Abstract
BACKGROUND:
Although Staphylococcus aureus is a major cause of bloodstream infections, population-based data on these infections in children are limited.
OBJECTIVE:
To describe the epidemiology of S aureus bacteremia in children.
METHODS:
Population-based surveillance for all incident S aureus bacteremias was conducted among children (18 years of age or younger) living in the Calgary Health Region (Alberta) from 2000 to 2006.
RESULTS:
During the seven-year study, 120 S aureus bloodstream infections occurred among 119 patients; 27% were nosocomial, 18% health care associated and 56% community acquired. The annual incidence was 6.5/100,000 population and 0.094/1000 live births. A total of 52% had a significant underlying condition, and this was higher for nosocomial cases. Bone and joint (40%), bacteremia without a focus (33%), and skin and soft tissue infections (15%) were the most common clinical syndromes. Infections due to methicillin-resistant S aureus were uncommon (occurring in one infection) and three patients (2.5%) died.
CONCLUSIONS:
S aureus bacteremia is an important cause of morbidity in the paediatric age group. Underlying medical conditions and implanted devices are important risk factors. Methicillin-resistant S aureus and mortality rates are low.
Keywords: Bloodstream infection, Clinical syndromes, Staphylococcus aureus
Abstract
HISTORIQUE :
Même si le Staphylococcus aureus est une cause importante d’infections sanguines, les données en population de ces infections chez les enfants sont limitées.
OBJECTIF :
Décrire l’épidémiologie de la bactériémie à S aureus chez les enfants.
MÉTHODOLOGIE :
Les chercheurs ont procédé à la surveillance en population de toutes les bactériémies à S aureus incidentes chez les enfants (de 18 ans et moins) qui habitaient sur le territoire de la régie régionale de Calgary, en Alberta, entre 2000 et 2006.
RÉSULTATS :
Pendant l’étude de sept ans, 120 infections sanguines à S aureus se sont déclarées chez 119 patients. De ce nombre, 27 % étaient d’origine nosocomiale, 18 % s’associaient aux soins et 56 % étaient d’origine non nosocomiale. L’incidence annuelle s’élevait à 6,5 cas pour 100 000 habitants et à 0,094 cas pour 1 000 naissances vivantes. Dans l’ensemble, 52 % des patients étaient atteints d’une maladie sous-jacente importante, et cette proportion était plus élevée pour ce qui est des maladies nosocomiales. Les infections des os et des articulations (40 %), les bactériémies sans foyer (33 %) et les infections de la peau et des tissus mous (15 %) étaient les principaux syndromes cliniques. Les infections causées par le S aureus méthicillinorésistant étaient peu courantes (un cas seulement), et trois patients (2,5 %) sont décédés.
CONCLUSIONS :
La bactériémie à S aureus est une cause importante de morbidité au sein du groupe d’âge pédiatrique. Les maladies sous-jacentes et les dispositifs internes constituent des facteurs de risque non négligeables. Le S aureus méthicillinorésistant et le taux de mortalité sont faibles.
Staphylococcus aureus is recognized as an important cause of invasive disease in children (1,2). It has assumed an increasing relative importance as an agent of community-acquired outpatient bacteremia, especially in regions where successful widespread implementation of infant conjugate vaccines has reduced the incidence of invasive Haemophilus influenzae and Streptococcus pneumoniae diseases (3). S aureus is a frequent cause of hospital-acquired bacteremia, with patients admitted to neonatal and paediatric intensive care units at the highest risk (4–6). While nosocomial infections due to methicillin-resistant S aureus (MRSA) have been recognized in many jurisdictions for decades, in recent years, severe invasive infections due to MRSA strains have increasingly been reported (7–11) in patients, including children, without significant previous health care exposure. A number of studies (1,12,13) have been published describing the occurrence, clinical attributes and outcomes of S aureus bloodstream infections among children admitted to hospitals. However, studies conducted in selected hospitals may be biased by referral patterns, and incidence rates cannot be calculated using these designs (14,15).
Population-based studies that identify all new cases of disease occurring among residents of a defined population at risk are optimal for defining the epidemiology of an infectious disease. However, only a few population-based studies of invasive or bacteremic S aureus infections have been conducted (2,16–25). To our knowledge, only two of these studies – one from Denmark (18) and the other from New Zealand (25) – have focused on children. The objective of the present study was to describe the incidence, clinical features and outcomes associated with S aureus bacteremia in all children living in a large Canadian health region from 2000 to 2006.
METHODS
Base population
The Calgary Health Region (CHR; currently named Alberta Health Services – Calgary and Area) administers virtually all medical and surgical care to the residents of Calgary, Airdrie and a large surrounding area (population 1.2 million; 270,000 are 18 years of age or younger) in the province of Alberta. The region is serviced by four major acute care centres that are all located in Calgary. The Alberta Children’s Hospital is the principal acute tertiary care paediatric institution and admits most children. Noncritically ill children are also admitted to a paediatric ward at the Peter Lougheed Centre. Level 2 neonatal special care nurseries are present at the Peter Lougheed Centre, the Rockyview General Hospital and the Foothills Medical Centre. High-risk maternity care is focused at the Foothills Medical Centre, and is supported by a neonatal intensive care unit. A broad range of services – from outpatient to acute tertiary care paediatric services – are provided within the region. Only children requiring open heart or acute liver, heart or lung transplant surgery are routinely referred elsewhere. The study population consisted of all children (18 years of age or younger) living in the CHR who developed bacteremic S aureus infection between January 1, 2000, and December 31, 2006. The Conjoint Health Research Ethics Board for the University of Calgary and the CHR approved the study and waived the requirement for individual written informed consent.
Study protocol
An active, population-based surveillance cohort design was used. The present study was nested within a larger cohort of all S aureus bacteremias at all ages occurring in the CHR for which the detailed surveillance methodology has been previously described (16).
In summary, patients with positive blood cultures for S aureus were identified by the microbiology laboratory. The patients identified were then linked to the corporate databases of the CHR. Data extracted from the corporate databases included basic demographic information, admission and comorbidity diagnoses based on the International Statistical Classification of Diseases and Related Health Problems (ICD-10). All admission and comorbidity codes were included for analysis and were grouped as demonstrated in the results. No chart review was performed for the study.
Calgary Laboratory Services is a regional laboratory system that receives more than 95% of all blood samples submitted for culture from the acute care sites as well as the community setting for the CHR. Dialysis in a paediatric population is performed as part of the inpatient nephrology service; therefore, infections associated with dialysis or the associated central venous catheters would be captured within the data set.
Bacteremic S aureus infection was defined by its isolation from at least one set of aseptically obtained blood culture bottles. Clinical isolates were confirmed as S aureus (Gram stain, colonial morphology, catalase test and tube coagulase test) and tested for antimicrobial susceptibility by standard techniques (26,27). Phenotypic MRSA strains were confirmed as mecA-positive by polymerase chain reaction assay (or detection of penicillin-binding protein 2a by latex agglutination). Typing of MRSA strains was performed using pulse-field gel electrophoresis as previously described (28).
Residency status was established using the 2003 boundaries of the CHR (29). Incident cases were defined by the new first isolation of methicillin-sensitive S aureus or MRSA; repeated isolation of the same organism (methicillin-sensitive S aureus or MRSA) within 365 days after the first positive culture was deemed to represent the same incident infection.
The Centers for Disease Control and Prevention (USA) guidelines (30) were used for defining health care association and noso-comial infections. Nosocomial bacteremias were those for which the first culture was positive 48 h or more after hospital admission or within 48 h of discharge. Infections occurring within the first two days of birth were also classified as nosocomial. Community-onset infections were those for which the first positive culture was obtained within 48 h of admission or 48 h or more after discharge from hospital. A health care-associated community-onset S aureus bacteremia had at least one of the following: attendance in a specialized hospital-based clinic or emergency room within the previous two to 30 days before bloodstream infection; or admitted to a CHR acute care hospital for two or more days within the previous 90 days before bloodstream infection. Community-acquired infections were defined as community-onset bacteremias that were not health care associated. Discharge coding (ICD-10) was used to determine whether the infection had a primary source (eg, musculoskeletal, soft tissue, intravascular catheter-associated or endovascular infections) compared with a primary bacteremia. Neonatal infections were defined by positive culture in patients younger than 30 days of age.
Statistical analysis
Analysis was performed using Stata version 9.2 (Stata Corp, USA). Non-normally distributed variables were reported as medians with interquartile ranges (IQRs), and compared using the rank-sum test for pairs or the median test for multiple groups. Differences in proportions among categorical data were assessed using Fisher’s exact test for pairwise comparisons and the χ2 test for multiple groups. The overall incidence, and age- and sex-specific incidences of bacteremic S aureus infection were calculated by dividing the number of incident cases by the regional population. The neonatal infection rate was also calculated per 1000 live births. Differential risks among different population subgroups were expressed as incidence rate ratios with 95% CIs. For all statistical comparisons, P<0.05 was deemed to represent statistical significance.
RESULTS
During the seven-year study, 120 bacteremic S aureus infections occurred among 119 patients; 32 (27%) were classified as nosocomial, 21 (18%) as health care associated and 67 (56%) as community acquired.
Incidence
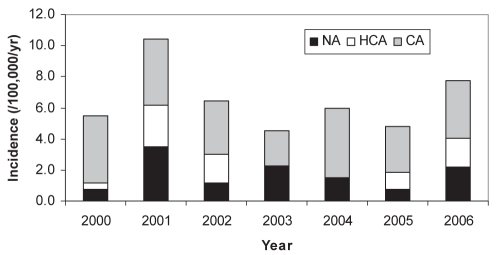
The annual incidence of bacteremic infection was 6.5/100,000 population. Ten neonates (two within the first six days of life) developed S aureus bacteremia yielding a rate of 0.094/1000 live births. The incidence of invasive bacteremic disease in children varied from year to year (Figure 1). The occurrence of S aureus bloodstream infection also demonstrated considerable monthly variability. While no clear seasonal trend was evident for community-acquired or health care-associated infections, most nosocomial infections (n=25 [78%]) occurred during the second half of the year.
Figure 1).
Annual incidence of Staphylococcus aureus bacteremia according to mode of acquisition in children living in the Calgary Health Region (Alberta), 2000 to 2006. CA Community acquired; HCA Health care-associated community onset; NA Nosocomial acquired; yr Year
The median age of the patients was 6.6 years (IQR 1.0 to 11.2 years) and 68 (57%) were boys. The risk for developing S aureus bacteremia was related to age, with the risk being highest in the neonatal period (younger than 30 days) (124.8 cases/100,000 population), abruptly dropping at one to 23 months (14.0 cases/100,000 population) and through later childhood (3.7 to 5.5 cases/100,000 population at two to 17 years). Overall, boys were at a modestly increased risk (incidence rate ratio 1.54 [95% CI 1.03 to 2.32]; P=0.03), but this excess risk was attributable to a two- to threefold increased risk in the younger groups compared with the two-year-old and older adolescent age groups, with no excess risk observed for the three- to nine-year-old age group.
Among the 10 neonatal infections, all were nosocomial, with the exception of one patient who developed a health care-associated S aureus bacteremia in the community at 29 days of age. Beyond the neonatal age group, there was a significant relationship between age and location of acquisition (P=0.03). For patients between 30 days and four years of age (n=39), nosocomial (n=14 [36%]) and community-acquired cases (n=16 [41%]) predominated. Older children and young adolescents five to 14 years of age (n=52) mainly had community-acquired disease (n=40 [77%]), with only seven (13%) and five (10%) cases being nosocomial and health care associated. In the oldest age group of 15 to 17 years (n=9), three (33%) were community acquired, five (56%) were health care associated and one (11%) was nosocomial.
Clinical characteristics
Clinical information was available for the 110 cases (92%) admitted to one of the four major regional hospitals. Approximately one-half of the patients (n=57 [52%]) had at least one significant underlying condition, and this was significantly higher for nosocomial (25 of 31 [81%]) and health care-associated (17 of 20 [85%]) cases compared with community-acquired cases (15 of 59 [25%]; P<0.0001). There was also a significant (P=0.001) inverse relationship observed between age and the presence of comorbid conditions; nine of 10 neonates (90%), 25 of 39 (64%) patients 30 days to four years of age, and 23 of 61 (38%) patients five years of age and older had a comorbid condition associated with their bacteremia.
The underlying comorbid conditions included congenital heart disease (16%), neoplastic disease (9%), bone marrow transplant (2%), asthma (5%) and diabetes (1%). Ten neonatal cases represented 10% of cases (all nosocomial), of which six cases were complicated by prematurity and five cases by an extremely low birth weight.
Bone and joint infections, bacteremia without a focus, and soft tissue infections were the most common clinical syndromes observed (Table 1). Endovascular, and bone and joint infections occurred more commonly in older children (P=0.005) (Table 1). The focus of infection was also related to the location of acquisition (P<0.001), with bone and joint infections, soft tissue infections and endovascular infections (endocarditis, conduit infections, septic thrombophlebitis or infected intravascular clots) predominantly community acquired, and respiratory and bacteremia without a focus either nosocomial or health care associated (Table 1). Only three patients were diagnosed with toxic shock syndrome, all of which were community-acquired disease in boys 10 to 12 years of age. Two of these infections involved endocarditis with metastatic complications and the other was a bacteremia without a focus in a cancer patient.
TABLE 1.
Clinical diagnosis and correlates of Staphylococcus aureus bacteremia in 110 children in the Calgary Health Region (Alberta), 2000 to 2006
| Primary focus of infection | n (%) | Age, years, median (IQR) | Comorbidity, n (%) | NA, n (%) | HCA, n (%) | CA, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone and joint | 44 | (40) | 9.2 | (5.8–13.2) | 9 | (20) | 4 | (9) | 6 | (14) | 34 | (77) |
| Skin and soft tissue | 16 | (15) | 3.6 | (1.7–11.1) | 7 | (44) | 5 | (31) | 1 | (6) | 10 | (63) |
| Endovascular | 5 | (5) | 12.4 | (10.6–13.7) | 3 | (60) | 0 | (0) | 0 | (0) | 5 | (100) |
| Lower respiratory | 3 | (3) | 1.7 | (0.1–2.9) | 3 | (100) | 2 | (67) | 1 | (33) | 0 | (0) |
| Intra-abdominal or pelvic | 3 | (3) | 8.7 | (0.1–14.0) | 1 | (33) | 0 | (0) | 1 | (33) | 2 | (67) |
| Ear, nose and throat | 2 | (2) | 0.3 | (0.1–0.5) | 1 | (50) | 1 | (50) | 1 | (50) | 0 | (0) |
| Central nervous system | 1 | (1) | 0.1 | 1 | (100) | 1 | (100) | 0 | (0) | 0 | (0) | |
| No focus | 36 | (33) | 3.4 | (0.2–8.3) | 30 | (83) | 18 | (50) | 10 | (28) | 8 | (22) |
CA Community acquired; HCA Health care-associated community onset; NA Nosocomial acquired
Microbiology
Detailed susceptibility testing results were available for all but one (n=109) of the incident infections for which clinical information was available. Only one infection was caused by MRSA and was a health care-associated bone and joint infection. The CHR’s local antibiogram in 2006 demonstrated the presence of MRSA in 8% of S aureus isolates. Five isolates (5%) demonstrated reduced susceptibility to trimethoprim/sulfamethoxazole, 13 (12%) to erythromycin, two (2%) to ciprofloxacin and one (1%) to gentamicin. Clindamycin susceptibility was not available because it is not reported in invasive isolates. No isolates were resistant to rifampin or linezolid.
Hospital course and outcome
The overall median length of stay in hospital was 12.2 days (IQR 6.9 to 27.0 days). The median time from admission to development of nosocomial bacteremia was 13.4 days (IQR 6.1 to 24.7 days). All cases were admitted for treatment, although an unknown number were outpatients when the blood culture was drawn. The median length of stay following diagnosis of an S aureus bloodstream infection was significantly (P<0.0001) longer for nosocomial (38.0 days [IQR 17.1 to 91.9 days]) compared with health care-associated (11.7 days [IQR 8.7 to 14.9 days]) and community-acquired (9.6 days [IQR 6.8 to 13.9 days]) infections. Three of 120 patients (2.5%) with an S aureus bacteremia infection died before discharge from hospital, for an overall annual mortality rate of 0.2/100,000 population. The proportion of patients requiring critical care could not be determined.
DISCUSSION
S aureus is an important pathogen in the paediatric population. The results of the present study provide important contemporary information regarding the epidemiology of this pathogen in the paediatric population of a large Canadian city.
We observed an overall S aureus bacteremia incidence of 5.9/100,000 in the paediatric population (younger than 18 years of age) and a neonatal incidence of 0.094/1000 live births. A similar incidence has been demonstrated among children in Denmark (8.4 cases/100,000 children) (18), Finland (five cases/100,000 children) (21) and the United States (2.3 cases/100,000 children) (31). A similar incidence has also been demonstrated in children younger than one year of age in Australia and New Zealand (0.08 cases/1000 live births) (4). Maori and Pacific Islander populations, in a New Zealand cohort (25), had significantly higher rates of disease. Our region has an invasive S aureus rate similar to that of other nondisadvantaged populations. The reason for the predominance of nosocomial infections in the second half of the year is unclear.
S aureus represented the third most commonly isolated blood culture organism (12%) in the paediatric CHR population from 2000 to 2006, following Streptococcus viridans (23%) and S pneumoniae (16%), with a prevalence similar to Escherichia coli (32). The proportion was significantly higher in the nosocomial and health care-associated groups. Our 12% nosocomial prevalence was similar to an Israeli cohort (5,32). Similarly, in a United States hospital-based surveillance study (6), S aureus represented 9.2% of all isolates.
Case fatality rates in staphylococcal bacteremia are significant. Our study demonstrated a case fatality rate of 2.5% (0.2 deaths/100,000 population). In the paediatric case series as well as population-based studies (1,12,18,21,25), case fatality is reported to be between 1% and 11%. Higher rates of case fatality have been described in the context of MRSA, disadvantaged populations and young neonates (4,18,25). This is consistent with the much higher case fatality rate in adults of 25% in our region and 15% to 30% elsewhere (2,16,21).
Invasive staphylococcal disease is associated with the risk factors in 25% to 89% of children (1,4,10,12,18,25,31). Our population demonstrated the peak incidence in the neonatal period, as described above, with an increased risk in male children (RR 1.5), most notably in the neonatal age group, with 57% of children in the population having significant underlying conditions. The breakdown of illnesses, representing risk factors, in our population mirrors that of the literature.
The prevalence of congenital heart defects of 16% in our population is significantly higher than the prevalence of 10.6 cases of major general heart defects per 100,000 live births in a Canadian birth cohort (33). The incidence rates of cancer in Canadian children was quoted by Howard et al (34) to be 144.2/1,000,000 children, again suggesting an increased risk for S aureus bacteremia in this population, with a prevalence of 9% in our study.
Clinical presentations of illness in our paediatric patients presenting with S aureus bacteremia revealed that bone and joint infections, followed by skin and soft tissue infections were the most common. This is similar to several other paediatric cohorts (18,25). No focus of infection was identified in approximately one-third of our patients. An Australian case series (1) also demonstrated bone and joint infections in 59% of otherwise healthy children, with endocarditis being a rare event in 1.4% of children compared with 30% of adults. The population with no identified focus of infection in our study was similar to the New Zealand data if one combines rates for their patients with no identified focus and those with intravascular catheter infections (27%); unfortunately, our administrative database does not reliably capture the presence of indwelling venous catheters. However, our rate of “no identified focus” is significantly less than the “unknown primary focus” group in the Denmark data (50% to 80%) (18,25).
MRSA is being reported with increasing frequency in many jurisdictions including Great Britain, Taiwan and the United States. In a Chicago jurisdiction, MRSA increased from 10/100,000 admissions to 259/100,000 admissions, while other studies demonstrated an increased number of cases during their surveillance (9–11,35). MRSA was an uncommon problem in our study.
A limitation of using administrative data is that they do not allow for case follow-up and, thus, sequelae related to S aureus bacteremia cannot be determined. The exact length of treatment (intravenous portion followed by oral step down) cannot be determined. It is possible that not all potential risk factors associated with invasive staphylococcal infection would be identified using administrative data; in particular, the presence of an indwelling central venous catheter might not be reliably captured via ICD-10 coding.
S aureus infection continues to be a significant disease in the paediatric population. Children with underlying risk factors, including central venous access devices, malignancies and chronic illnesses, continue to be at increased risk for this serious infection. Although a significant number of children were identified without a focus of infection, it remains essential that the focus is aggressively investigated because a large majority of staphylococcal bacteremias are secondary. Fortunately, the mortality rate associated with invasive staphylococcal illnesses in children remains much lower than in adults. The epidemiology of disease continues to change, and if the experience in other jurisdictions holds true, a changing pattern of disease will emerge as MRSA becomes a more significant problem.
REFERENCES
- 1.Suryati BA, Watson M. Staphylococcus aureus bacteraemia in children: A 5-year retrospective review. J Paediatr Child Health. 2002;38:290–4. doi: 10.1046/j.1440-1754.2002.00787.x. [DOI] [PubMed] [Google Scholar]
- 2.Laupland KB, Church DL, Mucenski M, Sutherland LR, Davies HD. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J Infect Dis. 2003;187:1452–9. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- 3.Herz AM, Greenhow TL, Alcantara J, et al. Changing epidemiology of outpatient bacteremia in 3- to 36-month-old children after the introduction of the heptavalent-conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2006;25:293–300. doi: 10.1097/01.inf.0000207485.39112.bf. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs D, Fraser S, Hogg G, Li HY. Staphylococcus aureus infections in Australasian neonatal nurseries. Arch Dis Child Fetal Neonatal Ed. 2004;89:F331–5. doi: 10.1136/adc.2002.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank M, Gur E, Givon-Lavi N, Peled N, Dagan R, Leibovitz E. Nosocomial bloodstream infections in children and adolescents in southern Israel: A 10-year prospective study (1992–2001) Scand J Infect Dis. 2005;37:177–83. doi: 10.1080/00365540410020956. [DOI] [PubMed] [Google Scholar]
- 6.Wisplinghoff H, Seifert H, Tallent SM, Bischoff T, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in pediatric patients in United States hospitals: Epidemiology, clinical features and susceptibilities. Pediatr Infect Dis J. 2003;22:686–91. doi: 10.1097/01.inf.0000078159.53132.40. [DOI] [PubMed] [Google Scholar]
- 7.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–8. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 8.Seal JB, Moreira B, Bethel CD, Daum RS. Antimicrobial resistance in Staphylococcus aureus at the University of Chicago Hospitals: A 15-year longitudinal assessment in a large university-based hospital. Infect Control Hosp Epidemiol. 2003;24:403–8. doi: 10.1086/502222. [DOI] [PubMed] [Google Scholar]
- 9.Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: A 14-year study at Driscoll Children’s Hospital. Arch Pediatr Adolesc Med. 2005;159:980–5. doi: 10.1001/archpedi.159.10.980. [DOI] [PubMed] [Google Scholar]
- 10.Chuang YY, Huang YC, Lee CY, Lin TY, Lien R, Chou YH. Methicillin-resistant Staphylococcus aureus bacteraemia in neonatal intensive care units: An analysis of 90 episodes. Acta Paediatr. 2004;93:786–90. doi: 10.1080/08035250410028084. [DOI] [PubMed] [Google Scholar]
- 11.Khairulddin N, Bishop L, Lamagni TL, Sharland M, Duckworth G. Emergence of methicillin resistant Staphylococcus aureus (MRSA) bacteraemia among children in England and Wales, 1990–2001. Arch Dis Child. 2004;89:378–9. doi: 10.1136/adc.2003.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakim H, Mylotte JM, Faden H. Morbidity and mortality of staphylococcal bacteremia in children. Am J Infect Control. 2007;35:102–5. doi: 10.1016/j.ajic.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Ladhani S, Konana OS, Mwarumba S, English MC. Bacteraemia due to Staphylococcus aureus. Arch Dis Child. 2004;89:568–71. doi: 10.1136/adc.2003.026781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laupland KB, Ross T, Pitout JD, Church DL, Gregson DB. Investigation of sources of potential bias in laboratory surveillance for anti-microbial resistance. Clin Invest Med. 2007;30:E159–66. doi: 10.25011/cim.v30i4.1777. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto VN, Rubenfeld GD. Attending to the lightness of numbers: Toward the understanding of critical care epidemiology. Crit Care. 2004;8:422–4. doi: 10.1186/cc2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: Risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis. 2008;198:336–43. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsson G, Dashti S, Wahlberg T, Andersson R. The epidemiology of and risk factors for invasive Staphylococcus aureus infections in western Sweden. Scand J Infect Dis. 2007;39:6–13. doi: 10.1080/00365540600810026. [DOI] [PubMed] [Google Scholar]
- 18.Frederiksen MS, Espersen F, Frimodt-Moller N, et al. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr Infect Dis J. 2007;26:398–405. doi: 10.1097/01.inf.0000261112.53035.4c. [DOI] [PubMed] [Google Scholar]
- 19.Benfield T, Espersen F, Frimodt-Moller N, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13:257–63. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 20.Collignon P, Nimmo GR, Gottlieb T, Gosbell IB. Staphylococcus aureus bacteremia, Australia. Emerg Infect Dis. 2005;11:554–61. doi: 10.3201/eid1104.040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyytikainen O, Ruotsalainen E, Jarvinen A, Valtonen V, Ruutu P. Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995–2001. Eur J Clin Microbiol Infect Dis. 2005;24:399–404. doi: 10.1007/s10096-005-1345-3. [DOI] [PubMed] [Google Scholar]
- 22.Frimodt-Moller N, Espersen F, Skinhoj P, Rosdahl VT. Epidemiology of Staphylococcus aureus bacteremia in Denmark from 1957 to 1990. Clin Microbiol Infect. 1997;3:297–305. doi: 10.1111/j.1469-0691.1997.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 23.Morgan M, Salmon R, Keppie N, Evans-Williams D, Hosein I, Looker DN. All Wales surveillance of methicillin-resistant Staphylococcus aureus (MRSA): The first year’s results. J Hosp Infect. 1999;41:173–9. doi: 10.1016/s0195-6701(99)90014-2. [DOI] [PubMed] [Google Scholar]
- 24.Mc Donald P, Mitchell E, Johnson H, et al. MRSA bacteraemia: North/South Study of MRSA in Ireland 1999. J Hosp Infect. 2002;52:288–91. doi: 10.1053/jhin.2002.1274. [DOI] [PubMed] [Google Scholar]
- 25.Hill PC, Wong CG, Voss LM, et al. Prospective study of 125 cases of Staphylococcus aureus bacteremia in children in New Zealand. Pediatr Infect Dis J. 2001;20:868–73. doi: 10.1097/00006454-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 8th edn. Wayne: Clinical and Laboratory Standards Institute; 2009. p. 29. Approved standard M07-A8. [Google Scholar]
- 27.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. Wayne: Clinical and Laboratory Standards Institute; 2009. p. 29. M100-S19. [Google Scholar]
- 28.Mulvey MR, Chui L, Ismail J, et al. Development of a Canadian standardized protocol for subtyping methicillin-resistant Staphylococcus aureus using pulsed-field gel electrophoresis. J Clin Microbiol. 2001;39:3481–5. doi: 10.1128/JCM.39.10.3481-3485.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Government of Alberta Health and Wellness Health Regions. 2007. < www.health.alberta.ca/services/health-regions.html> (Accessed on April 19, 2011).
- 30.Garner J, Jarvis W, Emori T, Horan T, Hughes J. CDC definitions for nosocomial infections. In: Olmsted R, editor. APIC Infection Control and Applied Epidemiology: Principles and Practice. St Louis: Mosby; 1996. pp. A1–20. [Google Scholar]
- 31.Morin CA, Hadler JL. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J Infect Dis. 2001;184:1029–34. doi: 10.1086/323459. [DOI] [PubMed] [Google Scholar]
- 32.Laupland KB, Gregson DB, Vanderkooi OG, Ross T, Kellner JD. The changing burden of pediatric bloodstream infections in Calgary, Canada, 2000–2006. Pediatr Infect Dis J. 2009;28:114–7. doi: 10.1097/INF.0b013e318187ad5a. [DOI] [PubMed] [Google Scholar]
- 33.Prsa M, Saroli T, Mackie AS, Dancea AB, Correa JA, Asgharian M. Birth prevalence of congenital heart disease. Epidemiology. 2009;20:466–8. doi: 10.1097/EDE.0b013e31819f3bc0. [DOI] [PubMed] [Google Scholar]
- 34.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112:461–72. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
- 35.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]