Abstract
In this study we report that the protein kinase CK2 phosphorylates survivin specifically on threonine 48 (T48) within its BIR domain, and that T48 is critical to both the mitotic and anti-apoptotic roles of survivin. Interestingly, during mitosis T48 mutants localise normally, but are unable to support cell growth when endogenous survivin is removed by siRNA. In addition, while overexpression of survivin normally confers inhibition of TRAIL-mediated apoptosis, this protection is abolished by mutation of T48. Furthermore in interphase cells depletion of endogenous survivin causes redistribution of T48 mutants from the cytoplasm to the nucleus and treatment of cells expressing survivin-GFP with the CK2 inhibitor TBB phenocopies this nuclear redistribution. Finally, we show T48 mutants have increased affinity for borealin, and that this association and cell proliferation can be restored by introduction of a second mutation at T97. To our knowledge these data are the first to identify T48 as a key regulatory site on survivin, and CK2 as a mediator of its mitotic and anti-apoptotic functions.
Key words: survivin, chromosomal passenger protein, CK2, mitosis, apoptosis, phosphorylation
Introduction
Survivin is a multitasking protein that is essential for mitosis and can inhibit cell death. Over the last decade, it has received much attention as a potential therapeutic target in cancer due to the correlation of its expression with tumorogenesis and the resistance of survivin positive tumors to conventional therapies.1,2 During mitosis and meiosis, survivin is a chromosomal passenger protein (CPP) that operates with aurora-B, borealin and INCENP in the chromosome passenger complex (CPC). This complex facilitates the correction of maloriented chromosomes during prometaphase congression, and the execution of cytokinesis at cell division.3,4 Post-translation, survivin's mitotic activity is modulated by many kinases, including Cdk1, aurora-B and plk1 on T34, T117 and S20 respectively,5–9 and its phosphostatus can also influence its ability to inhibit cell death.5 Most strikingly, a phosphomutant T34E, which behaves as a constitutively Cdk1 phosphorylated form, potently inhibits cell death via apoptosis or X-irradiation, while the non-phosphorylatable form, T34A, sensitises cells to these challenges.5,10–12 In addition to the key mitotic kinases survivin is phosphorylated by the constitutive kinase, PKA at S20, the same residue that is targeted by plk1, and it has been suggested that the non-phosphorylatable form, S20A, has increased affinity for XIAP, with which it may operate as an inhibitor of apoptosis protein (IAP; reviewed in ref. 13).
Casein kinase 2 (CK2) is an essential and highly conserved serine-threonine protein kinase. Since its discovery in 1954, over 300 CK2 substrates have been identified, therefore not surprisingly it is reported to affect multiple cellular processes including regulation of gene expression, protein synthesis, signal transduction and cell survival.14 In accordance with its omnipresence, CK2 has been implicated in all cell cycle transitions: G0–G1, G1-S and G2-M, and is thought to promote cell proliferation by regulating key cell cycle proteins including p53, Cdk1, p21 and p27.15–18 It can also promote cell survival by augmenting an antiapoptotic response, which it can achieve via phospho-activation of the apoptotic repressor, ARC,19 and the inhibition of pro-apoptotic proteins, such as the transcription factor CHOP.20 In addition CK2 can inhibit cell death by phosphorylating and thereby inhibiting cleavage of specific caspase target proteins such as Bid, HS1 and Max.21–23 During cell division CK2 is thought to operate at several points, first it regulates mitotic entry through mediating the degradation of wee1;24,25 second it phosphorylates a number of proteins during mitosis including topoisomerase II,26,27 and third it localises to mitotic spindle and centrosomes,28–30 where it is phosphorylated by Cdk1.31,32 Intriguingly, emerging data point to a key role for Cdk1-phosphorylated CK2 in regulating the spindle assembly checkpoint (SAC), as Litchfield and co-workers recently showed that U2OS cells expressing CK2 that is unable to be phosphorylated by Cdk1 evade the SAC, while those expressing a phosphomimetic mis-segregate their chromosomes.33 CK2 and the SAC have also been linked in yeast through CK2 phosphorylation of Mad2.34
As with survivin, increased levels of CK2 protein and activity are observed in cancer cells and are associated with increased cell proliferation and inhibition of apoptosis.35,36 Conversely, chemical inhibition of CK2 or overexpression of a catalytically inactive mutant of CK2 subunit α results in reduced cell viability and increased apoptosis.22,37 These data suggest a role for CK2 in the promotion of cell proliferation and inhibition of apoptosis, although exactly how CK2 regulates these processes is unknown. Thus as both survivin and CK2 are involved in cell division and protection against death, we were interested to determine whether they communicate with each other to effect their duties. While a link between CK2 and survivin has already been demonstrated at the transcriptional level through β-catenin and Tcf/ Lef-mediated induction of survivin expression,38 here we hypothesized that CK2 may regulate survivin activity post-translation. Our data reveal that survivin is phosphorylated by CK2 at a single site, T48, within its BIR domain, and that mutation of this site renders the protein non-functional, both in terms of mitosis and as an IAP.
Intriguingly, mutation of a second site in the linker region of survivin (T97) alleviates the repression caused by mutating T48. From in vitro evidence presented herein, we speculate that mutation of T48 alters the affinity of survivin for borealin, and that the revived proliferative potential brought about by T97 mutation, is due, at least in part, to the restoration of this interaction to normal.
Results
Survivin is phosphorylated by CK2 at T48 in vitro.
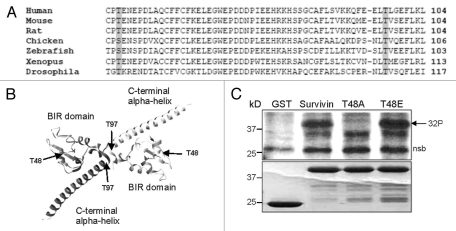
Using the online bioinformatics site, NetPhos, two highly conserved threonines in survivin were predicted to be potential phosphotargets of CK2: T48 within the BIR domain, and T97 in the central linker domain between the BIR and C-terminal alpha-helix, at the homodimerization interface (Fig. 1A and B). To establish whether survivin was indeed a CK2 target, an in vitro kinase assay was performed. Recombinantly expressed GST and GST-survivin were bound to glutathione-sepharose beads, and incubated with purified CK2 in the presence of 32P-γ-ATP. As shown in Figure 1C, GST-survivin incorporated 32P, but the GST control did not, indicating that wild-type survivin is a CK2 target in vitro. In addition, we made single mutations of T48 and T97, substituting the threonines for non-phosphorylatable alanines and tested whether these too could be phosphorylated by CK2. Interestingly, the T48A mutant did not incorporate 32P-γ-ATP, while radiolabelling of T97A was comparable to that of wild-type survivin. These data demonstrate that survivin is targetted by CK2 in vitro and is phosphorylated by it specifically at threonine 48 in its BIR domain.
Figure 1.
Survivin is phosphorylated by CK2 on T48 in vitro. (A) Sequence alignment of residues 46 to 104 of human survivin with survivin from other species demonstrates conservation of T48 and T97 and their contextual residues. (B) Crystal structure of the survivin homodimer indicating the positions of T48 within the BIR domain, and T97 within the central linker. The molecular graphic was produced using the UCSF Chimera package (www.cgl.ucsf.edu/chimera).65 (C) In vitro kinase assay using GST, GST-survivin, GST-T48A and GST-T97A as potential substrates, incubated with recombinant CK2 in the presence of γ-ATP labelled 32P. Lower Coomassie Blue part indicates equality of loading. 32P radiolabel incorporated into survivin and T97A but not GST or T48A, demonstrating that T48 is the principle CK2 phosphotarget of survivin. Nsb denotes a non-specific band. Note that the nsb is of higher mw than GST and that GST itself is not phosphorylated.
T48 mutants localise normally during mitosis but cannot support proliferation.
To determine whether T48 is important for survivin activity, we first examined the functional repertoire of T48 mutants during mitosis. HeLa cells stably overexpressing survivinT48A-GFP, or a substitution designed to potentially act as a constitutively phosphorylated form, survivinT48E-GFP, hereinafter referred to as T48A and T48E respectively, were generated and examined using fluorescence microscopy. Like wild-type survivin-GFP, both T48A and T48E localized to the centromeres during metaphase, the spindle midzone during anaphase and the midbody during telophase, indicating that altering threonine 48 does not affect the normal distribution of survivin during mitosis (Fig. 2A, left parts). Note that in all our assays T48A and T48E behaved similarly, suggesting T48E can probably be considered a second non-phosphorylatable form, rather than a phosphomimetic, as intended. In addition all cell lines grew similarly regardless of the survivin variant that was overexpressed demonstrating that their presence was not cytotoxic (see Fig. 6B).
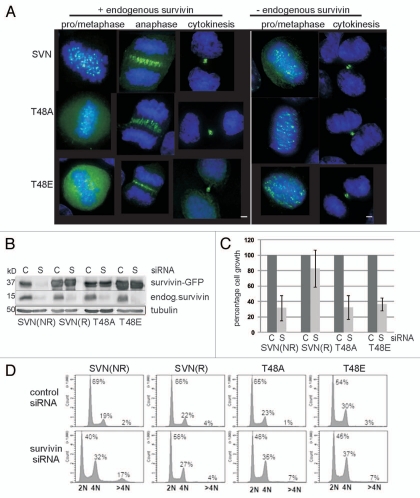
Figure 2.
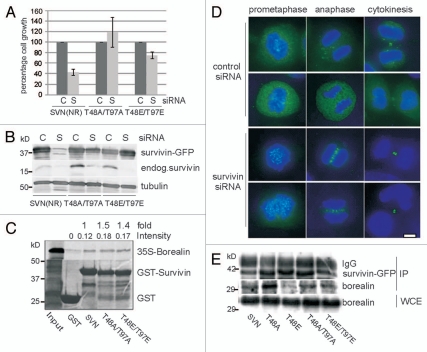
Survivin CK2 mutants localise normally in mitosis but cannot support proliferation. (A) Exponentially growing HeLa cells expressing wild-type or mutant versions of survivin-GFP (green), as indicated, were fixed and stained to localise the DNA with DAPI (blue). All forms localized normally to the centromeres, midzone and midbody, during (pro)metaphase, anaphase and cytokinesis, when the endogenous protein was present (left parts). Upon removal of the endogenous form by RNAi, localization was also normal (right parts); however, no anaphase cells were observed. Bars 5 µm. (B) Endogenous survivin was depleted from the HeLa lines indicated using siRNA, and immunoblotted with anti-survivin antibodies 72 h post-depletion. C, control siRNA; S, survivin-specific siRNA. (NR indicates non-resistant to survivin siRNA; R indicates the resistant form). Both mutants bear resistance to survivin siRNA. (C) Survival of each cell line 72 h post-RNAi treatment was assessed by trypan blue exclusion. Cell growth of the survivin depleted sample is expressed as a percentage of the control cell number, which is given as 100%. The positive control (SVNR) restored cell growth to 80%, while lines expressing T48A and T48E showed comparable response to the negative control population (SVNNR), demonstrating that neither mutant could restore cell proliferation. Results are representative of three independent experiments with error bars indicating standard deviation. (D) FACS profiles of T48 mutants: Asynchronous cell lines were fixed and stained with propidium iodide 72 h post-siRNA. After depletion of the endogenous protein, both T48 mutants displayed a decrease in 2N cells (G1 phase), an increase in 4N cells (G2, M or binucleated), and a modest increase in cells with >4N (polyploid). FACS profiles are respresentative of two independent experiments.
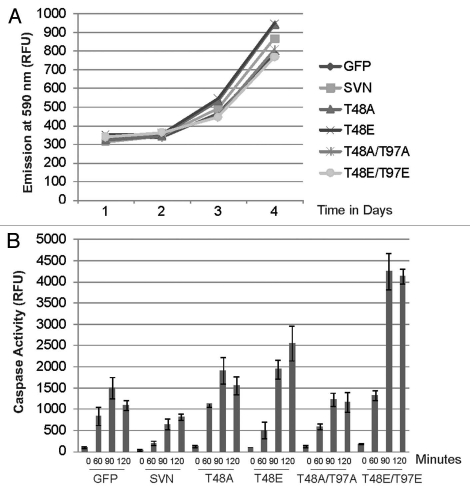
Figure 6.
T48 mutants and T48T97 double mutants abolish survivin's IAP activity. (A) A rezasurin cell proliferation assay was performed on cell lines overexpressing the survivin variants indicated; all cells grew normally. (B) Caspase-3 activity assay. Lysates prepared from the lines indicated after treatment for 0, 60, 90 or 120 minutes in TRAIL were incubated with Ac-DEVD-Amc, and fluorogenic release assessed using a Spectrophotometer in relative fluorescence units (RFU). Extracts prepared from cell lines expressing T48 mutants exhibited high caspase 3 activity, indicating that these forms were unable to protect cells against apoptosis. Second site mutations did not recover cytoprotection. Data is representative of three independent experiments. Average values are plotted with error bars indicating standard deviation within a single experiment performed in triplicate.
To assess whether these versions were mitotically competent we used an RNAi complementation assay. First we re-examined the localization of T48 mutants 72 h after removal of endogenous survivin using survivin-specific dsRNA oligonucleotides. As shown in Figure 2A (right parts), despite depletion of the endogenous form, as evidence by immunoblotting (Fig. 2B), T48A/E mutants still localized to the centromeres and midbody. Note, however, that the mitotic indices in these populations declined (see below), and we were unable to detect any cells in anaphase, suggesting defects in mitotic progression. To assess their proliferative potential, a trypan blue exclusion assay was conducted 72 h post-transfection with control or survivin-specific siRNA, and viable cells counted (Fig. 2C). In this experiment cells expressing an RNAi sensitive version of survivin-GFP failed to proliferate after treatment with siRNA specific to survivin (SVNNR), while those expressing an RNAi resistant wild-type form (SVNR) grew similarly when treated with either control or survivin-specific siRNA. These controls were included to demonstrate both the efficacy of depletion (see also Fig. 2B), and of complementation with wild-type survivinR-GFP in this system. Intriguingly, despite their normal localization neither T48A nor T48E supported cell proliferation in the absence of the endogenous form (Fig. 2C). Consistent with this the mitotic indices of both T48A and T48E populations declined from 1.9 to 0.7% and 1.5 to 0.7% respectively when the endogenous form was removed, whilst wild-type SurvivinR-GFP expressing cells showed little deviation between control (1.9%) and survivin-specific (1.6%) siRNA, as judged by immunofluorescence imaging with phospho-H3 antibodies.
To begin to understand why growth ceased in populations expressing T48 mutants we examined their DNA content by FACS profiling 72 h post transfection (Fig. 2D). Here cells expressing wild-type survivin that was not resistant to RNAi showed a normal cell cycle profile 72 h after transfection with control siRNA G1 = 69%, S = 9%, G2 & M = 19% but, as expected from previous studies,40,44 this pattern changed radically upon elimination of endogenous survivin with a notable decrease in cells with 2N DNA content (G1; 40%), and a concomittant increase in cells with 4N content (G2, M or with 4N ploidy; 32%). In addition, the abundance of cells with a DNA content >4N increased dramatically from 2 to 17%. By contrast, cells exhibiting restored proliferation by expression of the siRNA resistant form of wild-type survivin, showed little difference in FACS profile, whether transfected with control or survivin-specific siRNA. Similarly to the siRNA sensitive control cells, in the absence of endogenous survivin, cells expressing T48A or T48E exhibited a decreased G1 population (46 and 45% respectively), an increased G2 & M population (35 and 37%), and an elevation in the number of cells with >4N DNA content (both 7%), as compared with their profiles in the presence of control siRNA. Collectively these data demonstrate that neither T48A nor T48E can substitute for wild-type survivin to support cell proliferation.
Localization of survivin-GFP in the absence of CK2 phosphorylation.
Next we looked at the interphase localization of T48A and T48E mutants in our stable HeLa cell lines. In the presence of endogenous survivin both forms were cytoplasmic, (Fig. 3B and C, left parts); however, upon siRNA depletion they relocated to the nucleus where they formed discrete subnuclear foci (Fig. 3B and C, right parts). Given this relocation we wondered whether the distribution of survivin-GFP in the absence of CK2 activity would phenocopy this behavior. To this end HeLa cells expressing GFP or survivin-GFP were treated with the CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) for 6 h (100 µM). As with the T48 mutants TBB treatment did not affect the mitotic localization of survivin-GFP in HeLa cells (Fig. 3D), but did induce nuclear accumulation and focus formation in interphase cells (Fig. 3E, middle part). By contrast the distribution of the GFP control was unaffected by TBB (Fig. 3E, right part). As inhibition of CK2 activity has been shown to induce G2 arrest, we were concerned that the nuclear foci were simply a manifestation of G2 arrest, thus we examined the cell cycle distribution of untreated control and 100 µM TBB-treated cells by DNA-FACS-profiling. However, as indicated in Table 1, no difference in cell cycle distribution was apparent. Immunoprobing with anti-CENP-A antibodies revealed that some of these foci were centromeres (Fig. 3E, central part and insert). These data suggest that the response of survivin-GFP to TBB phenocopies the behavior of T48 mutants in HeLa cells, and that CK2 facilitates survivin exclusion from the nucleus in interphase.
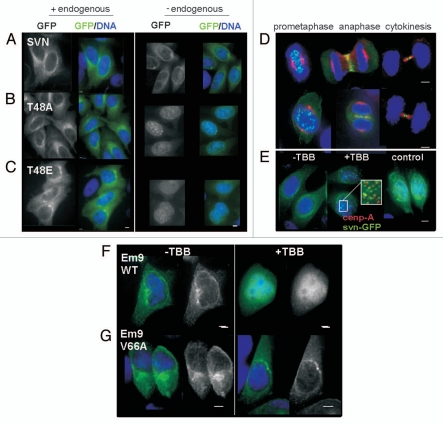
Figure 3.
TBB treatment phenocopies relocation of T48 mutants to the nucleus. (A–C) Interphase localization of GFP tagged SVN, T48A or T48E (green) in formaldehyde fixed, DAPI (blue) stained HeLa cell lines. All forms are distributed in the cytoplasm when endogenous survivin is present (left parts), but after its depletion with siRNA (right parts), T48A and T48E redistribute to the nucleus where they form punctuate foci. (D) Mitotic HeLa cells expressing survivin-GFP (green) were mock-treated (upper part), or treated with 100 µM TBB for 6 h to inhibit CK2 activity (lower part), then fixed and immunoprobed to localise the microtubules (red) and counterstained to localise the DNA (blue). Survivin-GFP localization during mitosis was unaltered by TBB. (E) Interphase cells expressing survivin-GFP were treated with TBB, which caused survivin-GFP to relocate to the nucleus and form discrete foci, some of which were centromeres, as indicated by CENP-A immunostaining (E, middle part inset, red). (E, right part) The localization of GFP alone is unaffected by TBB treatment. (F and G) EM9 cells expressing a TBB-sensitive CK2, or a TBB resistant version of CK2, V66A, were transfected with survivin-GFP then treated with 100 µM TBB for 48 h. Survivin-GFP redistributed from the cytoplasm to the nucleus in TBB-sensitive EM9 cells (F) but not in the TBB resistant cells (G). Bars 5 µm.
Table 1.
Cell cycle analysis of TBB-treated HeLa cells using FACS
| TBB (µM) | G1 | S | G2/M |
| 0 | 64.6 ± 0.1 | 16.7 ± 0.6 | 16 ± 0.4 |
| 100 | 64.2 ± 0.3 | 16.9 ± 0.5 | 13 ± 0.6 |
To control for TBB off-target effects, we carried out similar experiments in EM9 cells harboring TBB-sensitive CK2 or CK2 engineered to be insensitive to TBB via substitution of valine 66 for alanine (V66A). When transiently transfected with survivin-GFP then treated with 100 µM TBB, survivin-GFP became nuclear in EM9 cells expressing TBB-sensitive CK2 (Fig. 3F), but was retained in the cytoplasm in TBB insensitive EM9-V66A cells (Fig. 3G). In contrast to our data in HeLa cells, however, we did not witness the accumulation of survivin-GFP foci in TBB-sensitive cells, thus collectively our data suggest that CK2 activity contributes to the maintenance of survivin in the cytoplasm during interphase.
Survivin T48 mutants display altered affinities for survivin and borealin in vitro.
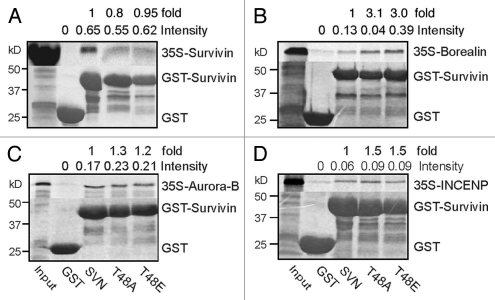
As survivin can self-associate and is an integral component of the chromosomal passenger complex (CPC) during mitosis we next asked whether mutation of T48 compromised its interaction with itself or any other CPP. To this end recombinant GST, GST-survivin and GST-T48 survivin variants were purified and incubated on glutathione beads in the presence of in vitro translated 35S-methionine labelled CPPs, in a series of in vitro pull down assays (Fig. 4). Using this method both T48 mutants interacted with all CPCs examined. Most noteably, after normalizing band intensities of IVT-CPC abundance with GST-protein loadings in each sample, borealin was found to have a three-fold increase in affinity for the mutant forms, compared with the wild-type protein (Fig. 4B). These data suggest that mutation of T48 enhances the affinity of survivin for borealin.
Figure 4.
Mutation of T48 alters the affinity of survivin for borealin in vitro. Recombinant GST, GST-survivin, GST-T48A, or GST-T48E were bound to glutathione sepharose beads and incubated with the in vitro translated 35S-labelled CPP of interest: (A) survivin, (B) borealin, (C) aurora-B or (D) INCENP. While affinity for borealin was three-fold higher with the mutants (B), little difference was observed in their associations with survivin, aurora-B or INCENP. Data is representative of two independent experiments. Lane splicing was carried out in (A and D) to remove irrelevant samples. Pixel intensity of bands was measured using a Storm Phosphoimager, and normalized against the intensity of Coomassie GST-bands. Quantitation is presented as intensity in relative units, and fold difference from wild type.
Mutation of T97, restores the proliferative potential of T48 mutants.
Prior to determining that T48 was the sole phosphorylation target of CK2 in survivin we analyzed the activity of T97 mutants, see reference 45, and double mutants, T48AT97A and T48ET97E. Intriguingly, this second mutation restored proliferation of T48 mutants in our siRNA assay (Fig. 5A and B). By microscopy, we observed that both double mutants localized to the centromeres, midzone and midbody in the absence of endogenous survivin (Fig. 5C, lower parts), although their localization to these sites was impeded when the endogenous form was present (Fig. 5C, upper parts). As our in vitro data in Figure 4B showed that mutation of T48 increased the affinity of survivin for borealin, we next asked whether the additional mutation of T97 could return this interaction to normal. Using a direct GST-pull down assay with IVT-borealin, we found that although T48AT97A and T48ET97E had 1.5- and 1.4-fold increased affinity for borealin over the wild-type form (Fig. 5D), these interactions were greatly reduced when compared with the three-fold increase in the interaction observed for the single mutants (Fig. 4B). Next we analyzed the interaction between the survivin variants and borealin by immunoprecipitation from asynchronous cells cotransfected with cDNA encoding the survivin variant of interest and cDNA to borealin (Fig. 5E). Using this method we discovered that all forms could interact with borealin in vivo, but that T48A exhibited the strongest affinity. As in the in vitro assay, the strength of T48A interaction was diminished when combined with the mutation T97A. Collectively these data suggest that mutation of T97 may restore cell proliferation in the presence of mutations in T48, by altering the binding of survivin to its partner borealin.
Figure 5.
Mutation of T97A/E alleviates repression of survivin activity conferred by T48A/E. (A and B) Cell lines indicated were subjected to RNAi with control (C) or survivin-specific (S) oligos for 72 h. (A) Number of viable cells is presented as a percentage of the control. (B) Immunoblot indicating the efficacy of depletion and confirming resistance of the double mutant forms. (C) Localization of double mutants (green) in the presence (upper parts) and absence (lower parts) of endogenous survivin in HeLa lines after fixation with formaldehyde and staining with DAPI (blue). Bar 5 µm. (D) GST-pull down with 35S-methionine labelled in vitro translated borealin with the GST-constructs indicated. (E) Asynchronous HeLa cells were cotransfected with pcDNA constructs encoding the relevant survivin-GFP variants and pcDNA-borealin. Anti-survivin antibodies and protein A/G beads were used to immunoprecipitate the ectopically expressed survivin variants and immunoblots interrogated for the presence of borealin. Exogenous borealin expression in the WCE, and IP'd GFP tagged version of survivin are shown to demonstrate equality in abundance.
Mutation of T48 abrogates the anti-apoptotic activity of survivin.
Finally, having established that T48 mutants lack the ability to support mitosis, we asked whether the anti-apoptotic activity of survivin was compromised by A/E substitution at this site. First we noted that ectopic expression of any of the survivin variants did not alter the rate of cell proliferation, indicating that they were not cytotoxic (Fig. 6A). Next asynchronous HeLa cells expressing the GFP, or the GFP tagged survivin forms indicated, were treated with TRAIL for 0, 60, 90 or 120 minutes, whole cell extracts were prepared and used in a caspase-3 activity assay to monitor cleavage of the tetrapeptide target DEVD-AMC (Fig. 6B). In this assay lysates from cells overexpressing survivin-GFP inhibited caspase-3 activity as expected. By contrast those prepared from T48A and T48E expressing cells showed elevated caspase-3 activity compared with cells expressing survivin-GFP (or GFP), indicating that they were unable to inhibit (and possibly enhanced) apoptosis. For comparative levels of expression, see control lanes in Figure 2B. When the assay was conducted with cells expressing the double mutants, T97A was found to attenuate caspase activity observed by cells expressing T48A alone; however, its expression did not confer protection against apoptosis. Combining T97E with T48E exacerbated apoptosis. Hence, we conclude that neither double mutant could restore the antiapoptotic activity of survivin once abrogated by mutation of T48.
Discussion
Survivin is a CK2 substrate.
From the bioinformatics service, NetPhos, survivin was predicted to be phosphorylated by CK2 at two sites, T48 and T97 (reviewed in ref. 46). Both these sites and their adjacent residues have been highly conserved throughout evolution, suggesting that they play a fundamental role in survivin function. In this study we report that survivin is indeed phosphorylated by CK2, but find that T48 in the globular BIR domain unique residue that is targeted. Consistent with these findings, for optimal phosphorylation of its substrates CK2 prefers acidic residues downstream of the target S/T, a consensus to which T48, but not T97, conforms.14 The context of T97 actually fits precisely the consensus for plk1;47 however, as with CK2, we recently showed that, T97A can still be phosphorylated by plk1 in vitro, and that S20 the NH2 terminus of survivin is the principle site targeted by plk1.9
CK2 regulates the mitotic function of survivin.
Here, using mutagenic analysis we have demonstrated that T48 is a critical residue for the proper functioning of survivin during mitosis as neither T48A nor T48E were able to support cell proliferation. Curiously, as mentioned above, T48 lies within the BIR domain of survivin, a region considered characteristic of proteins that inhibit apoptosis, the inhibitor of apoptosis (IAP) proteins. Indeed, survivin was assigned membership to the IAP family by the presence of this domain.48,49 However, emerging data support the notion that the BIR fold is not exclusive to anti-apoptotic proteins, but is involved in many protein-protein interactions and multiple pathways.50 Excitingly, Higgins and co-workers51 recently reported that two aspartic acids (D70D71) at the very tip of the BIR domain of survivin, previously found to aid association with aurora-B kinase,52 are responsible for positioning the CPC to the centromere during mitosis where they interact with Haspin-phosphorylated histone H3, see also reference 53. T48 is the second phosphorylation site identified in the BIR domain of survivin with mitotic relevance: Cdk1 targets T34, and mutation of T34 influences both the mitotic and anti-apoptotic activities of survivin.5,6,10–12 In contrast to Cdk1 which is only active during mitosis, CK2 is a constitutively active “house-keeping” kinase, thus it will be interesting to determine whether survivin is phosphorylated throughout the cell cycle by CK2.
CK2 phosphorylation regulates the interphase localization of survivin and its IAP activity.
When overexpressed in interphase cells survivin is predominantly cytoplasmic. This localization is regulated, at least in part, by a nuclear exportation sequence in its central linker domain.45,54 T48 mutants retain this cytoplasmic location provided the endogenous protein is present; however, in its absence, they enter the nucleus where they form speckles, some of which are associated with centromeres. These data are interesting as we recently showed that when forcibly expressed in the nucleus by NLS fusion, survivin is rapidly degraded in a cdh1 dependent manner.55 The presence of T48 mutants in the nucleus (whether focal or diffuse) after depletion of the endogenous protein suggests that these versions are more resistant to degradation than NLS-survivin-GFP, thus it is formally possible that modification of T48 could in some way regulate its turnover. Moreover, survivin needs to be cytoplasmic, as opposed to nuclear, in order to protect cells from death threats.45,55 Here we have reported that T48 mutants, despite being positioned in the cytoplasm in the presence of the endogenous protein, are unable to protect cells against apoptosis, demonstrating that although cytoplasmic localization is necessary for cytoprotection, phosphorylation of T48 is also required. As CK2 activation has been linked to the inhibition of apoptosis,21,23,56,57 it is tempting to speculate that its contribution is through phosphorylation of survivin, but with a plethora of substrates, it is unlikely that the cascade is so simplistic. Further possibilities are that phosphorylation of T48 facilitates binding to another protein that is needed to inhibit apoptosis, such as XIAP,58 or prevents binding to Smac/ DIABLO, and a non-acidic mutant form, D53A, like T48 mutants, is also unable to inhibit apoptosis.59 However, preliminary GST pulldown data from our lab suggest that T48 mutants can interact with XIAP in vitro, and the importance of the Smac/DIABLO-survivin interaction in the inhibition of apoptosis is not entirely clear.60 Indeed, how survivin prevents apoptosis at the molecular level is yet to be understood.
Phosphorylation of survivin at T48 influences its association with borealin.
Our in vitro and immunoprecipitation data suggest that T48 regulates heterodimerization of survivin and borealin. Localization of T48 mutants to the centromeres is consistent with their ability to bind borealin, as it has been reported that survivin is not configured as a homodimer when part of the CPC.61,62 However, another implication from our data is that for completion of mitosis, survivin may need to be phosphorylated at T48 and allowed to reassociate with itself. Crystallographic data has shown that survivin interacts with itself,63,64 and with borealin,61,62 via its central, “linker” region, between the BIR domain and the C-terminal alpha helix, thus it is interesting that modification of the BIR domain could influence these interactions. Intriguingly, that the loss in mitotic potential observed by T48 mutants can be compensated for by a second mutation within the linker region, T97, further suggests cooperation between the BIR domain and the dimerization site. Although we cannot exclude the possibility that a conformational change is required before T97 is accessed by a kinase, such as plk1 or CK2, it will be interesting to determine whether it is indeed phosphoregulated and which kinase is responsible for modification of this pivotal site.
In conclusion, these data show for the first time that survivin is a CK2 substrate and identify T48 as its target residue in vitro. Using mutagenic analysis in combination with overexpression, RNAi complementation and apoptosis assays, we have demonstrated that this residue is not only critical for survivin to inhibit cell death, but is also crucial for mitosis. Our protein interaction assays show that mutation of T48 alters the binding affinity of survivin for borealin. Collectively these data suggest that the phospho-status of survivin at T48, which can be regulated by CK2, alters its binding to borealin and thereby may influence its ability to promote cell proliferation and inhibit cell death.
Materials and Methods
Molecular biology.
Site directed mutants were generated using relevant primers and QuikChange site-directed mutagenesis (Stratagene) with wild-type human survivin (accession number NM001168), bearing a siRNA resistant mutation (C54G) in pBluescript as template, see reference 39 and 40. Once generated, mutants were subcloned into pGEX4T1 (G.E.Healthcare) for recombinant expression, or into pcDNA3.1 (Invitrogen), with a C-terminal GFP tag, for analysis in tissue culture cells. All mutants were confirmed by sequencing of the final construct.
In vitro kinase assays.
Recombinant GST, GST-Survivin, GST-Survivin-T48A and GST-Survivin-T97A (see GST pull down for expression), were incubated for 20 minutes at 37°C with purified CK2 (a gift from Prof. E. Pinna), 32P-γATP (5 µCi) and 0.1 mM ATP in kinase buffer (25 mM Tris-HCL pH 7.5, 5 mM MgCl2, 1 mM DTT, 0.25 mM EDTA, 50 mM NaCl) in a final volume of 20 µl.
Cell lines, transfection and cell proliferation assay.
All cells were cultured at 37°C and 5% CO2 in DMEM with 10% FCS, 1% penicillin-streptomycin and 1% fugizome. HeLa cell lines stably expressing GFP, survivin-GFP, survivinR-GFP or survivinRT48A-GFP and survivinRT48E-GFP (referred to as T48A and T48E) were established as described previously in reference 10. Clones were selected in 500 µg/ml G418, then pooled and FACS sorted to yield homogeneous populations.
EM9 cells were a gift from Prof. Keith Caldecott.41 Where indicated cells were transfected with pcDNA3.1 constructs using FuGene 6 (Roche) diluted in Optimem, and cultured in antibiotic-free medium, according to the manufacturer's guidelines. Cell viability and proliferation was assessed either using trypan blue exclusion and a hemocytometer or using a resazurin assay. Resazurin assay: cells were grown in 96-well plates then incubated for 1 h at 37°C in 10 µg/ml resazurin prepared in complete medium. Using a spectrophotometer (Fluostar Galaxy) fluorescence emission was read at 590 nm after excitation at 530 nm. All samples were prepared in triplicate.
Immunofluorescence and fluorescence imaging.
Cells were grown on polylysine coated coverslips then fixed and permeabilized using 4% formaldehyde and 0.15% triton in PBS as previously described in reference 9. For immunoprobing, cells were blocked with 1% BSA in PBS, then incubated sequentially for 1 h at RT with primary, then texas-red conjugated secondary antibodies (1/200, Vector). Centromeres were detected with anti-CENPA (1/1,000, AbCam, Ab13939), mitotic spindles with tubulin (B512, 1/2,000, Sigma, T5168), mitotic indices were determined using anti-phospho-H3 (1/500, Upstate, 06-570). All samples were counterstained with DAPI in Vectashield (Vector Labs). Image stacks were acquired using an Olympus inverted microscope fitted with an x63 (NA 1.4) oil immersion objective and Deltavision Software (Applied Precision). Images presented are 2D projections of 0.3 µm stepped Z-stacks.
RNAi.
Exponentially growing HeLa cells were seeded immediately before RNAi transfection at a density of 5 × 104 per well of a 24-welled plate and cultured in antibiotic free DMEM with 10% FCS. Control and survivin-specific oligonucleotides (Ambion) diluted in OptiMEM (Invitrogen) were transfected into cells at a final concentration of 10 nM, using siPORT NeoFX (ABI Biosystems, AM4511), and cells allowed a minimum of 48 h to grow in antibiotic free medium before analysis. RNAi insensitive versions of survivin were made resistant to siRNA knockdown by a base substitution C54G, in the siRNA targeting region see reference 40, and is denoted “R” for clarity where appropriate. All mutant forms are “R.”
Immunoblotting/immunoprecipitation.
Standard procedures were used for immunoblotting with 0.22 µm nitrocellulose, and the enhanced chemiluminescence method of detection (Pierce). Anti-survivin antibodies were used (in-house; 1/1,000) and anti-tubulin (B512, 1/2,000, Sigma, T5168), to assess loading. HRP-conjugated secondary antibodies were from DAKO. Immunoprecipitation: pcDNA3.1 encoding 1 µg each of survivin variants tagged to GFP and untagged borealin were transiently co-transfected into 5 × 105 HeLa cells using TransIT LT1 (Mirus, MIR2300) and whole cell lysates prepared 24 h post-transfection in RIPA buffer. To immunoprecipitate survivin-GFP and its variants lysates were incubated for 2 h at room temperature with 4 µg polyclonal anti-survivin antibodies (in-house) then incubated overnight at 4°C with 25 µl protein A/G beads (Pierce, 20421). After a series of RIPA-buffer based washes, see reference 42, proteins were boiled off the beads in Laemmli sample buffer, separated by SDS-PAGE and transferred to nitrocellulose. Subsequent blots were immunoprobed with anti-borealin (polyclonal, in-house; 1/500), and goat anti-survivin (1/1,000, R & D Systems, AF6471) antibodies.
Flow cytometry.
The DNA content of cells was assessed by propidium iodide staining. 500,000 cells were harvested, washed in PBS, then resuspended in 1 ml of ice cold 70% ethanol and left on ice for 2 h at 4°C. Cells were pelletted by gentle centrifugation, washed with PBS and resuspended in 100 µg/ml propidium iodide (Sigma) and 100 µg/ml RNase (Sigma) diluted in PBS. Cells were analyzed using a Fluorescence Activated Cell Sorter (FACSCanto, BD Biosciences) with FACS Diva software.
Inhibition of CK2 activity.
CK2 activity was inhibited in cultured cells by treatment with 4,5,6,7-tetrabromo-2-azabenzamidazole (TBB), initially a gift from Prof. E. Pinna, latterly from Sigma.43 TBB was diluted directly into culture medium to a working concentration of 100 µM from a 10 mM stock in DMSO.
Caspase assays.
Cells were seeded at 1 × 105 per well in 24-well plates, and cultured for 16 h before induction of apoptosis. Cells were treated with 250 ng/ml recombinant TRAIL (PeproTech EC Ltd., 310-04) for the times indicated, then lysed in 150–200 µl of MPER buffer (Pierce) containing 1 mM EDTA, 1 µg/µl pepstatin A, 1 mM AEBSF for 45 minutes and centrifuged at 13,000 rpm to remove debris. Caspase activity assays were performed in a 96-well microtiter plate: 5 µg of caspase-3 substrate (Ac-DEVD-AMC; Biomol, P-411) was incubated with 20 µl of cell lysate and 200 µl of reaction buffer (20 mM HEPES, 10% glycerol, 2 mM DTT, pH 7.5) at 37°C for 1 h. Fluorescent emission was measured using a Spectrofluorometer (Fluostar Galaxy) with excitation and emission wavelengths set at 390 and 450 nm respectively.
GST pull downs.
All genes encoding GST tagged recombinant proteins were cloned in pGEX4T1, and protein expression induced in BL21 E. coli with 0.5 mM IPTG at 18–20°C, using standard methods, see reference 42. Recombinant proteins were bound to glutathione-sepharose 4B beads (G.E Healthcare) in lysis buffer (PBS, 10 mM EDTA (pH 7.5), 0.05% triton X-100, 100 µM PMSF and 1 µg/ml CLAP) by incubation for 1 h at 4°C with rotation. For in vitro translation proteins were transcribed and translated using the T7 or T3 TNT coupled reticulocyte lysate system (Promega) with approximately 0.04 mCi of 35S methionine (G.E Healthcare) as tracer. After binding to the beads, GST-lysates were washed with PBS then incubated with the in vitro translated protein of choice for 1 h at 4°C. Beads were washed with 1 ml of lysis buffer x3, then boiled in SDS loading buffer and analyzed by SDS-PAGE and autoradiography.
Acknowledgements
The majority of this work was supported by a Cancer Research-UK through a Senior Fellowship held by S.P.W. and a Ph.D., studentship held by RB. We thank CR-UK for this funding. We would also like to thank Prof. K. Caldecott for EM9 cells; Prof. E. Pinna for TBB; Prof. W.C. Earnshaw for initial the pBS-borealin from which pcDNA-borealin was derived; Ms. N. Lovegrove for FACS sorting cells and Mr. Jamie Webster for technical support.
Abbreviations
- CPP
chromosomal passenger protein
- IAP
inhibitor of apoptosis
- CK2
casein kinase 2
- CPC
chromosomal passenger complex
- SAC
spindle assembly checkpoint
References
- 1.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 2.Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression. Cell Cycle. 2009;8:2708–2710. doi: 10.4161/cc.8.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: Conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 4.Sun SC, Wei L, Li M, Lin SL, Xu BZ, Liang XW, et al. Perturbation of survivin expression affects chromosome alignment and spindle checkpoint in mouse oocyte meiotic maturation. Cell Cycle. 2009;8:3365–3372. doi: 10.4161/cc.8.20.9855. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor DS, Grossman D, Plescia J, Li FZ, Zhang H, Villa A, et al. Regulation of apoptosis at cell division by p34(cdc2) phosphorylation of survivin. PNAS. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor DS, Wall NR, Porter ACG, Altieri DC. A p34cdc2 survival checkpoint in cancer. Cancer Cell. 2002;2:43–54. doi: 10.1016/s1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley SP, Henzing AJ, Dodson H, Khaled W, Earnshaw WC. Aurora-B phosphorylation in vitro identifies a residue of survivin that is essential for its localization and binding to INCENP in vivo. J Biol Chem. 2004;279:5655–5660. doi: 10.1074/jbc.M311299200. [DOI] [PubMed] [Google Scholar]
- 8.Wheatley SP, Barrett RMA, Andrews PD, Medema RH, Morley SJ, Swedlow JR, et al. Phosphorylation of aurora-B negatively regulates survivin function during mitosis. Cell Cycle. 2007;6:1220–1230. doi: 10.4161/cc.6.10.4179. [DOI] [PubMed] [Google Scholar]
- 9.Colnaghi R, Wheatley SP. Liaisons between survivin and plk1 during cell division and cell death. J Biol Chem. 2010;285:22592–22604. doi: 10.1074/jbc.M109.065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett RMA, Osborne TP, Wheatley SP. Phosphorylation of survivin at threonine 34 inhibits its mitotic function and enhances its cytoprotective activity. Cell Cycle. 2009;8:278–283. doi: 10.4161/cc.8.2.7587. [DOI] [PubMed] [Google Scholar]
- 11.Jones MK, Padilla OR, Webb NA, Norng M. The antiapoptosis protein, survivin, mediates gastric epithelial cell cytoprotection against ethanol-induced injury via activation of the p34cdc2 cyclin-dependent kinase. J Cell Physiol. 2008;215:750–764. doi: 10.1002/jcp.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marusawa H, Matsuzawa S, Welsh K, Zou H, Armstrong R, Tamm I, et al. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J. 2003;22:2729–2740. doi: 10.1093/emboj/cdg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohi T, Xia F, Altieri DC. Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol Cell. 2007;27:17–28. doi: 10.1016/j.molcel.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 15.Meek DW, Simon S, Kikkawa U, Eckhart W. The p53 tumor suppressor protein is phosphorylated at serine 389 by casein kinase II. EMBO J. 1990;9:3253–3260. doi: 10.1002/j.1460-2075.1990.tb07524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo GL, Vandenberg MT, Yu IJ, Bae YS, Franza BR, Jr, Marshak DR. Casein kinase II phosphorylates p34cdc2 kinase in G1 phase of the HeLa cell division cycle. J Biol Chem. 1992;267:20317–20325. [PubMed] [Google Scholar]
- 17.Romero-Oliva F, Allende JE. Protein p21(WAF1/CIP1) is phosphorylated by protein kinase CK2 in vitro and interacts with the amino terminal end of the CK2 beta subunit. J Cell Biochem. 2001;81:445–452. doi: 10.1002/1097-4644(20010601)81:3<445::aid-jcb1058>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Tapia JC, Bolanos-Garcia VM, Sayed M, Allende CC, Allende JE. Cell cycle regulatory protein p27KIP1 is a substrate and interacts with the protein kinase CK2. J Cell Biochem. 2004;91:865–879. doi: 10.1002/jcb.20027. [DOI] [PubMed] [Google Scholar]
- 19.Li PF, Li J, Muller EC, Otto A, Dietz R, von Harsdorf R. Phosphorylation by protein kinase CK2: A signaling switch for the caspase-inhibiting protein ARC. Mol Cell. 2002;10:247–258. doi: 10.1016/s1097-2765(02)00600-7. [DOI] [PubMed] [Google Scholar]
- 20.Ubeda M, Habener JF. CHOP transcription factor phosphorylation by casein kinase 2 inhibits transcriptional activation. J Biol Chem. 2003;278:40514–40520. doi: 10.1074/jbc.M306404200. [DOI] [PubMed] [Google Scholar]
- 21.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, et al. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 22.Ruzzene M, Penzo D, Pinna LA. Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J. 2002;364:41–47. doi: 10.1042/bj3640041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krippner-Heidenreich A, Talanian RV, Sekul R, Kraft R, Thole H, Ottleben H, et al. Targeting of the transcription factor Max during apoptosis: phosphorylation-regulated cleavage by caspase-5 at an unusual glutamic acid residue in position P1. Biochem J. 2001;358:705–715. doi: 10.1042/0264-6021:3580705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe N, Arai H, Iwasaki J, Shiina M, Ogata K, Hunter T, et al. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc Natl Acad Sci USA. 2005;102:11663–11668. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yde CW, Olsen BB, Meek D, Watanabe N, Guerra B. The regulatory beta-subunit of protein kinase CK2 regulates cell cycle progression at the onset of mitosis. Oncogene. 2008;27:4986–4997. doi: 10.1038/onc.2008.146. [DOI] [PubMed] [Google Scholar]
- 26.Daum JR, Gorbsky GJ. Casein kinase II catalyzes a mitotic phosphorylation on threonine 1342 of human DNA topoisomerase IIalpha, which is recognized by the 3F3/2 phosphoepitope antibody. J Biol Chem. 1998;273:30622–30629. doi: 10.1074/jbc.273.46.30622. [DOI] [PubMed] [Google Scholar]
- 27.Escargueil AE, Plisov SY, Filhol O, Cochet C, Larsen AK. Mitotic phosphorylation of DNA topoisomerase II alpha by protein kinase CK2 creates the MPM-2 phosphoepitope on Ser-1469. J Biol Chem. 2000;275:34710–34718. doi: 10.1074/jbc.M005179200. [DOI] [PubMed] [Google Scholar]
- 28.Yu IJ, Spector DL, Bae YS, Marshak DR. Immunocytochemical localization of casein kinase II during interphase and mitosis. J Cell Biol. 1991;114:1217–1232. doi: 10.1083/jcb.114.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krek W, Maridor G, Nigg EA. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992;116:43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faust M, Gunther J, Morgenstern E, Montenarh M, Gotz C. Specific localization of the catalytic subunits of protein kinase CK2 at the centrosomes. Cell Mol Life Sci. 2002;59:2155–2164. doi: 10.1007/s000180200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litchfield DW, Lozeman FJ, Cicirelli MF, Harrylock M, Ericsson LH, Piening CJ, et al. Phosphorylation of the beta subunit of casein kinase II in human A431 cells. Identification of the autophosphorylation site and a site phosphorylated by p34cdc2. J Biol Chem. 1991;266:20380–20389. [PubMed] [Google Scholar]
- 32.Litchfield DW, Luscher B, Lozeman FJ, Eisenman RN, Krebs EG. Phosphorylation of casein kinase II by p34cdc2 in vitro and at mitosis. J Biol Chem. 1992;267:13943–13951. [PubMed] [Google Scholar]
- 33.St Denis NA, Derksen DR, Litchfield DW. Evidence for regulation of mitotic progression through temporal phosphorylation and dephosphorylation of CK2αλπηα. Mol Cellular Biol. 2009:2068–2081. doi: 10.1128/MCB.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada M, Yamamoto A, Murakami-Tonami A, Nakanshi M, Yoshida T, Aiba H, et al. Casein kinase II is required for the spindle assembly checkpoint regulating Mad2p in fission yeast. Biochem Biophys Res Comm. 2009;388:529–532. doi: 10.1016/j.bbrc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Munstermann U, Fritz G, Seitz G, Lu YP, Schneider HR, Issinger OG. Casein kinase II is elevated in solid human tumors and rapidly proliferating non-neoplastic tissue. Eur J Biochem. 1990;189:251–257. doi: 10.1111/j.1432-1033.1990.tb15484.x. [DOI] [PubMed] [Google Scholar]
- 36.Duncan JS, Litchfield DW. Too much of a good thing: The role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochimica et Biophysica Acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Lebrin F, Chambaz EM, Bianchini L. A role for protein kinase CK2 in cell proliferation: evidence using a kinase-inactive mutant of CK2 catalytic subunit alpha. Oncogene. 2001;20:2010–2022. doi: 10.1038/sj.onc.1204307. [DOI] [PubMed] [Google Scholar]
- 38.Tapia JC, Torres VA, Rodriguez DA, Leyton L, Quest AF. Casein kinase 2 (CK2) increases survivin expression via enhanced beta-catenin-T cell factor/lymphoid enhancer binding factor-dependent transcription. PNAS. 2006;103:15079–15084. doi: 10.1073/pnas.0606845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheatley SP, Carvalho A, Vagnarelli P, Earnshaw WC. INCENP is required for proper targeting of survivin to the centromeres and the anaphase spindle during mitosis. Curr Biol. 2001;11:886–890. doi: 10.1016/s0960-9822(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in response to loss of spindle tension in HeLa cells. J Cell Sci. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- 41.Loziou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, et al. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell. 2004;117:17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- 42.Noton EA, Colnaghi R, Tate S, Starck C, Carvalho A, Ferrigno PK, et al. Molecular analysis of survivin isoforms: evidence that alternatively spliced variants do not play a role in mitosis. J Biol Chem. 2006;281:1286–1295. doi: 10.1074/jbc.M508773200. [DOI] [PubMed] [Google Scholar]
- 43.Sarno S, Reddy H, Meggio F, Ruzzene MSPD, Donella-Deana A, et al. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of CK2 (“casein kinase-2”) FEBS Lett. 2001;496:44–48. doi: 10.1016/s0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- 44.Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, et al. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J. 2003;22:2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colnaghi R, Connell CM, Barrett RMA, Wheatley SP. Separating the anti-apoptotic and mitotic roles of survivin. J Biol Chem. 2006;281:33450–33456. doi: 10.1074/jbc.C600164200. [DOI] [PubMed] [Google Scholar]
- 46.Mahotka C, Wenzel M, Springer E, Gabbert HE, Gerharz CD. Survivin-{{Delta}}Ex3 and Survivin-2B: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res. 1999;59:6097–6102. [PubMed] [Google Scholar]
- 47.Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for plk (polo-like kinase) phosphorylation reveals myt1 as a plk1 substrate. J Biol Chem. 2003;278:25277–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- 48.Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem. 1998;273:11177–11182. doi: 10.1074/jbc.273.18.11177. [DOI] [PubMed] [Google Scholar]
- 49.Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001;2:1–10. doi: 10.1186/gb-2001-2-7-reviews3009. 3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: More than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7:1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- 51.Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, et al. Histone H3 Thr-3 phosphorylation by haspin positions Aurora B at centromeres in mitosis. Science. 2010;330:231–235. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao L, Yan X, Wu Y, Hu H, Li Q, Zhou T, et al. Survivin mutant (Surv-DD70,71AA) disrupts the interaction of survivin with aurora-B and causes multinucleation in HeLa cells. Biochem Biophys Res Comm. 2006;346:400–407. doi: 10.1016/j.bbrc.2006.05.131. [DOI] [PubMed] [Google Scholar]
- 53.Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 Threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330:235–239. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knauer SK, Mann W, Stauber RH. Survivin's dual role: An exports view. Cell Cycle. 2007;6:518–521. doi: 10.4161/cc.6.5.3902. [DOI] [PubMed] [Google Scholar]
- 55.Connell CM, Colnaghi R, Wheatley SP. Nuclear survivin has reduced stability and is not cytoprotective. J Biol Chem. 2008;238:3289–3296. doi: 10.1074/jbc.M704461200. [DOI] [PubMed] [Google Scholar]
- 56.Yamane K, Kinsella TJ. CK2 inhibits apoptosis and changes its cellular localization following ionizing radiation. Cancer Res. 2005;65:4362–4367. doi: 10.1158/0008-5472.CAN-04-3941. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: An emerging role for protein kinase CK2. Trends Cell Biol. 2002;12:226–230. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- 58.Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, et al. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- 59.Song Z, Liu S, He H, Hoti N, Wang Y, Feng S, et al. A single amino acid change (Asp53-Ala53) converts survivin from anti-apoptotic to pro-apoptotic. Mol Biol Cell. 2004;15:1287–1296. doi: 10.1091/mbc.E03-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKay TR, Bell S, Tenev T, Stoll V, Lopes R, Lemoine NR, et al. Procaspase 3 expression in ovarian carcinoma cells increases survivin transcription which can be countered with a dominant-negative mutant, survivin T34A: A combination gene therapy strategy. Oncogene. 2003;22:3539–3547. doi: 10.1038/sj.onc.1206417. [DOI] [PubMed] [Google Scholar]
- 61.Bourhis E, Hymowitz SG, Cochran AG. The mitotic regulator survivin binds as a monomer to its functional interactor borealin. J Biol Chem. 2007;282:35018–35023. doi: 10.1074/jbc.M706233200. [DOI] [PubMed] [Google Scholar]
- 62.Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a survivin-borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 63.Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2000;7:602–608. doi: 10.1038/76838. [DOI] [PubMed] [Google Scholar]
- 64.Chantalat L, Skoufias DA, Kleman JP, Jung B, Dideberg O, Margolis RL. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alphahelical extensions. Mol Cell. 2000;6:183–189. [PubMed] [Google Scholar]
- 65.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]