scholar]
- Basu S, Haldar DP. Observations on three new species of Myxobolus Butschli, 1882 from hybrid carps of West Bengal, India. Indian J Environ Ecoplan. 2002;6(3):629–640. [Google Scholar]
- Basu S, Haldar DP. Description of three new species (Myxozoa: Myxosporea: Bivalvulida) of the genera Myxobilatus Davis, 1944 and Myxobolus Butschli, 1882. Acta Protozool. 2004;43:337–343. [Google Scholar]
- Butschli O (1882) Myxosporidia. In: Borns Klass Ordnungen, vol 1. Protozoa pp 590–603
- Casal G, Matos E, Azevedo C. Ultrastructural data on the spore of Myxobolus maculates n sp. (phylum Myxozoa), parasites from the Amazonian fish Metynnis maculates (Teleostei) Dis Aquat Org. 2002;51:107–111. doi: 10.3354/dao051107. [DOI] [PubMed] [Google Scholar]
- Chakravarty M. Studies on Myxosporidia from the fishes of Bengal, with a note on the myxosporidea infection in aquarium fishes. Arch Protistenkd. 1939;92:169–178. [Google Scholar]
- Chakravarty M. Studies on myxosporidia from the common food fishes of Bengal. Proc Indian Acad Sci. 1943;18:21–35. [Google Scholar]
- Cone DK, Yang J, Sun G, Easy R. Taxonomy and molecular phylogeny of Myxobolus bilobus n. sp. (Myxozoa) parasitizing Notemigonus crysoleucas (Cyprinidae) in Algonquin Park, Ontario, Canada. Dis Aquat Org. 2005;66(3):227–232. doi: 10.3354/dao066227. [DOI] [PubMed] [Google Scholar]
- Eiras JC, Molnar K, Lu YS. Synopsis of the species of Myxobolus Butschli, 1882 (Myxozoa: Myxosporea: Myxobolidae) Syst Parasitol. 2005;61:1–46. doi: 10.1007/s11230-004-6343-9. [DOI] [PubMed] [Google Scholar]
- Fomena A, Bouix G. New myxosporidia species from freshwater water teleosts in Southern Cameroon (Central Africa) J Afr Zool. 1994;108:481–491. [Google Scholar]
- Gupta S, Khera S (1988) Review of the genus Myxobolus Butschli, 1882. Res Bull (Sci) 39(I-II): 45–48
- Gupta S, Khera S. Observations on Myxobolus haldari sp. nov. (Myxozoa: Myxosporea) from freshwater fishes of North India. Res Bull Sci. 1989;40:281–291. [Google Scholar]
- Haldar DP, Samal KK, Mukhopadhyay D. Studies in the protozoan parasites of fishes in Orissa: five new species of the genera Henneguya, Thelohanellus and Unicauda (Myxozoa: Bivalvulida) J Beng Nat Hist Soc. 1997;16(2):50–63. [Google Scholar]
- Hoshina T. On a new myxosporidian parasite of the genus Myxosoma, M salmonis n. sp., infecting on the scales of the dog salmon. Seibutsu. 1949;4:106–109. [Google Scholar]
- Jayasri M, Paryateesam M, Mathur PN. Myxosoma mathurii n sp. (Protozoa: Myxosporidia) parasitic on Puntius sarana (Ham.) Curr Sci. 1981;50:736–739. [Google Scholar]
- Jurine L (1825) Histoire abregee des poissons du lac Léman. Soc Phys Geneve Mem 3. vu, 32
- Kalavati C, Nandi NC. Handbook of Myxosporidean parasites of Indian fishes. Kolkata: ZSI; 2007. p. 293. [Google Scholar]
- Kandilov NK. New species of myxosporidia occurring in Varicorhinus capoeta of the river Kura basin Izvestiya Akademii Nauk Azerbaidzhanskoi SSR. Seriya Biolgicheskikh I Meditsinskikh Nauk. 1963;6:75–76. [Google Scholar]
- Kaur H, Singh R (2008) Observation on one new species of the genus Myxobolus (Myxozoa: Myxosporea: Bivalvulida) and Redescription of Myxobolus magauddi (Bajpai, 1981) Landsberg and Lom, 1991 recorded from freshwater fishes of Kanjali Wetland of Punjab (India). Proc Natl Congr Parasitol 75–79
- Kaur H, Singh R. A new myxosporean species, Myxobolus eirasi sp. nov. and a known species M. venkateshi Seenappa and Manohar, 1981 from the Indian major carp fish Cirrhina mrigala (Ham.) Protistology. 2009;6(2):126–130. [Google Scholar]
- Kaur H, Singh R. One new myxosporidian species, Myxobolus slendrii sp. nov., and one known species, M. punjabensis Gupta and Khera, 1989, infecting freshwater fishes in wetlands of Punjab, India. Parasitol Res. 2009;106(5):1043–1047. doi: 10.1007/s00436-010-1746-9. [DOI] [PubMed] [Google Scholar]
- Kaur H, Singh R (2010/2011) Two new species of Myxobolus (Myxosporea, Bivalvulida) from the Indian major carp Labeo rohita Hamilton, 1822. Protistology 6(4): 264–270
- Kaur H, Singh R, et al. A new myxosporean species Myxobolus sclerii sp. nov. and one known species M. stomum Ali et al. (2003) from two Indian major carp fishes. J Parasit Dis. 2010;34(1):33–39. doi: 10.1007/s12639-010-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Singh R (2010b) Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) infecting Indian freshwater fishes in Punjab Wetlands (India). Parasitol Res. doi 10.1007/s00436-011-2307-6 [DOI] [PubMed]
- Kaur H, Singh R (2011) Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) from freshwater fishes of Punjab Wetlands (India). J Parasit Dis. doi 10.1007/s12639-011-0024-9 [DOI] [PMC free article] [PubMed]
- Kudo R. Studies on some protozoan parasites of fishes of Illinois. Ill Biol Monogr. 1934;13:7–44. [Google Scholar]
- Kundu TK. Myxosoma indirae sp n. (Myxozoa: Myxosomatidae) from the head cartilage, scale and tail fin of Cirrhina mrigala. Acta Protozool. 1985;24:333–337. [Google Scholar]
- Landsberg JH, Lom J. Taxonomy of the genera of the Myxobolus/Myxosoma group (Myxobolidae: Myxosporea), current listing of species and revision of synonyms. Syst Parasitol. 1991;18:155–186. doi: 10.1007/BF00009358. [DOI] [Google Scholar]
- Levsen A, Alvik T, Grotmol S. Neurological symptoms in tricolor shark minnow Balantiocheilos melanopterus associated with Myxobolus balantiocheili n sp. infecting the central nervous system. Dis Aquat Org. 2004;59:135–140. doi: 10.3354/dao059135. [DOI] [PubMed] [Google Scholar]
- Li L, Desser SS. The protozoan parasites of fish from two lakes in Algonquin Park, Ontario. Can J Zool. 1985;63:1846–1858. doi: 10.1139/z85-275. [DOI] [Google Scholar]
- Lom J, Arthur JR. A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis. 1989;12:151–156. doi: 10.1111/j.1365-2761.1989.tb00287.x. [DOI] [Google Scholar]
- Lom J, Dykova I. Myxozoan genera: Definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol. 2006;53:1–36. [PubMed] [Google Scholar]
- Muller J. Uber Psorospermien. Arch Anat Physiol Wissensch Med. 1841;5:477–496. [Google Scholar]
- Nie DS, Li LX. On the myxosporidians of freshwater fishes from Lake Huama, Hubei Province II. Descriptions of new species (Myxosporea: Bivalvulida) Acta Zootaxon Sinica. 1992;17:133–150. [Google Scholar]
- Pugachev ON. Parasitic fauna of the sucker (Catostomus catostomus) from the Kolima River. Parazitologiya. 1980;14:511–513. [Google Scholar]
- Qadri SS. New myxosporidia from Indian freshwater fish Labeo fimbriatus I. Gyrospora crucifila gen. n. sp. n. Z Parasitenkd. 1962;21:513–516. doi: 10.1007/BF00260256. [DOI] [PubMed] [Google Scholar]
- Reuss H. Neue Myxosporidien von Susswasserfischen. Bull Acad Imp Sci. 1906;25:199–205. [Google Scholar]
- Sarkar NK. Some Coelozoic Myxosporida (Myxozoa: Myxosporea) from a freshwater water teleost fish of River Padma. Acta Protozool. 1985;24(1):47–53. [Google Scholar]
- Sarkar NK (2009) Thelohanelloid bengalensis gen. and sp. nov. (Myxosporea: Thelohanellidae) from the gall bladder of marine catfish of the Bay of Bengal, India. UP J Zool 29(2):251–254
- Seenappa D, Manohar L. Myxobolus vanivilasae n sp. parasitic in Cirrhina mrigala (Ham.) Proc Indian Acad Sci. 1980;89:485–491. doi: 10.1007/BF03179134. [DOI] [Google Scholar]
- Seenappa D, Manohar L. Five new species of Myxobolus (Myxosporea: Protozoa), parasitic in Cirrhina mrigala (Hamilton) and Labeo rohita (Hamilton), with a note on a new host record for M. curmucae Seenappa and Manohar, 1980. J Protozool. 1981;28:358–360. [Google Scholar]
- Shulman SS. Myxosporidia. In: Pavlovskii EN, editor. Key to parasites of freshwater fish of USSR. Leningrad: Publishing House of the Academy of Sciences of the USSR; 1962. pp. 47–130. [Google Scholar]
- Shulman SS. Parasitic Protozoa. In: Baeur ON, editor. Key to parasites of freshwater fishes of the USSR. Leningrad: Nauka Publishers; 1984. pp. 1–428. [Google Scholar]
- Smothers JF, Dohlen CD, Smith LH, Spall RD. Molecular evidence that the myxozoan protist are metazoan. Science. 1994;265:1719–1721. doi: 10.1126/science.8085160. [DOI] [PubMed] [Google Scholar]
- Srivastava SP. A new species of Myxobolus from scales of Cirrhina mrigala (Hamilton) Sci Cult. 1979;45:444–445. [Google Scholar]
- Stolc A. Actinomyxidia, eine neue Gruppe der Mesozoa, der Myxosporidien verwandt. Abh Bohm Ges Wissensch. 1899;8:1–12. [Google Scholar]
- Szekely C, Shaharom-Harrison F, Cech G, Mohamed K, Molnar K. Myxozoan infections in fishes of the Tasik Kenyir water resevoir, Terengganu, Terengganu, Malaysia. Dis Aquat Org. 2009;83:37–48. doi: 10.3354/dao01991. [DOI] [PubMed] [Google Scholar]
- Thelohan P. Recherches sur les Myxosporidies. Bull Sci France Belgique. 1895;26:100–365. [Google Scholar]
- Tripathi YR. Studies on the parasite of Indian fishes I. Protozoa. Myxosporidia together with a checklist of parasitic protozoa described from Indian fishes. Rec Indian Mus. 1952;50:63–88. [Google Scholar]
- Ward HB. Notes on North American myxosporidia. J Parasitol. 1919;6:49–64. doi: 10.2307/3270895. [DOI] [Google Scholar]
- Weill R. L ‘interpretation des Cnidosporidies et Ia valeur taxonomique de Ieur cnidome Leur cycle compare ii Ia phase Iarvaire des Narcomeduses Cuninides. Travaux de la Station Zoologique de Wimereaux. 1938;13:727–744. [Google Scholar]
- Weiser J. Parasites of freshwater fish II. Vestnık Ceskoslovenske Spolecnosti Zoologicke. 1949;13:364–371. [Google Scholar]