Abstract
There is a delicate balance between the host, pathogen and environment. Aquatic organisms, including shellfish, respond directly to climatic changes in their biological environment as their metabolic processes are influenced by temperature, salinity, and oxygen levels. Certain environmental conditions are more conducive to diseases than others among which water temperature is significantly associated with disease outbreak. The present study showed that Peneaus monodon of Sundarbans serve as a host for many protozoan parasites and epibionts including ciliates, gregarines and microsporidia. The protozoan parasites also require a particular environmental condition for their maximum growth and survival. The intensity of infection significantly increases with rise in temperature (P < 0.05) following a definite trend but no significant relationship between infection rate of ciliates and pH of water. In case of gregarine parasites significance (P < 0.05) exists among infection rate and temperature as well as pH of the farm water. Microsporidian parasites do not follow any significant seasonal trend in infecting the host P. monodon.
Keywords: Penaeus monodon, Environmental stress, Parasitic prevalence, Vorticella sp., Gregarines, Microsporidia
Introduction
Black tiger shrimp Peneaus monodon serve as the host for wide range of protozoan parasites and commensals. Protozoan parasites and commensals occur both inside and outside the host body. Peritrichous ciliates like Zoothamnium sp. and Voritcella sp. are ecto-commensals and microsporidia and gregarines are endo-commensals.
The ectosymbionts peritrichous ciliates remain attached to the gills and limbs. A possible relationship between the peritrich ciliate, Zoothamnium sp., and mortality of host following stress was discussed previously (Overstreet 1973). Their abundant presence can interfere with the breathing and mobility of the host. The Zoothamnium infections are at their peak during monsoon and post-monsoon months and these infections appear to be governed directly by salinity of the habitat and indirectly by the rainfall (Jayasree et al. 2001).
Gregarine belonging to the phylum Apicomplexa, class Sporozoea, order Eugregarinida Leger, 1900 are all pathogenic although they cause some local damage and occlude passage in the inhibited organ with serious results (Sprague and Couchi 1971). They reside in the gut of the decapods crustaceans and may lead to reduced absorption of food or occasional intestinal blockage and possibly mortality of their host (Lightner 1933). Two types of gregarines, Nematopsis sp. and Cephalolobus sp. are encountered in penaeid shrimps (Bower et al. 1994; Prema and Janardan 1990). Invasion with gregarinids vary with temperature. The infection rate with gregarinids is high in summer than winter (Timofeev 2001)
Microsporidians belonging to the phylum Microspora, class Microsporea, order Microsporida are another type of obligate, intercellular parasites of shrimps and prawns (Johny et al. 2006). They can infect a wide range of invertebrate and vertebrate taxa (Didier et al. 2000) but almost half of the described species have insect as the host (Becnel and Andreadis 1999). They may infect gut, hepatopancreas, muscle, reproductive tissue, and nervous tissue (Walker and Hinsch 1972; Becnel and Andreadis 1999; Solter and Becnel 2000). There is no seasonal trend in the prevalence of the parasite in crustaceans (Childers et al. 1996).
Materials and methods
Sampling
The present study was done during the period of March 2008 to February 2009. Live shrimps Peneaus monodon were randomly sampled from the adjacent fish markets around the bheries (local brackishwater fishery comprises impounding the fry and juveniles of fishes and prawns in embanked tidal flats, saline swamps, and paddy fields) of Kharibari (22.70 N, 88.45 E) and Baduria (22.74 N, 88.79 E) of 24 Parganas (North), West Bengal.
Isolation of pathogens
Animals were dissected and scrapings from gills, intestine, and vital organs like hepatopancreas, gonads were taken on clean slides. The smeared slides were air dried, fixed in acetone free methanol and stained with Giemsa. The slides were examined under microscope to observe the protozoan parasites. From the monthly collected data, variations in the prevalence of infection by each species of parasites were determined.
Estimation of physicochemical parameters of water
Water samples were collected from the bheries in 500 ml polypropylene bottles for analysis of physico–chemical parameters of farm water like dissolved oxygen, pH, salinity etc. Dissolved oxygen of water was estimated by Winkler’s method while pH was determined in the field by pH meter. Water temperature was measured in the field using thermometer.
Statistical analysis
Relationship between parasitic intensities and physico-chemical parameters of water were determined by calculating correlation coefficient (r) and the statistical significance of the relationship was determined by employing student’s t-test. The level of significance was set at P = 0.05 (5% level of significance).
Results
Protozoans belonging to three groups namely epibiontic peritrichous ciliates, gut dwelling gregarines and muscle dwelling microsporidians were encountered from the sampled and examined hosts, P. monodon.
Relationship between water quality parameters and ciliate parasites
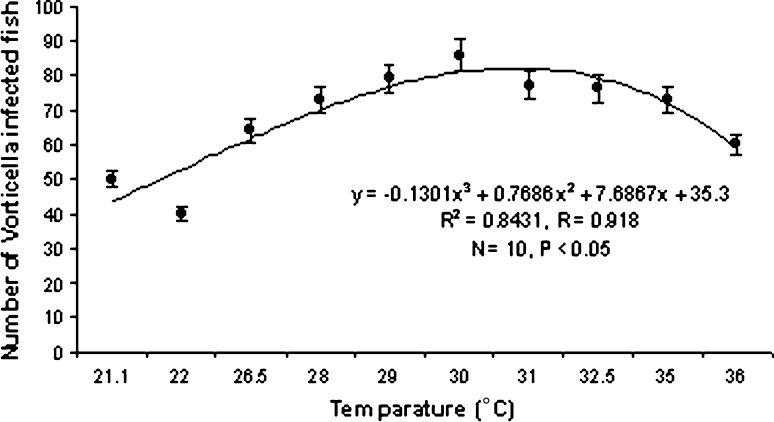
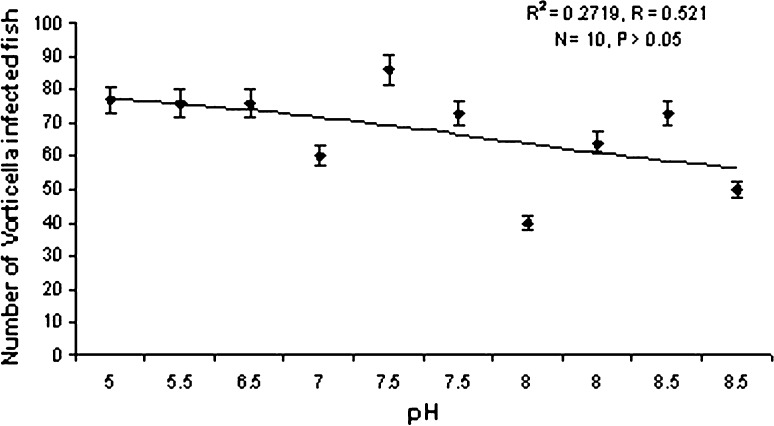
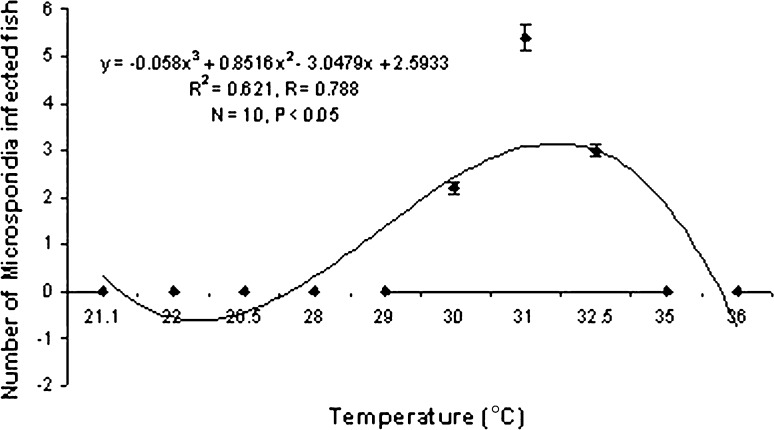
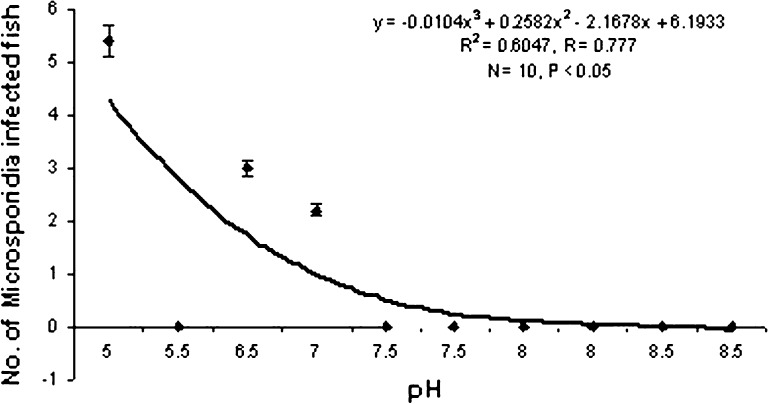
Ciliates isolated from the infected hosts belonging to the genus Vorticella (Fig. 1) measuring 9–18.5 μm in length and 3.5–5 μm in width. They were frequently found on the gills and occasionally appendages. The intensity of infection significantly increases with rise in temperature of the water (P < 0.05) following a definite trend (Graph 1). It becomes highest during the month of July i.e. 76% of the P. monodon were infected. So the prevalence of infection is positively correlated (r = +0.918) with water temperature. No significant relationship was found between the rate of infection and pH of water (Graph 2).
Fig. 1.
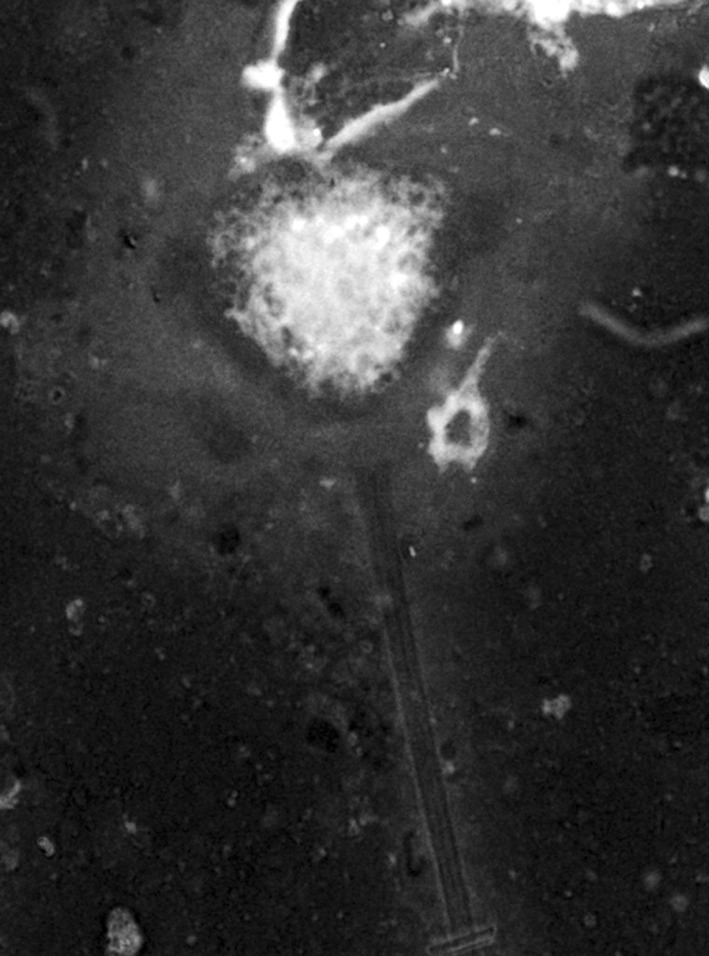
Vorticella sp. isolated from gill of P. monodon
Graph 1.
Correlation between Temperature and Vorticella infection
Graph 2.
Correlation between pH and Vorticella infection
Relationship between water quality parameters and gregarine parasites
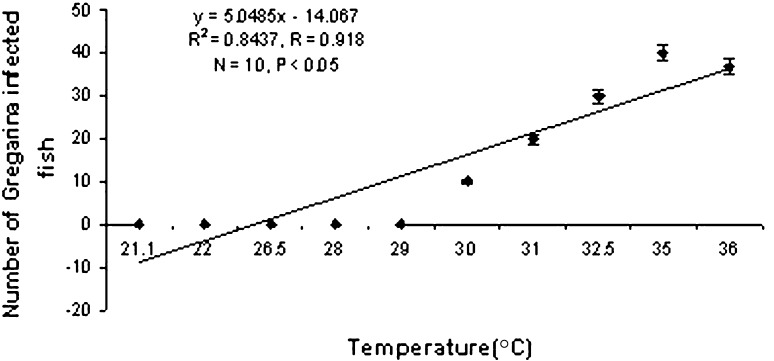
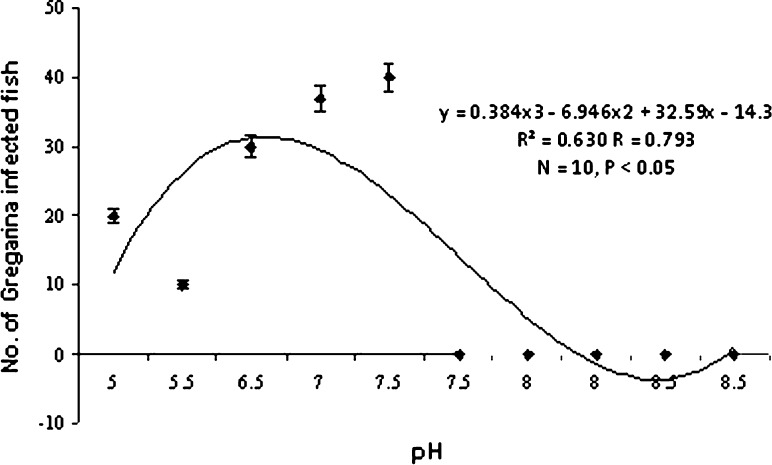
Gregarines found from the gut are segmented, the body was divisible into three parts,epimerite,protomerite,duotomerite. The gregarines remain attached with the gut by dint of protomerite, i.e. first part of the gregarines (Fig. 2). Chakraborti and Bandyopadhyay 2010 described a new species of the genus Nematopsis from tiger prawns of Sundarbans. It was observed that 40% of the hosts were infected with gregarine parasites. Highest infection was observed during April. The parasite follows a seasonal pattern of infection varying significantly (P < 0.05) with temperature (Graph 3) and pH (Graph 4) of the water. Heavy infection was recorded in the pre-monsoon or summer months i.e. from April to June when water pH ranges from 6.5 to 7.5. Thus it shows a negative correlation with pH of water.
Fig. 2.
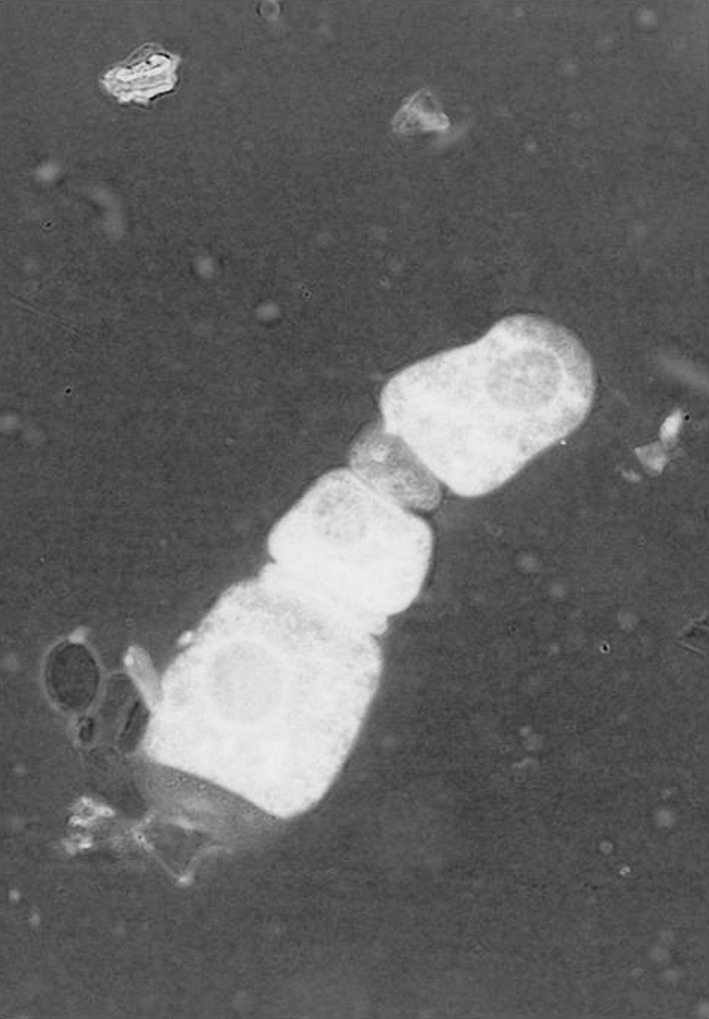
Gregarine isolated from gut of P. monodon
Graph 3.
Correlation between temperature and gregarine infection
Graph 4.
Correlation between pH and gregarine infection
Relationship between water quality parameters and microspridian parasites
Microsporidian parasites measuring 0.5–1.5 μm in diametre (Fig. 3) were isolated from the muscles and hepatopancreas of the decapod crustacean, P. monodon. It does not follow any significant seasonal trend in infecting the host (Graphs 5 and 6). Infection only occurred during summer and was totally absent during spring and autumn.
Fig. 3.
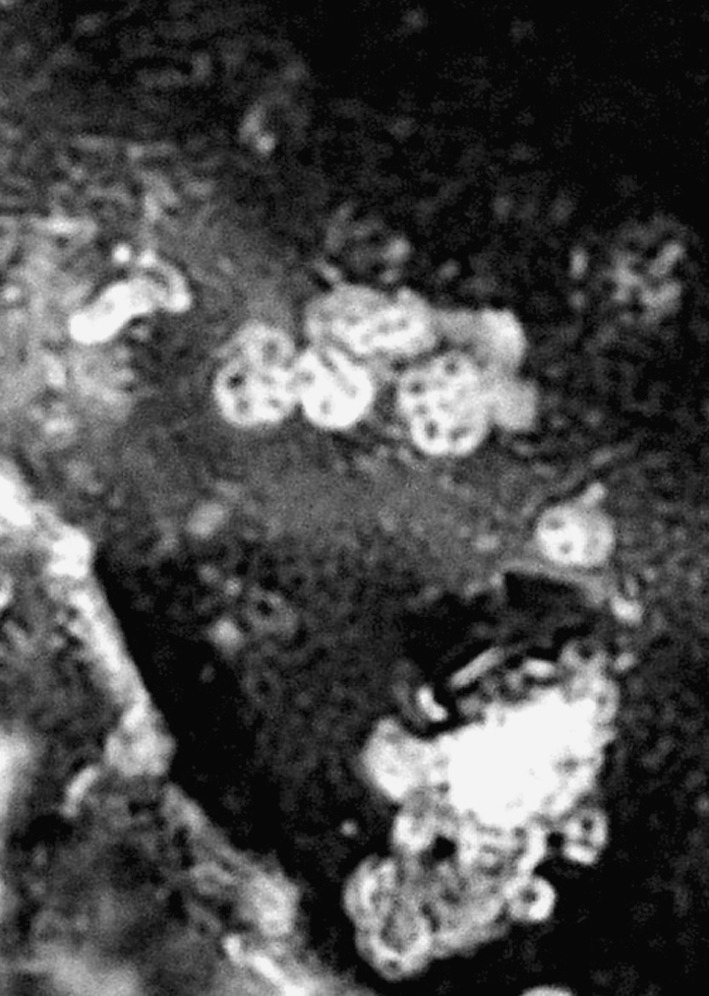
Microsporidia isolated from muscle of P. monodon
Graph 5.
Correlation between temperature and microsporidia infection
Graph 6.
Correlation between pH and microsporidia infection
Discussion
The present study revealed a heterogenity in the parasitic fauna of black tiger shrimp of Sundarbans. It consists of both freshwater and marine protozoan species. The former category being represented by peritrichous ciliates and microsporidians while gregarine represents the other category. All these parasites were earlier reported from different geographical areas of India (Bower et al. 1994; Prasadan and Janardan 2001; Johny et al. 2006). Pronounced seasonal variations were observed in the occurrence of all these parasites.
Infections with ectosymboints peritrichous ciliates, belonging to the genus Vorticella were frequently recorded from the tiger shrimp of Sundarbans. The infection rate reached its peak during summer when water temperature ranged from 29–31°C, below and above this temperature the infection rate decreased. This work corroborated with the work done by Feigenbaum 1975); Hudson and Lester 1992. Thus an optimum temperature of 29–31°C is required for growth and survival of the protozoan parasites. Since they are epibionts, the infection caused by them at a low density is non pathogenic but heavy infection may cause fouling of gills and appendages. They may even cause death since they interfere with respiration and locomotion (Overstreet 1973).
Transmission of ciliate parasites increases during summer in tropical or subtropical climates because of the physicochemical conditions of the farm (Norma et al. 2009), increasing metabolism and molting of decapods host occurred due to heavy amount of solar energy (Rhode 1992; Jayasree et al. 2001).
Septate gregarines are the intestinal parasite of penaeid shrimp (Kruse 1959; Overstreet 1973 and Lightner 1993). The black tiger shrimp of Sundarbans was found to be infected by a gregarine parasite, Nematopsis sundarbanensis (Chakraborti & Bandyopadhyay 2010). The prevalence of the protozoan parasite was observed in summer following the same seasonal trend as ciliates. This parasite is highly pathogenic to shrimp and heavy infection may cause loss of appetite, growth retardation and ultimately death of the host in the culture system (Lightner 1993). Incase of microsporidian infections, heavy parasite load leads to mortality. Thus from the above discussion it can be concluded that heavy infestations of epibionts and parasites are expected when the rate of transmission is increased due to high stocking density and variations of environmental parameters (e.g., amount of ammonium, nitrate, and dissolved oxygen levels) and the consequences of pond fertilization (Kautsky et al. 2000; Gualteros-Rodríguez 2003). During summer months due to high intensity of solar energy the moulting process is favoured (Rhode 1992; Jayasree et al. 2001), thus enhancing the invasion of parasites. So adequate measures should be taken in the susceptible months to avoid protozoan infections.
Acknowledgments
One of the authors (JC) is thankful to University Grants Commission, New Delhi for financial support in the form of a scholarship to run the project (Project no. F.32-483/2006 (SR).
References
- Becnel JJ, Andreadis TG. Microsporidia in insects. In: Wittner M, Weiss LM, editors. The microsporidia and microsporidiosis. Washington, DC: American Society for Microbiology Press; 1999. pp. 447–501. [Google Scholar]
- Bower SM, McGladdery SE, Price IM. Synopsis of infectious diseases and parasites of commercially exploited shellfish. Annu Rev Fish Dis. 1994;4:1–199. doi: 10.1016/0959-8030(94)90028-0. [DOI] [Google Scholar]
- Chakraborti J, Bandyopadhyay PK. First record of a parasitic septate gregarines (Apicomplexa: Sporozoea) in the shrimp Peneaus monodon in Sundarbans of West Bengal. J Parasit dis. 2010;34(1):40–43. doi: 10.1007/s12639-010-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers RK, Reno PW, Olson RE. Prevalance and geographic range Nadelspora canceri (Microspora) in Dungeness crab cancer magister. Dis Aquatic Org. 1996;24:135–142. doi: 10.3354/dao024135. [DOI] [Google Scholar]
- Didier ES, Didier PJ, Snowden KF, Shadduck JA. Microsporidiosis in mammals. Microbes Infect. 2000;2:709–720. doi: 10.1016/S1286-4579(00)00354-3. [DOI] [PubMed] [Google Scholar]
- Feigenbaum DL. Parasites of the commercial shrimp Penaeus vannamei Boone and P. brasiliensisLatreille. Bull Mar Sci. 1975;25:491–514. [Google Scholar]
- Gualteros-Rodríguez W (2003) Estudio de la comunidad de nemátodos meiobentónicos en 2 estanques de producción de camarón. Golfo de Guayaquil, Ecuador. MS thesis. Escuela Superior Politécnica del Litoral, Facultad de Ingeniería Marítima y Ciencias del Mar. Guayaquil, Ecuador
- Hudson D, Lester R. Relationships between water quality parameters and symbiont ciliates on prawns (Penaeus japonicus Bate) in aquaculture. Aquaculture. 1992;105:269–280. doi: 10.1016/0044-8486(92)90092-Y. [DOI] [Google Scholar]
- Jayasree L, Janakiram P, Madhavi R. Epibionts and parasites of Macrobrachium rosenbergii and Metapenaeus dobsoni from Gosthani estuary. J Nat Hist. 2001;35:157–167. doi: 10.1080/00222930150215297. [DOI] [Google Scholar]
- Johny S, Kanginakudru S, Muralirangan MC, Nagaraja J (2006) Morphological and molecular characterization of a new microsporidian (Protozoa: Microsporidia) isolated from Spodoptera litura (Fabricus) (Lepidoptera: Noctuidae). Parasitology 92(2):59–65 [DOI] [PubMed]
- Kautsky N, Rönnbäck P, Tedengren M, Troell M. Ecosystem perspectives on management of diseases in shrimp pond farming. Aquaculture. 2000;191:145–161. doi: 10.1016/S0044-8486(00)00424-5. [DOI] [Google Scholar]
- Kruse DN (1959) Parasites of the commercial shrimps Penaeus aztecus Ives, P. duorarum, Burkenroad, and P. setiferus (Linnaeus). Tulane Stud Zool 7:123–144
- Lightner DV. Diseases of cultured peneaid shrimp. In: McVey JP, Lightner DV, editors. CRC handbook of mariculture: crustacean aquaculture. Boca Raton, FL: CRC Press; 1993. pp. 303–346. [Google Scholar]
- Norma A, López-Téllez, Vidal-Martínez VM, Overstreet RM. Seasonal variation of ectosymbiotic ciliates on farmed and wild shrimps from coastal Yucatan, Mexico. Aquaculture. 2009;287:271–277. doi: 10.1016/j.aquaculture.2008.11.003. [DOI] [Google Scholar]
- Overstreet RM. Parasites of some penaeid shrimps with emphasis on reared hosts. Aquaculture. 1973;2:105–140. doi: 10.1016/0044-8486(73)90140-3. [DOI] [Google Scholar]
- Prasadan PK, Janardan PK. Three new species of gregarines (Apicoplexa: Sporozoea: Porosporidae) in the Estuarine crabs from Kerala, India. Acta Protozool. 2001;40:303–309. [Google Scholar]
- Prema S, Janardan KP. Two new species of cephaline gregarines (Apicomplexa, Sporozoa) from the marine prawn, Peneaus indicus H. Milne Edwards. Acta Parasitilogica. 1990;29:365–373. [Google Scholar]
- Rhode K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos. 1992;65:514–527. doi: 10.2307/3545569. [DOI] [Google Scholar]
- Solter LF, Becnel JJ. Entomopathogenic microsporidia. In: Lacey LA, Kaya H, editors. Field manual of techniques for the evaluation of entomopathogens. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 231–254. [Google Scholar]
- Sprague V, Couchi J. An annotated list of protozoan parasites, hyperparasites and commensals of Decapod crustacean. J Protozool. 1971;18:526–537. [Google Scholar]
- Timofeev SF. Quantitative analysis of the invasion with gregarinids (Sporozoa: Gregarina) of the euphausiid Thysanoessa raschii (Crustacea: Euphausiacea) from the Barentsev sea. Parazitologiia. 2001;35:235–240. [PubMed] [Google Scholar]
- Walker MH, Hinsch GW. Ultrastructural observations of a microsporidian protozoan parasite in Libinia dubia (Decapoda) Parasitol Res. 1972;39:17–26. [PubMed] [Google Scholar]