Cancers become clinically obvious when they have overcome numerous mechanisms of anti-tumor immunity. Immune evasion in the tumor microenvironment therefore represents a hallmark of probably all established solid cancers. While overcoming tumor-induced immunosuppression has resulted in indisputable clinical responses against certain tumors, multiple mechanisms of immune evasion still prevent the implementation of effective immunotherapies against lethal epithelial cancers.
Negative co-stimulatory molecules present in tumor cells and leukocytes at tumor locations prevent the control of cancer progression by T lymphocytes. These molecules include multiple members of the B7 family, such as PD-L1 and B7-H4.1 Interestingly, butyrophilin (BTNs) and BTN-like (BTNL) proteins share significant sequence homology and predicted structural features with B7 molecules. A recent publication from our group now demonstrates that members of the BTN3 subfamily recognized by anti-CD277 antibodies are expressed by myeloid leukocytes and tumor cells in all ovarian carcinoma specimens analyzed and weaken human T-cell responses.2 In our hands, individual expression of a BTN3 protein significantly impaired, although not completely abrogated T-cell proliferation and Th1 cytokine secretion. However, there are at least 16 different BTN and BTNL molecules in humans, suggesting that their combined immunosuppressive effect could act in concert to drive immune privilege at tumor locations. BTN and BTNL molecules therefore emerge as potentially crucial negative modulators of anti-tumor immunity.
The first suggestion that BTN and BTNL molecules could both play a role in preventing excessive immune responses came from the identification of BTNL2 mutations in sarcoidosis patients.3 An inhibitory role for BTNL2 on T cells was confirmed soon after.4 However, it was not until this year that the inhibition of T-cell responses by members of the BTN1 and BTN2 subfamilies was experimentally confirmed.5 Our recent work has extended the suppressive effect on T cells to members of the remaining BTN subfamily. Then, immediately after the publication of our findings, another BTNL molecule was shown to be involved in negative T-cell costimulation.6 Cumulative evidence therefore points to redundant activity of as many as 16 different inhibitory mediators that modulate the intensity of T-cell activation in health and disease. The extent of their additive inhibitory effect in multiple physiological and pathological processes remains to be investigated, but the identification of BTN/BTNL polymorphisms associated with multiple autoimmune conditions suggests a crucial role in preventing uncontrollable T-cell responses.7
Because tumors utilize non-mutated cells to dampen and eventually abrogate immune pressure, finding inhibitory BTNs in the tumor microenvironment was not completely unexpected. However, two facts suggest that these molecules could be especially important to understand the immunobiology of various cancers: First, polymorphisms of a BTN3 molecule inversely correlate with ovarian cancer risk.8 This suggests that their inhibitory effects could be pivotal to allow tumor initiation and/or promote malignant progression. Second, the fact that tolerogenic activities have been so far found for all BTN/BTNL molecules investigated suggests that their combined suppressive effect in vivo might go way beyond the individual activity of each molecule (Fig. 1). Even if individual BTN/BTNL molecules are not redundantly expressed, many of them converge at tumor locations through different microenvironmental cells. Thus, our unpublished studies confirm that BTN2A2 and BTNL2 are additionally expressed in the ovarian cancer microenvironment, at least at the mRNA level. In addition, although the regulation of the expression of most individual BTN/BTNL proteins remains unknown, at least BTN3A1 and/or highly similar BTN3A3 are upregulated by cytokines (CCL3) and hypoxia-induced mediators (VEGF) blatantly overexpressed at tumor locations.
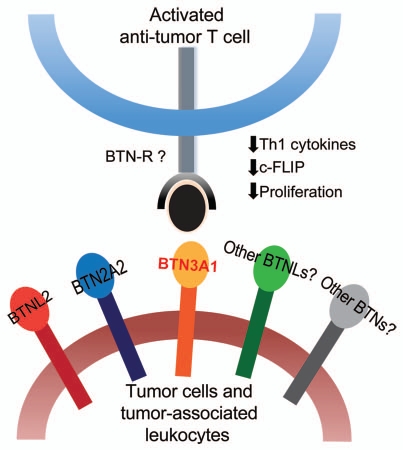
Figure 1.
BTN and BTNL molecules impair T-cell responses. Expression of multiple BTNs and BTNLs by tumor cells or tumor-associated leukocytes may act in concert to potently hinder the function of anti-tumor T cells. BTN3A1, for instance, interacts with a so far unidentified receptor expressed on activated T cells to downregulate anti-apoptotic c-FLIP and abrogate their TCR-induced proliferation. BTN3A1 also inhibits secretion of Th1 cytokines by activated T cells. Future research is aimed at identifying a novel putative T-cell receptor(s) for BTN and BTNL molecules.
In understanding how BTN/BTNL molecules exert their inhibitory function and the magnitude of this effect, the major challenge is the repeated failure to identify BTN/BTNL binding partners in T cells. We found that BTN3A1 only interacts with the surface of activated T cells, suggesting that the receptor is turned on during T-cell activation and is not present in naïve lymphocytes. PD-1 would be therefore an obvious candidate, if it had not been already ruled out as a receptor for BTN3 subfamily members by others.9 Other potential inhibitory partners such as CTLA-4 and BTLA have been also excluded.9 Finding T-cell receptors for BTN/BTNL proteins is critical for understanding the real contribution of these suppressive mediators to tumor-mediated immune evasion and for designing novel therapeutic interventions. For instance, if the same receptor promiscuously bound other members of the extended family, it could be targeted with the same blocking antibodies.
Finally, agonistic antibodies targeting the extracellular domain of a BTN3 molecule also impair T-cell responses,10 suggesting that the intracellular domain of BTNs can also mediate tolerogenic responses in the cells were it expressed. Under physiological conditions, BTN/BTNL molecules appear to be expressed by T cells. This implies a different reciprocal inhibitory mechanism and incorporates an additional layer of complexity to the role of these proteins. We should note, however, that at least in the ovarian carcinoma microenvironment, the expression of BTN3A1/CD277 is restricted to MHC-II+ myeloid leukocytes and tumor cells.
The coming years will reveal an exciting comprehensive view of the role of BTNs and BTNLs in the regulation of anti-tumor immunity.
Comment on: Cubillos-Ruiz JR, et al. Oncotarget. 2010;1:329–338. doi: 10.18632/oncotarget.165.
References
- 1.Zou W, et al. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 2.Cubillos-Ruiz JR, et al. Oncotarget. 2010;1:329–338. doi: 10.18632/oncotarget.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valentonyte R, et al. Nat Genet. 2005;37:357–364. doi: 10.1038/ng1519. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen T, et al. J Immunol. 2006;176:7354–7360. doi: 10.4049/jimmunol.176.12.7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith IA, et al. J Immunol. 2010;184:3514–3525. doi: 10.4049/jimmunol.0900416. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki T. J Immunol. 2010;185:5907–5914. doi: 10.4049/jimmunol.1000835. [DOI] [PubMed] [Google Scholar]
- 7.Arnett HA, et al. J Immunol. 2007;178:1523–1533. doi: 10.4049/jimmunol.178.3.1523. [DOI] [PubMed] [Google Scholar]
- 8.Peedicayil A, et al. PLoS One. 5:8884. [Google Scholar]
- 9.Compte E, et al. Eur J Immunol. 2004;34:2089–2099. doi: 10.1002/eji.200425227. [DOI] [PubMed] [Google Scholar]
- 10.Yamashiro H, et al. J Leukoc Biol. 2010;88:757–767. doi: 10.1189/jlb.0309156. [DOI] [PubMed] [Google Scholar]