Summary
Homeostatic control of body fluid CO2 is essential in animals but is poorly understood. C. elegans relies on diffusion for gas exchange and avoids environments with elevated CO2. We show that C. elegans temperature, O2, and salt-sensing neurons are also CO2 sensors mediating CO2 avoidance. AFD thermosensors respond to increasing CO2 by a fall and then rise in Ca2+ and show a Ca2+ spike when CO2 decreases. BAG O2 sensors and ASE salt sensors are both activated by CO2 and remain tonically active while high CO2 persists. CO2-evoked Ca2+ responses in AFD and BAG neurons require cGMP-gated ion channels. Atypical soluble guanylate cyclases mediating O2 responses also contribute to BAG CO2 responses. AFD and BAG neurons together stimulate turning when CO2 rises and inhibit turning when CO2 falls. Our results show that C. elegans senses CO2 using functionally diverse sensory neurons acting homeostatically to minimize exposure to elevated CO2.
Highlights
► The major temperature, O2, and salt-sensing neurons of C. elegans are CO2 sensors ► AFD, BAG, and ASE neurons have unique CO2-response properties ► O2-sensing atypical soluble guanylate cyclases also mediate CO2 neuronal responses ► CO2 sensing involves both transient and persistent neuronal responses
Introduction
As the major by-product of oxidative metabolism, CO2 is ubiquitous in nature. Although CO2 comprises only ∼0.038% of Earth's atmosphere, it can accumulate to higher levels in environments with high respiration rates (Lahiri and Forster, 2003). Organisms have evolved CO2-sensing mechanisms to monitor both external and internal CO2 concentrations, but how these systems function to control physiology and behavior remain poorly understood.
Mice can smell environmental CO2 concentrations as low as 0.066% CO2 using specialized olfactory neurons that express carbonic anhydrase II (Hu et al., 2007). Carbonic anhydrases catalyze hydration of CO2 to generate H+ and HCO3−. HCO3− is thought to stimulate the mouse olfactory neurons by activating a guanylate cyclase, GC-D (Hu et al., 2007; Sun et al., 2009). In humans the GC-D homolog is a pseudogene, and we cannot smell CO2 (Young et al., 2007). However, we can taste CO2 in carbonated solutions via sour-sensing cells on our tongues (Chandrashekar et al., 2009). In rodents, CO2 levels of 10% or more elicit an innate fear response in which animals freeze and avoid open spaces (Ziemann et al., 2009). This response requires activation of the acid-sensing ion channel ASIC-1A in cells of the amygdala (Ziemann et al., 2009). High concentrations of inhaled CO2 also modulate wakefulness by stimulating midbrain neurons (Williams et al., 2007; Richerson, 2004; Buchanan and Richerson, 2010).
Insects also sense and respond to environmental CO2. Drosophila adults and larvae avoid CO2 levels as low as 0.1% (Suh et al., 2004; Faucher et al., 2006). Like the CO2-evoked fear behavior in mice, Drosophila CO2 avoidance is innate (Suh et al., 2004) and may be part of an alarm response: stressed flies release 3- to 4-fold more CO2 than unstressed flies (Suh et al., 2004). Drosophila senses gaseous CO2 using two olfactory receptors, Gr21a and Gr63a, which are expressed in antennal sensory neurons (Jones et al., 2007; Kwon et al., 2007). Like other insect olfactory receptors, these do not have homologs in vertebrates or worms (Vosshall and Stocker, 2007). Artificial activation of the Gr21a/Gr63a-expressing neurons elicits an avoidance response (Suh et al., 2007). Whether the Gr21a/Gr63a receptor binds molecular CO2 or a CO2 derivative is not known. Interestingly, some food-associated odorants inhibit Gr21a/Gr63a CO2 receptor function, and the presence of food reduces CO2 avoidance (Turner and Ray, 2009). Although Drosophila avoids gaseous CO2, it is attracted to carbonated substrates, a response mediated by HCO3−-sensitive neurons in the proboscis (Fischler et al., 2007).
Besides monitoring external CO2, many animals also monitor internal CO2. Internal CO2 levels are regulated by respiratory gas exchange (Lahiri and Forster, 2003; Feldman et al., 2003; Bustami et al., 2002), but when left unregulated can lead to toxic changes in body fluid pH and death (Richerson, 2004). Mammalian respiratory CO2 chemoreception occurs in the brain and carotid bodies (Lahiri and Forster, 2003). The molecular mechanisms are unclear, but CO2-sensitive cells express carbonic anhydrases (Coates et al., 1998; Cammer and Brion, 2000), and changes in extracellular or intracellular pH modulate signaling via H+-sensitive ion channels (Lahiri and Forster, 2003; Richerson et al., 2005; Buckler et al., 2000; Feldman et al., 2003; Richerson, 2004; Jiang et al., 2005). Insects achieve respiratory gas exchange by opening and closing spiracles, but the control mechanisms involved are not known (Hetz and Bradley, 2005; Lehmann and Heymann, 2005).
Many small animals, including the nematode C. elegans, lack a specialized respiratory system and use diffusion for gas exchange. As in other animals, high CO2 levels are toxic (Sharabi et al., 2009). C. elegans appears to control internal CO2 by avoiding environments where this gas exceeds ∼0.5%. Avoidance requires cGMP-gated ion channels containing the TAX-2 and TAX-4 subunits (Bretscher et al., 2008; Hallem and Sternberg, 2008). Also implicated are the BAG sensory neurons, required for acute avoidance of a high CO2 and low O2 mixture (Hallem and Sternberg, 2008). Recent work indicates that the BAG neurons are transiently activated when ambient O2 levels fall below 10% (Zimmer et al., 2009).
Here, we show that the C. elegans head sensory neurons AFD, BAG, and ASE are primary CO2 sensors. AFD, BAG, and ASE were previously only known to detect changes in temperature, O2, and salt ion levels, respectively. Using Ca2+ imaging, we describe the CO2 responses of these neurons, which include ON, OFF, and perduring responses. We show that some, but not all, of the Ca2+ responses to CO2 depend on a cGMP-gated ion channel. Finally, we dissect how the C. elegans CO2 sensory system regulates CO2-evoked behavior. We find that the contribution of different sensors to behavior varies widely, depending on both context and stimulus dynamics.
Results
Multiple Sensory Neurons Mediate C. elegans Avoidance of CO2
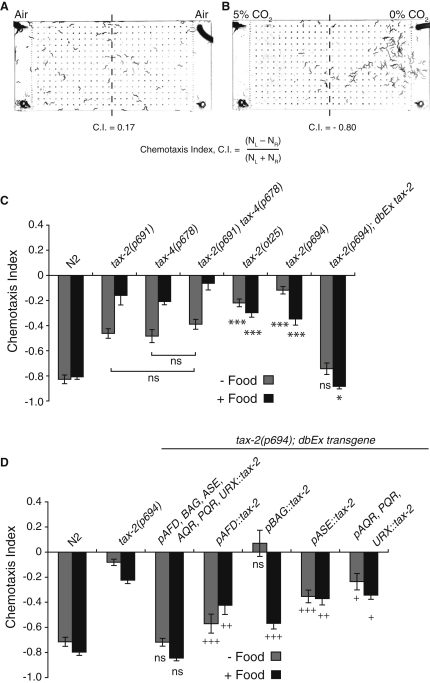
When placed in a 5%-0% CO2 gradient, C. elegans migrate away from high CO2 (Figures 1A and 1B) (Bretscher et al., 2008). We used this assay to identify potential CO2-sensing neurons. Mutants defective in either the TAX-4 α or TAX-2 β cGMP-gated ion channel subunits show reduced CO2 avoidance, both in the presence and absence of E. coli food (Figure 1C) (Bretscher et al., 2008; Hallem and Sternberg, 2008). The defects of tax-2; tax-4 double mutants recapitulated those of single mutants (Figure 1C), consistent with α and β subunits functioning together. tax-2 and tax-4 are coexpressed in 14 of 40 C. elegans sensory neuron classes (White et al., 1986; Komatsu et al., 1996; Coburn and Bargmann, 1996), implicating a subset of these neurons in CO2 sensing. A tax-2 promoter mutation, tax-2(p694), also disrupted CO2 avoidance (Figure 1C). Previous work reported that this allele deletes exon 1 and ∼1.6 kb of tax-2 upstream sequences (Coburn and Bargmann, 1996). However, our sequencing data suggest that it removes only 365 bp in this interval (details in Supplemental Experimental Procedures available online). tax-2(p694) mutants have deficits in behaviors mediated by the AFD, BAG, ASE, AQR, PQR, and URX neurons but appear wild-type for responses mediated by other tax-2 expressing neurons (Dusenbery et al., 1975; Hedgecock and Russell, 1975; Coburn and Bargmann, 1996; Coates and de Bono, 2002). Selectively expressing tax-2 cDNA in AFD, BAG, ASE, AQR, PQR, and URX in tax-2(p694) mutants restored CO2 avoidance to the same extent as a full-length tax-2 genomic fragment (Figures 1C and 1D). We next attempted to rescue the tax-2(p694) defect by expressing tax-2 cDNA from neuron-specific promoters, confirming appropriate expression by polycistronic constructs that coexpress tax-2 and gfp (Coates and de Bono, 2002). Expressing tax-2 cDNA in the AFD thermosensory neurons strongly rescued CO2 avoidance, both on and off food (Figure 1D). In contrast, restoring tax-2 to the BAG O2-sensing neurons rescued CO2 avoidance on food, as shown previously (Hallem and Sternberg, 2008), but not off food. Expressing tax-2 cDNA in the ASE taste neurons or in the AQR, PQR, and URX O2-sensing neurons also partially rescued CO2 avoidance, both on food and off food (Figure 1D). These data implicate functionally diverse sensory neurons in CO2 avoidance.
Figure 1.
The cGMP-Gated Ion Channel Subunit TAX-2 Acts in Multiple Neurons to Promote CO2 Avoidance
(A and B) Wild-type C. elegans distribute uniformly in air (A) but avoid 5% CO2 (B) over 10 min. NL and NR, the number of animals in the left and right halves of the chamber, respectively.
(C) Mutations in the cGMP-gated ion channel subunits tax-2 and tax-4 disrupt avoidance of 5% CO2 both on and off food. The null allele tax-2(ot25) and promoter deletion allele tax-2(p694) almost completely abolish CO2 avoidance. A transgene containing tax-2 genomic DNA restores CO2 avoidance to tax-2(p694) mutants. In this and all subsequent figures, unless otherwise stated: a 5%-0% CO2 gradient was used; data points represent an average of nine assays; error bars indicate standard error of the mean (SEM); ns, not significantly different; ∗∗∗ or +++ indicates p < 0.001; ∗∗ or ++ indicates p < 0.01; ∗ or + indicates p < 0.05; and significance comparisons were made using the two-tailed Student's t test.
(D) Expressing full-length tax-2 cDNA in the AFD, BAG, ASE, and AQR, PQR, and URX neurons rescues CO2 avoidance in tax-2(p694) mutants. Expressing tax-2 cDNA from the neuron-specific promoters pgcy-8 (AFD), pflp-17 (BAG), and an ASE-specific pflp-6 fragment or pgcy-32 (AQR, PQR, URX) gives varying degrees of rescue. Significance comparison of the transgenic line expressing tax-2 in all six neurons is with N2. For all other genotypes, significance markers indicate comparison with tax-2(p694).
The AFD Thermosensory Neurons Sense CO2
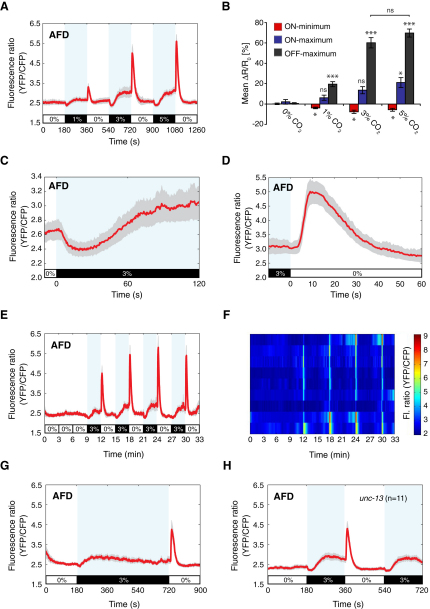
The AFD neurons are transiently activated when temperatures exceed cultivation levels (Kimura et al., 2004; Clark et al., 2006). To test whether AFD also responds to CO2, we monitored AFD intracellular Ca2+ levels during CO2 exposure using the ratiometric Ca2+ sensor cameleon YC3.60, expressed in AFD under control of the gcy-8 promoter (Yu et al., 1997). Animals expressing the Ca2+ sensor retained wild-type CO2 responses (Figure S1A; see Experimental Procedures). To deliver CO2 stimuli, we used a Y-shaped microfluidic chamber that enables the gas phase over an immobilized animal to be switched in less than 3 s (Persson et al., 2009). In all experiments, O2 was maintained at 21%, with nitrogen (N2) completing the balance. AFD Left and AFD Right neurons responded equally to CO2 (Figure 2A; data not shown). On CO2 exposure the AFD neurons exhibited a fall in intracellular Ca2+ that slowly reversed to rise above baseline levels (“CO2-ON” response) within 2 min of CO2 coming on (Figures 2A and 2C). Thus, the AFD CO2-ON response has two components to it, an “ON-minimum” and an “ON-maximum.” Strikingly, AFD also responded to removal of CO2 with a fast Ca2+ spike that peaked within 10 s (“CO2-OFF” response, Figures 2A and 2D). The OFF-maximum was the largest feature of the AFD Ca2+ pattern, being on average 3- to 4-fold greater than the ON-maximum (Figure 2B). All three components of the AFD CO2 response were concentration dependent (Figure 2B). To exclude the possibility that the observed activity could be due to AFD temperature sensing, we exposed animals to 0%-0%-0% CO2 mock switches. Under these conditions AFD gave no responses (first 9 min, Figure 2E).
Figure 2.
The AFD Thermosensory Neurons Sense CO2
(A) Mean fluorescence ratio (YFP/CFP) of AFD neurons expressing cameleon YC3.60 across a 0%-1%-0%-3%-0%-5%-0% CO2 stimulus. In this and all subsequent figures, blue shading indicates presence of CO2, and gray shading indicates the SEM (n = 26 traces).
(B) Mean ratio change, ΔR, expressed as a percentage of the initial fluorescence ratio, R0, for the AFD ON and OFF responses for mock, 1%, 3%, and 5% CO2 concentrations. ΔR = Rf − R0, where Rf is the fluorescence ratio after gas shift. Time intervals for calculation of Rf, and corresponding intervals for R0, were chosen according to peaks in ratio change. For ON-minima, a 20 s time interval was used, for ON-maxima a 30 s interval, and for OFF-maxima an 8 s interval. Data for 1%, 3%, and 5% steps from (A); data for mock step from (E). Details for 1%, 3%, and 5% steps: ON-minima (150–170 s and 190–210 s, 510–530 s, and 550–570 s, 870–890 s and 910–930 s, used for R0 and Rf, respectively); ON-maxima (150–180 s and 330–360 s, 510–540 s, and 690–720 s, 870–900 s, and 1050–1080 s, used for R0 and Rf, respectively); OFF-maxima (344–352 s and 368–376 s, 704–712 s and 728–736 s, 1064–1072 s and 1088–1096 s, used for R0 and Rf, respectively). Significance markers indicate comparisons with responses to a mock 0% CO2 gas switch. Error bars indicate SEM.
(C and D) Expanded view of mean AFD response to a 0%-3% CO2 increase (C) and 3%-0% CO2 decrease (D). Data from (A).
(E and F) Mean AFD response to multiple 3% CO2 stimuli (E) and individual responses (F) plotted in a heat map (n = 9 traces).
(G) Mean AFD response to a 3% CO2 stimulus lasting 9 min (n = 9 traces).
(H) Mean AFD response to 3% CO2 in an unc-13 mutant.
We next examined whether repeated stimulation altered AFD Ca2+ responses. Some C. elegans sensory neurons, such as the ALM anterior touch neurons, habituate upon repeated stimulation (Kindt et al., 2007). The AFD OFF response remained undiminished upon repeated exposure to 3% CO2 (Figures 2E, 2F, and S1B). We also asked whether prolonged CO2 exposure affects AFD responses. After a 9 min exposure to 3% CO2, the ON-maximum had decayed to baseline levels, whereas the OFF-maximum was unaltered (Figure 2G).
CO2-evoked activity in AFD could be due to synaptic input to AFD. To test this, we imaged CO2 responses in unc-13 mutants, which have severe defects in synaptic release (Richmond et al., 1999). The AFD CO2 responses of unc-13 animals were indistinguishable from wild-type (Figures 2H and S1C). These data suggest that, as well as being a thermosensory neuron (Mori and Ohshima, 1995; Kimura et al., 2004; Clark et al., 2007), AFD is a CO2 sensor with both ON and OFF responses. The sensory endings of AFD have many finger-like projections, potentially providing a large surface for CO2 and temperature reception (Ward et al., 1975).
AFD only responds to a temperature rise above the cultivation temperature (Kimura et al., 2004; Clark et al., 2006). If AFD temperature and CO2-sensing are distinct, AFD might be expected to respond to CO2 at temperatures below the cultivation temperature. To test this, we built a temperature-controlled stage (see Supplemental Experimental Procedures). In animals grown at 22°C, AFD responded to CO2 both at 15°C and at 22°C (Figures S1E and S1F). The shape of the response was similar at the two temperatures but smaller at 15°C than at 22°C. These data support the idea that AFD CO2 and temperature-sensing pathways are at least partly distinct.
The BAG O2 Sensory Neurons Sense CO2
Recent work has shown that the BAG neurons are transiently activated when O2 levels drop below 10% (Zimmer et al., 2009). Hallem and Sternberg (2008) showed that feeding animals lacking the BAG neurons have reduced avoidance of a 10% CO2/10% O2 mixture. We have previously shown that O2 responses can modulate CO2 avoidance (Bretscher et al., 2008). These data suggest that either BAG responds exclusively to O2 but modulates neural circuits mediating CO2 responses or that BAG is a primary sensor of both O2 and CO2.
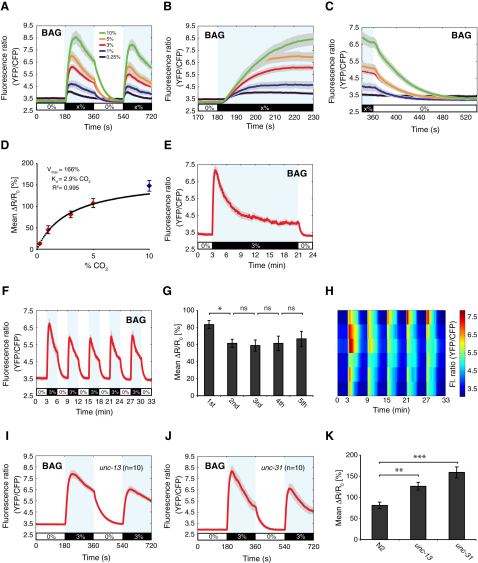
To test BAG neuron CO2 sensitivity, we created animals expressing cameleon YC3.60 in BAG from a pflp-17::YC3.60 transgene and imaged Ca2+ levels. The BAGL and BAGR neurons were exquisitely sensitive to a rise in CO2 (Figures 3A–3C). Cameleon reported a rise in Ca2+ that peaked after ∼30 s and then decayed (Figures 3A and 3B). The excitability threshold of BAG was below 0.25% CO2. A plot of mean fluorescence ratio change against percent (%) CO2 suggests that BAG reaches half-maximal activity at ∼2.9% CO2 (Figure 3D). Thus, BAG neurons respond to both O2 and CO2.
Figure 3.
The BAG Neurons Are Highly Sensitive to CO2
(A–C) The BAG neurons exhibit a large “CO2-ON” response. Mean BAG responses to 0%-x%-0%-x% CO2 stimuli for x = 0.25%, 1%, 3%, 5%, or 10% CO2. Shown are the full response (A), a 60 s interval across CO2 introduction (B), and a 180 s interval across CO2 removal (C) (n = 10 or more traces for all concentrations).
(D) Dose-response curve for the BAG CO2 response. The mean fluorescence ratio change, ΔR, is plotted as a percentage of the mean baseline fluorescence ratio, R0. ΔR = Rf − R0. Rf was calculated from the peak of the BAG Ca2+ response at 200–260 s and R0 from 120–180 s. Curve fit of the standard equation for a single-site binding process (Michaelis-Menten, y = Vmaxx/(Kd + x), where Vmax and Kd are constants with units of [% mean ratio change] and % CO2, respectively) to the red data points using least-squares regression analysis. The blue data point (10% CO2) was omitted from the curve fit because at 10% CO2 the BAG fluorescence ratio (YFP/CFP) falls outside of the linear dynamic range of the Ca2+ sensor YC3.60. Curve fit gives Kd = 2.9% CO2, and Vmax = 166% mean ΔR/R0, with a goodness of fit R2 regression value of 0.995. Error bars indicate SEM.
(E) Mean BAG response to a 3% CO2 stimulus lasting 18 min (n = 9 traces).
(F–H) BAG responses to a 0%-3%-0%-3%-0%-3%-0%-3%-0%-3%-0% CO2 stimulus. (F) Mean fluorescence ratio (YFP/CFP), (G) mean percent (%) ΔR/R0, and individual BAG Ca2+ traces plotted in a heat map (H) (n = 6 traces).
(I–K) Mean BAG Ca2+ responses in unc-13 mutants (I) and unc-31 mutants (J). (K) Mean percent (%) ΔR/R0 values for (I) and (J). Asterisks indicate significance compared to wild-type.
Elevated CO2 persistently stimulates locomotory activity in feeding C. elegans, suggesting that some CO2-sensing circuits can signal tonically in high CO2 (Bretscher et al., 2008). During prolonged high CO2 the BAG Ca2+ spike decayed to a plateau that persisted until CO2 removal, at which point Ca2+ returned to resting levels (Figure 3E). Thus, BAG exhibits both a transient peak and a perduring Ca2+ plateau in response to elevated CO2. As with AFD, we asked whether BAG neurons habituate. During five stimulus cycles of 3% CO2, BAG showed a decrement in response amplitude after the first CO2 stimulus, but no habituation thereafter (Figures 3F–3H).
To test if the BAG neurons are primary CO2 sensors, we disrupted synaptic input to BAG using the unc-13 and unc-31 mutations. unc-31 mutants are defective in dense-core vesicle release, but not synaptic vesicle release (Speese et al., 2007). Neither the unc-13 nor the unc-31 mutations disrupted BAG Ca2+ responses, suggesting that BAG neurons are intrinsically CO2 sensitive (Figures 3I–3K). However, the magnitude of Ca2+ responses in these mutants was significantly enhanced, particularly in unc-31 animals, suggesting that BAG activity is normally inhibited by neuromodulators.
The Asymmetric ASEL and ASER Taste Neurons Are Both Activated by CO2
We next examined CO2 responses in the ASE neurons that mediate chemotaxis to water-soluble cues, including salt ions such as Na+ and Cl− (Bargmann and Horvitz, 1991; Ortiz et al., 2009). ASEL and ASER are functionally asymmetric (Hobert et al., 2002). ASEL is activated by a rise in the concentration of NaCl, whereas ASER is activated by a drop (Suzuki et al., 2008). For NaCl responses, activation of ASEL inhibits animals from reversing, whereas activation of ASER increases reversal likelihood (Suzuki et al., 2008).
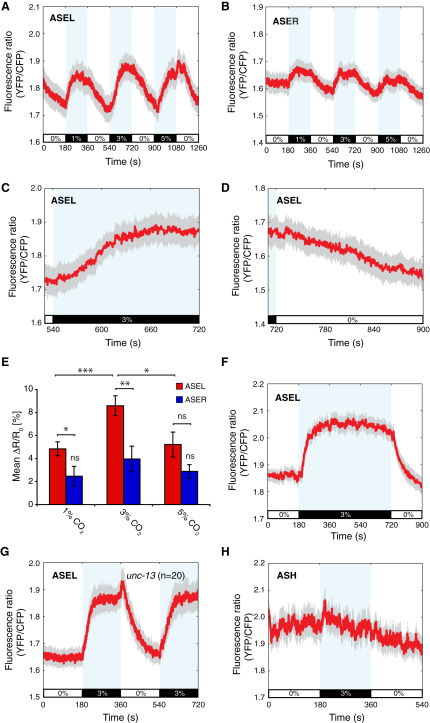
We imaged ASEL and ASER Ca2+ responses to CO2, using animals expressing the Ca2+ sensor YC2.12 in ASE from a pflp-6::YC2.12 transgene (Suzuki et al., 2008). Both ASEL and ASER were activated by 1%, 3%, and 5% CO2 (Figures 4A–4E), although the responses of ASEL were generally ∼2-fold larger than those of ASER (Figure 4E). ASE responses to CO2 were slow, taking around 2 min for Ca2+ levels to peak (Figure 4F). Sustained elevated CO2 led to sustained increases in Ca2+ (Figure 4F). As for AFD and BAG, ASE neurons appeared to be intrinsically CO2 sensitive because Ca2+ responses were intact in unc-13 mutants (Figures 4G and S1D). In summary, ASEL and ASER both respond to CO2 by a slow rise in Ca2+ that persists while CO2 is high and returns to baseline when CO2 returns to baseline.
Figure 4.
The ASEL and ASER Neurons Are Activated by CO2
(A and B) Mean responses of ASEL (A) and ASER (B) to 0%-1%-0%-3%-0%-5%-0% CO2. ASEL, n = 26 traces; ASER, n = 19 traces.
(C and D) Expanded view of mean ASEL response to a 0%-3% CO2 increase (C) and 3%-0% CO2 decrease (D).
(E) Mean percent (%) ΔR/R0 for ASEL and ASER for 1%, 3%, and 5% CO2 stimuli. Baseline ratio, R0, was calculated from the 60 s before CO2 exposure, and peak ratio, Rf, was calculated from the 60 s before CO2 removal. ASER responses to 1% and 5% CO2 are not significantly different from responses to 3% CO2. ASEL responses to 1% and 5% CO2 are significantly different from responses to 3% CO2. ASEL responses are significantly different from ASER responses for 1% and 3% CO2, but not 5% CO2.
(F) Mean ASEL response to a 3% CO2 stimulus lasting 9 min (n = 14 traces).
(G) Mean ASEL response to 3% CO2 in an unc-13 mutant.
(H) The ASH neurons are not activated by 3% CO2 (n = 21 traces).
AQR, PQR, and URX O2-Sensing Neurons Are Weakly CO2 Responsive
We examined whether the AQR, PQR, and URX O2-sensing neurons (Persson et al., 2009; Zimmer et al., 2009) respond to CO2 because our tax-2 rescue data indicated that these neurons contribute, albeit weakly, to CO2 avoidance. Average Ca2+ traces indicated that unlike AFD, BAG, and ASE, none of these neurons respond reliably to CO2 (Figures S2A–S2D). URX most consistently showed CO2-evoked activity, and this was retained in unc-13 mutants (Figures S2A, S2E, and S2F). AQR and PQR occasionally showed a Ca2+ rise associated with an increase in CO2 but also showed apparent spontaneous activity that lay out of synchrony with the CO2 stimulus (Figures S2B–S2D). The response of PQR to a 0%-3%-0%-3% CO2 stimulus was dwarfed by its response to a 21%-11%-21%-11% O2 stimulus (Figure S2C).
Having identified three C. elegans neuron classes that responded strongly to CO2 and a further three that responded weakly to CO2, we considered the possibility that all sensory neurons show some CO2 responsiveness. Therefore, we imaged Ca2+ responses to CO2 in the ASH neurons that respond to various aversive stimuli (Hilliard et al., 2005). ASH showed no response to 3% CO2 (Figure 4H). This suggests that AFD, BAG, and ASE are functionally specialized as CO2 sensors.
CO2 Sensitivity in BAG and AFD Requires a cGMP-Gated Ion Channel
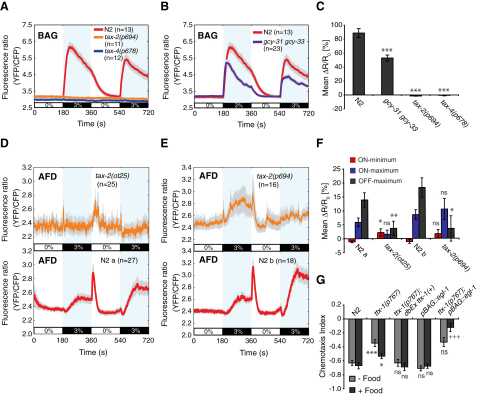
Our tax-2 rescue data suggested that CO2 sensing in BAG and AFD neurons involves cGMP signaling. To examine this further we imaged BAG responses to CO2 in tax-2(p694) and tax-4(null) mutants. Both mutations completely abolished CO2-evoked Ca2+ responses in BAG (Figures 5A and 5C). This suggests that BAG CO2 sensory transduction is mediated by TAX-2/TAX-4 cGMP-gated channels and by extension, upstream guanylate cyclases (gcy).
Figure 5.
A cGMP Pathway Couples CO2 to BAG and AFD Activation, and These Neurons Are Required for CO2 Avoidance
(A) Mutations in the tax-2 and tax-4 cGMP-gated ion channel subunits abolish BAG responses to 3% CO2.
(B) Mean BAG responses to 3% CO2 in wild-type and gcy-31(ok296) gcy-33(ok232) double-mutant animals.
(C) Mean percent (%) ΔR/R0 values for the BAG responses in (A) and (B).
(D and E) Mean AFD response to 3% CO2 of tax-2(ot25) null (D) and tax-2(p694) promoter deletion (E) mutants and their wild-type controls. Longer exposure times were used in imaging AFD in tax-2(ot25) animals due to weak expression of YC3.60.
(F) Mean percent (%) ΔR/R0 values for the AFD ON-minima, ON-maxima, and OFF-maxima of tax-2(ot25), tax-2(p694), and wild-type. Significance markers indicate comparisons against wild-type.
(G) AFD and BAG both contribute to CO2 avoidance in shallow spatial gradients. ttx-1 mutants have defects in CO2 avoidance both on and off food. These defects are fully rescued by ttx-1(+) genomic DNA. Genetic ablation of BAG alone does not disrupt CO2 avoidance, but loss of BAG when AFD is absent further disrupts CO2 avoidance on food. Asterisks (∗) and “ns” indicate significance comparisons against N2 wild-type. Plus signs (+) and “ns” indicate significance comparisons against ttx-1(p767) mutants.
The only gcy genes known to be expressed in BAG are the atypical soluble guanylate cyclases gcy-31 and gcy-33 (Yu et al., 1997; Zimmer et al., 2009; Ortiz et al., 2006). These appear to be O2 regulated (Gray et al., 2004; Boon and Marletta, 2005) because both are required for BAG O2 responses (Zimmer et al., 2009). To examine if GCY-31, GCY-33, or both are required in CO2 sensory transduction, we imaged BAG responses to 3% CO2 in gcy-31; gcy-33 double-deletion mutants. Loss of gcy-31 and gcy-33 reduced the CO2-evoked BAG Ca2+ response (Figures 5B and 5C). This suggests that GCY-31 and/or GCY-33 forms part of the CO2 sensory system in BAG, although other molecules are likely to be involved.
We next imaged AFD responses in tax-2(null) and tax-2(p694) animals. Expression from the gcy-8 promoter is markedly reduced in tax-2 and tax-4 mutants (Satterlee et al., 2004), and YC3.60 expression was correspondingly low in AFD in tax-2(ot25null) animals. In contrast, expression in tax-2(p694) animals was similar to wild-type (data not shown). Both tax-2 mutations significantly reduced the AFD CO2 response, but neither completely abolished it (Figures 5D–5F). The AFD ON-minimum appeared to be absent in both tax-2 mutants, whereas the AFD ON-maximum was absent in tax-2(null) animals but enhanced in tax-2(p694) animals (Figures 5D–5F). Our data suggest that all three components of the AFD CO2 response involve TAX-2 mediated cGMP pathways but that other pathways also contribute.
C. elegans Carbonic Anhydrases Are Expressed in Several Neurons, Including BAG
To further investigate molecular mechanisms of CO2 sensing, we asked whether C. elegans CO2 sensors express carbonic anhydrases, hallmarks of CO2-responsive neurons in other animals (Hu et al., 2007; Wang et al., 2002; Ridderstrale and Hanson, 1985; Coates et al., 1998). Database searches indicate that the C. elegans genome encodes eight predicted carbonic anhydrases. Six, cah-1 to cah-6, belong to the alpha family, and two, bca-1 and bca-2, to the beta family. Because many members of the beta family are mitochondrial (Syrjänen et al., 2010; Fasseas et al., 2010), we focused our studies on the alpha family. We fused upstream promoter regions of each gene to gfp and examined the resulting expression patterns. We found that cah-1, 2, 3, and 6 show strong neuronal expression in adults (Figure S3A). cah-4 was primarily expressed in the hypodermis (excluding the seam cells) and in the excretory cell, consistent with a kidney-like function for this cell. cah-3 and cah-5 show expression in intestinal cells, with cah-3 expression being especially strong. Using a pBAG::mCherry marker, we showed that cah-2, but not apparently any of the other five cah genes, was expressed in BAG (Figure S3B). cah-2 was also expressed in a set of four quadrant head neurons, other unidentified head neurons, the canal neurons CANL/R, whose processes run parallel to the tracts of the excretory cell, and a pair of tail neurons (Figure S5). Previous data suggest that cah-2 is also expressed in AFD (Colosimo et al., 2004). These data suggest that BAG and AFD neurons are specialized CO2 sensors that coexpress carbonic anhydrases and CO2-regulated cGMP pathways. They also raise the possibility that other C. elegans neurons and tissues respond to CO2.
AFD and BAG Direct Avoidance Behavior in Spatial CO2 Gradients
To investigate how CO2 sensors contribute to avoidance in spatial gradients, we genetically ablated neurons. We focused on AFD and BAG neurons because the Ca2+ responses of ASE to CO2 stimuli were slow, and those of AQR, PQR, and URX, weak. Specification of the AFD neurons requires the otd/Otx homeodomain transcription factor ttx-1, which is expressed only in AFD (Satterlee et al., 2001). ttx-1 mutants show thermotactic defects equivalent to those of animals in which AFD has been removed by laser ablation (Mori and Ohshima, 1995). ttx-1 mutants had a strong CO2 avoidance defect off food, and a weaker defect on food (Figure 5G). Wild-type avoidance was restored to ttx-1 mutants by a transgene containing ttx-1 genomic DNA (Figure 5G). These data suggest that the AFD neurons promote CO2 avoidance in spatial CO2 gradients.
To ablate BAG we expressed the egl-1 programmed cell death activator from a BAG-specific gcy-33 promoter (Conradt and Horvitz, 1998; Yu et al., 1997) (we thank M. Beverly and P. Sengupta for this line). Both BAGL and BAGR neurons were absent in greater than 90% of animals bearing this transgene (Table S1 available online). Surprisingly, the CO2 avoidance of BAG-ablated animals was not significantly different from wild-type, both on and off food (Figure 5G). We asked if combined genetic ablation of AFD and BAG causes a synthetic CO2 avoidance phenotype. Ablating the BAG neurons disrupted the residual CO2 avoidance of ttx-1(p767) mutants on food (Figure 5G). However, in the absence of food, ttx-1(p767); pgcy-33::egl-1 animals showed no greater defect than ttx-1(p767) single mutants (Figure 5G). These data show that AFD and BAG promote CO2 avoidance in spatial gradients on food, and that AFD and at least one other neuron that is not BAG promote avoidance when food is absent. Thus, the importance of different sensory neurons for CO2 avoidance in spatial gradients depends on context.
AFD and BAG Control Discrete Aspects of the C. elegans Response to CO2
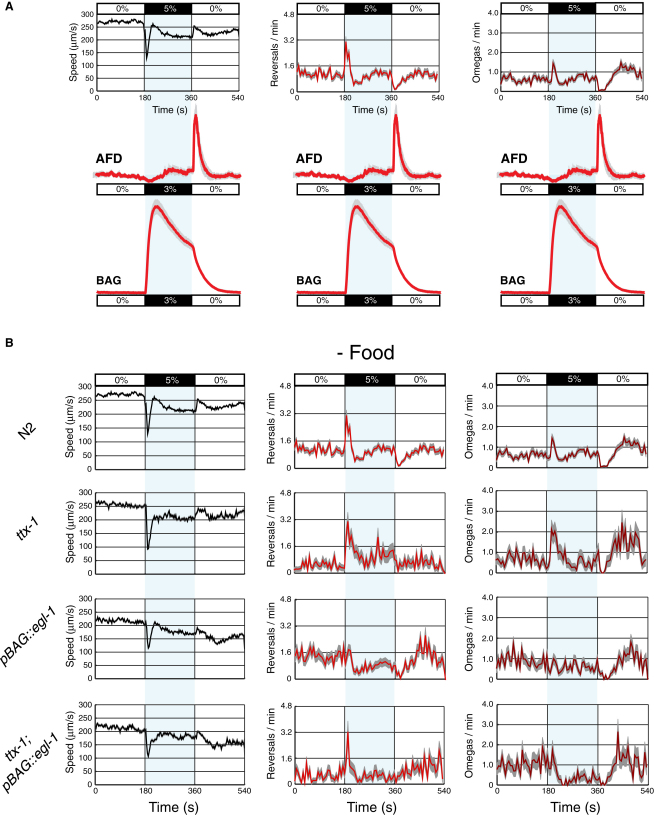
In 5%-0% CO2 spatial gradients (Figure 1), a C. elegans moving at ∼0.3 mm/s experiences a change of 0%-0.05% CO2/s, depending on bearing relative to the gradient. In our Ca2+-imaging experiments, immobilized animals experienced much sharper temporal gradients of ∼1% CO2/s. In the wild, animals are likely to encounter a variety of CO2 gradients. To analyze behavioral responses to sharp CO2 gradients, we designed a square-shaped microfluidic chamber that enables CO2 levels over freely moving animals to be switched rapidly (Movie S1 available online). We recorded responses and used custom software to extract instantaneous speed, reversal rate, and rate of omega turns, turns in which an animal's head and tail touch to form an “Ω” shape (N2, Figure 6B). In the absence of food, a rise in CO2 from 0% to 5% elicited a brief slowing followed by a transient increase in reversals and omega turns (Figure 6B). A rapid drop in CO2, from 5% to 0%, elicited an acceleration that coincided with suppression of reversals and omega turns.
Figure 6.
AFD and BAG Control Behavioral Responses to Changes in Percent (%) CO2
(A) AFD and BAG CO2-evoked neuronal events correlate with CO2-evoked behavioral events. Behavioral plots reproduced from (B). (B) Average speed, reversal, and omega rates of wild-type (N2), AFD-ablated (ttx-1), BAG-ablated (pgcy-33::egl-1), and AFD-ablated BAG-ablated (ttx-1; pgcy-33::egl-1) animals off food across a 0%-5%-0% CO2 stimulus. Stimulus bar and light blue shading indicate the timing of gas switches. Gray shading indicates SEM. Speed (μm/s, black line) calculated in 3 s bins. Reversal (orange line) and omega rates (maroon line) are in event initiations per animal per minute calculated in 6 s bins. N2, n = 59 movies; ttx-1(p767), n = 20 movies; pgcy-33::egl-1, n = 15 movies; ttx-1; pgcy-33::egl-1, n = 16 movies.
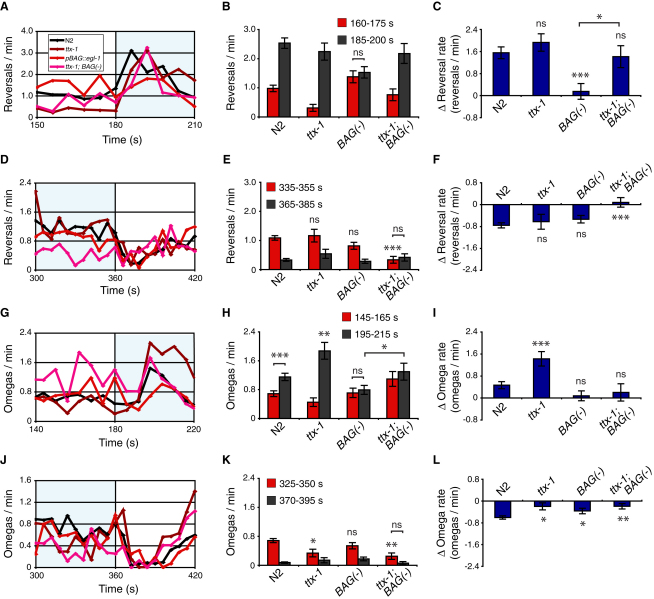
The timing of CO2-evoked Ca2+ responses in both AFD and BAG correlated with peaks in locomotory activity (Figure 6A). We investigated these correlations directly by ablating AFD and/or BAG and examining behavioral responses (Figure 6B). For statistical comparison, we chose time intervals before and after gas switches according to the occurrence of peaks in wild-type behavioral rates. In the absence of food, neither AFD nor BAG ablation abolished modulation of speed across shifts in CO2 (Figures 6B and S4). Stronger phenotypes were observed for reversal and omega rates (Figure 6B). Unexpectedly, ablation of AFD increased reversal and omega rates following a sharp CO2 rise (ttx-1, Figures 6B, 7B, 7C, 7H, and 7I) and reduced suppression of omega turns following a CO2 fall (ttx-1, Figures 6B, 7K, and 7L), suggesting that AFD acts to suppress reversals and omega turns at these two time points. Ablation of BAG abolished reversal and omega responses to a rise in CO2 (pBAG::egl-1, Figures 6B, 7B, 7C, 7H, and 7I) and reduced the suppression of omega turns following a CO2 fall (pBAG::egl-1, Figures 6B, 7K, and 7L), consistent with BAG excitation promoting reversals and omega turns. Coablation of AFD and BAG abolished the suppression of reversals and omega turns following a fall in CO2 (ttx-1; pBAG::egl-1, Figures 7F and 7L). This effect was due to reduced reversal and omega rates under prolonged high CO2 (ttx-1; pBAG::egl-1, red bars, Figures 7E and 7K). These data suggest that together BAG and AFD act to suppress reversals and omega turns when CO2 decreases.
Figure 7.
AFD and BAG Together Promote Turning When CO2 Levels Rise and Inhibit Turning When CO2 Levels Fall
(A–L) Statistical analysis of reversal and omega turns of wild-type and ablated animals during 0%-5% CO2 increases and 5%-0% CO2 decreases. Average behavioral traces are shown at left, time-averaged behavioral rates before and after gas switches are shown at middle, and average changes in behavioral rates are shown at right. Rates are in initiations of reversals or omega events per animal per minute. (A, D, G, and J) Average reversal and omega rates during 0%-5% CO2 and 5%-0% CO2 gas switches. Error bars omitted for clarity. (B, E, H, and K) Time-averaged reversal (B and E) and omega (H and K) rates before (red bars) and after (dark gray bars) an increase (B and H) or a decrease (E and K) in percent (%) CO2. Intervals for comparison coincide with stationary points in wild-type behavioral rates. Error bars indicate SEM. (C, F, I, and L) Average change in reversal (C and F) and omega (I and L) rates across an increase (C and I) or a decrease (F and L) in percent (%) CO2. Difference calculations based on data in (B), (E), (H), and (K), and error bars calculated from SEM values in (B), (E), (H), and (K) using error propagation formulae. Significance markers indicate comparisons against wild-type, unless otherwise indicated.
Curiously, AFD-ablated BAG-ablated animals continued to show a transient increase in reversals following a CO2 rise (ttx-1; pBAG::egl-1, Figures 6B, 7B, and 7C). This result suggests that there is at least one other CO2 “ON” sensory neuron, XYZ, that promotes reversals in response to a CO2 rise. It also suggests that after a CO2 rise, AFD acts antagonistically to both BAG and the hypothetical XYZ neuron to inhibit reversals. We investigated whether the ASE or AQR, PQR, URX neurons could be XYZ by ablating them together with AFD and BAG. Ablating ASEL/R had no significant effect on the reversal rate of AFD-ablated BAG-ablated animals immediately following a CO2 rise (che-1; ttx-1; pBAG::egl-1, Figures S5A–S5D) but did alter reversal rates under prolonged high CO2 (Figures S5E and S5F). The ablation of AQR, PQR, URX by an integrated pgcy-36::egl-1 transgene caused an increase in the reversal rate of AFD-ablated BAG-ablated animals in air alone (Figures S5A–S5D). These data suggest that the ASE neurons suppress reversals under prolonged high CO2 and that the AQR, PQR, URX neurons suppress reversals in the absence of CO2. However, even animals defective in AFD, BAG, ASE, AQR, PQR, and URX retained some CO2 responsiveness, suggesting that C. elegans has additional CO2 sensors.
The Presence of Food Modulates the Neural Circuit Controlling CO2 Avoidance
Wild-type C. elegans (N2) exhibit distinct locomotory patterns in the presence and absence of food (de Bono and Bargmann 1998; Sawin et al., 2000). Animals move slowly and reverse frequently on food, whereas in its absence they move rapidly with fewer reversals. The escape mechanisms elicited by a CO2 rise on and off food were correspondingly different (Movies S1 and S2 and Figure S6). Feeding animals still briefly slowed down when CO2 levels rose but then switched to a high locomotory rate as high CO2 persisted (Figure S6) (Bretscher et al., 2008). Coupled to the slowing response was a much stronger transient increase in omega turns (Figure S6). Feeding animals also persistently suppressed reversals in high CO2. These mechanisms increased the exploratory behavior of feeding animals, presumably helping them to escape from high CO2.
To investigate whether AFD and BAG contribute to differences between on- and off-food behavior, we ablated them. AFD ablation abolished the increased speed response to high CO2 and resulted in inappropriately high-reversal and omega rates under high CO2 (ttx-1, Figure S6). In contrast, ablating only BAG had little or no effect (pBAG::egl-1, Figure S6). Ablating neither AFD nor BAG alone abolished the dramatic spike in omega turns following a CO2 rise, but ablating both neurons together nearly did (ttx-1; pBAG::egl-1, Figure S6). As for off food, loss of AFD and BAG did not eliminate CO2 responses, suggesting that other neurons contribute to rapid CO2-evoked behavior on food.
In summary, genetic ablation suggests that AFD and BAG account for much of the different behavioral strategies employed in CO2 avoidance on and off food. In both contexts one or more other neurons also contribute to CO2 avoidance.
Discussion
The AFD, BAG, and ASE Sensory Neurons Exhibit Distinct CO2 Responses
C. elegans, like mammals, monitors CO2 using multiple neuron types. CO2 sensors include the ASE neurons with sensory endings directly exposed to the external environment and AFD and BAG neurons whose dendrites lie within the animal. All three neuron types are primary CO2 sensors: their CO2 responses are unimpaired in unc-13 mutants defective in synaptic release. Each neuron type has a unique CO2 response. In AFD, a rise in CO2 triggers an initial drop in intracellular Ca2+ levels (AFD ON-minimum), then a rise above baseline (AFD ON-maximum), and when CO2 is removed, a spike (AFD OFF-maximum). This complexity may reflect multiple CO2-transduction mechanisms. In contrast, BAG and ASE neurons are activated by a rise, but not a fall, in CO2. In BAG, Ca2+ peaks within 60 s of a rise in CO2, then decays to a plateau that persists as long as CO2 remains high; Ca2+ drops back to baseline upon CO2 removal. ASE responds slowly to CO2 exposure: Ca2+ takes 2 min to peak but remains elevated while CO2 is high. The tonic activity of BAG and ASE neurons in high CO2 may allow C. elegans to modify responses to other cues, perhaps by affecting sensory pathways or interneuron networks.
AFD, BAG, and ASE also sense other stimuli. AFD senses temperature (Kimura et al., 2004), BAG senses ambient O2 (Zimmer et al., 2009), and ASE senses salt (Suzuki et al., 2008). This may enable sensory integration within sensory neurons. For each of the three neurons, CO2 and non-CO2 stimuli evoke distinct Ca2+ responses. When temperature rises above the cultivation level, AFD responds with a monophasic Ca2+ spike that lasts a few seconds (Kimura et al., 2004; Clark et al., 2007). The dissimilar CO2 and temperature responses suggest that the two stimuli are sensed differently. Supporting this, AFD responds to CO2 below the cultivation temperature. The Ca2+ responses of BAG to high CO2 and low O2 are more similar in shape (Figure 3) (Zimmer et al., 2009). In contrast, the responses of ASE to CO2 and NaCl differ markedly (Figure 4) (Suzuki et al., 2008). First, unlike CO2, NaCl evokes an asymmetric response in ASEL and ASER: a rise in NaCl triggers a Ca2+ spike in ASEL but a drop in Ca2+ in ASER. Second, ASEL/R Ca2+ responses to NaCl adapt rapidly, whereas sustained CO2 stimulation leads to sustained high Ca2+ in ASE (Figure 4F). Third, whereas ASE responses to CO2 are slow, taking around 2 min for Ca2+ to peak, responses to NaCl peak within 30 s of stimulus exposure. The slowness of ASE CO2 responses could reflect rate-limiting hydration of environmental CO2.
cGMP Signaling Mediates CO2 Responses
CO2 sensing in AFD, BAG, and ASE involves cGMP signaling. Mutating the cGMP-gated channel subunit tax-2 partially abolishes the AFD Ca2+ response to CO2 and completely abolishes CO2-evoked activity in BAG (Figure 5). CO2-evoked Ca2+ responses in ASE likely also depend on cGMP-gated channels because expression of tax-2 cDNA in ASE in tax-2 mutants partially restores CO2 avoidance (Figure 1). In mouse olfactory epithelia, CO2 sensing requires the transmembrane guanylate cyclase GC-D, which is activated by HCO3− (Hu et al., 2007; Sun et al., 2009). The hallmarks that make GC-D HCO3− regulated are unknown, but the C. elegans genome encodes 27 transmembrane guanylate cyclase (gcy), a subset of which could be similarly regulated (Yu et al., 1997; Ortiz et al., 2006). The AFD neurons express gcy-8, gcy-18, gcy-23, and gcy-29. gcy-8 gcy-18 gcy-23 triple mutants have a thermotaxis defect similar to that of the AFD specification mutant ttx-1 (Inada et al., 2006), but have no defect in CO2 avoidance in a 5%-0% CO2 gradient (data not shown). ASE neurons express 11 transmembrane guanylate cyclases, nine of which are expressed asymmetrically either in ASEL or ASER (Ortiz et al., 2006).
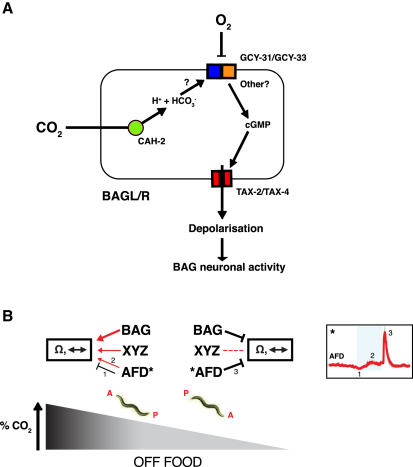
Transmembrane guanylate cyclase expression has not been reported in BAG. However, BAG expresses the atypical soluble guanylate cyclases GCY-31 and GCY-33 (Yu et al., 1997). Simultaneously disrupting gcy-31 and gcy-33 reduced the CO2-evoked Ca2+ response amplitudes in BAG, suggesting that GCY-31 and/or GCY-33 contribute to CO2 sensing. GCY-31 and GCY-33 are thought to function as heterodimers that have an O2-binding heme cofactor (Boon and Marletta, 2005) and are required for BAG O2-evoked Ca2+ responses when O2 drops below 10% (Zimmer et al., 2009). An intriguing possibility is that the GCY-31/GCY-33 heterodimer is inhibited by O2 and activated by CO2, making it a sensory integrator of CO2 and O2 signals in BAG (Figure 8A); however, we cannot rule out the possibility of a linked mutation disrupting BAG responses.
Figure 8.
Models of CO2 Sensory Neuron Function
(A) Model of CO2 and O2-evoked excitability in the BAG neurons. As in mouse CO2 olfactory neurons, a carbonic anhydrase CAH-2 catalyzes hydration of CO2 in BAG. HCO3− ions or H+ protons may activate the GCY-31/GCY-33 heterodimer as well as another guanylate cyclase. Elevated cGMP levels open the TAX-2/TAX-4 channel causing Ca2+ influx and BAG depolarization.
(B) AFD, BAG, and the postulated CO2-ON sensory neuron XYZ ensure turning probability increases when the CO2 gradient is positive and decreases when negative. As an animal moves up a CO2 gradient, increasing CO2 activates BAG and XYZ, which activate turning. AFD initially inhibits turning (ON-minimum, 1), but as Ca2+ levels rise (ON-maximum, 2), AFD promotes turning. As an animal moves down a CO2 gradient, a decrease in CO2 causes deactivation of BAG and the AFD OFF-response (3), which suppress turning. Activation is represented by red arrows, and inhibition, by black “T” symbols. The worm at left is heading up the gradient, and the worm at right, down the gradient. A, anterior; P, posterior. “Ω” represents omega turns and the double-headed arrow, reversals.
AFD, BAG, and ASE are unlikely to be the only CO2-responsive neurons in C. elegans. The AQR, PQR, and URX O2-sensing neurons showed sporadic responses to CO2 (Figure S2), and selective expression of tax-2 cDNA in these neurons partially restored CO2 avoidance to tax-2(p694) mutants, suggesting that they are CO2 sensitive. Moreover, more than ten C. elegans neurons express carbonic anhydrases, some of which may be unidentified CO2 sensors.
The Contribution of Different Sensors to CO2 Avoidance Varies with Stimulus Dynamics and Context
Why does C. elegans have multiple CO2 sensors? One reason is that sensors are deployed differently according to the dynamics of the CO2 stimulus. For example, when food is absent, BAG mediates responses to sharp CO2 gradients but is less important for navigating shallow gradients (compare Figures 5G and 6B). A second reason is that context modifies the behavioral changes needed to escape CO2. For example, when food is present, C. elegans move slowly and reverse frequently. To efficiently escape high CO2 in a food-containing environment, C. elegans increase speed and suppress reversals relative to the “on food” ground state. By contrast when food is absent, animals are already moving quickly and reversing less frequently. Correspondingly, the importance of BAG for CO2 avoidance depends on both stimulus shape and food context. Whereas BAG-ablated animals respond poorly to rapid CO2 changes when food is absent, they respond like wild-type animals when food is present (pBAG::egl-1, Figures 6 and S6). Conversely, in shallow gradients BAG acts redundantly with AFD to promote CO2 avoidance when food is present but is not important when food is absent, even when AFD is ablated (Figure 5G).
How do the Ca2+ responses of CO2 sensory neurons encode behavior? CO2-evoked neuronal events in AFD and BAG correlate with peaks and troughs in locomotory rates (Figure 6A). To investigate these relationships, we ablated CO2 sensors. One caveat of neuronal ablation is that it can only remove a neuron in its entirety, and not individual components of its responses. Ablation of AFD and BAG neurons one at a time and together suggests that: (1) BAG activation and the AFD ON-minimum act antagonistically, promoting and suppressing reversal and omega rates, respectively (Figures 7C and 7I); (2) BAG plateau activity and the AFD ON-maximum both act to promote reversal and omega rates during maintained high CO2 (ttx-1; BAG(-), Figures 7E and 7K); and (3) decay of BAG activity and the AFD OFF-maximum act together to suppress reversals and omega turns following CO2 removal (ttx-1; BAG(-), Figures 7F and 7L). Together our data suggest that when an animal is migrating up a CO2 gradient, BAG and AFD trigger turning, whereas when an animal is migrating down a CO2 gradient, AFD and BAG suppress turning (Figure 8B). Therefore, it appears that the three different components of the AFD CO2 response may differentially regulate behavior (1, 2, 3, AFD, Figure 8B). Because AFD(−) BAG(−) animals still respond to CO2, we also infer the existence of an additional sensory neuron, XYZ, that is neither ASE nor AQR, PQR, URX, that promotes turning when CO2 rises (Figure 8B).
CO2 Avoidance Behavior in C. elegans Appears to Be a Homeostatic Mechanism
Elevated tissue CO2 is toxic (Richerson, 2004). In C. elegans, CO2 levels exceeding 9% disrupt body muscle organization and general development and reduce fertility (Sharabi et al., 2009). The CO2 responses of AFD, BAG, and ASE neurons do not habituate upon multiple exposures to CO2 (Figures 2 and 3; data not shown). C. elegans CO2 avoidance in spatial gradients is also nonhabituating over a similar period (data not shown). By contrast, C. elegans attraction to benzaldehyde (L'Etoile et al., 2002), response to noxious Cu2+ ion stimuli (Hilliard et al., 2005), and response to nose touch (Kindt et al., 2007) all habituate. Moreover, BAG and ASE neurons show tonic signaling while CO2 levels are high, at least over 20 min. We speculate that C. elegans CO2 avoidance habituates slowly and performs a homeostatic function by preventing CO2 poisoning of body tissues. C. elegans CO2 avoidance provides an opportunity for detailed examination of a CO2 homeostatic system with comparative ease relative to the systems of more complex animals.
Experimental Procedures
Strains
Strains were grown at 22°C under standard conditions (Brenner, 1974). Mutant combinations were made by following visible phenotypes or using PCR to confirm genotype. A full list of strains can be found in Supplemental Experimental Procedures.
Behavioral Assays
Spatial CO2 gradient assays were as described (Bretscher et al., 2008). Briefly, polydimethylsiloxane (PDMS) chambers connected to gas syringe pumps were placed over adult worms on a 9 cm agar plate. After 10 min the distribution of worms was used to calculate a chemotaxis index (Figure 1). Chemotaxis bar graphs represent the average of nine independent assays performed over 3 days.
For temporal gradient assays a square 11 × 11 × 0.2 mm PDMS chamber was placed over adult worms on 6 cm agar plates. For off-food assays, ∼40 animals were picked after washing in M9 Buffer to remove adhering E. coli. For on-food assays, a 2-day-old 20 μl E. coli lawn was used. Worms were allowed to crawl on food for 1 hr. After placing the chamber, animals were left for 4 min before exposure to a 0%-5%-0% CO2 stimulus. Behavior was captured using a Grasshopper CCD camera (Point Grey Research). A TTL-output from a frame counter (custom built) controlled opening and closing of Teflon™ pinch valves (Automate Scientific) at defined time points, controlling the switching of gases. Worms were tracked using DIAS Software (Solltech), and worm object paths were created. The centroid X and Y coordinates, maximum length, mean width, perimeter, and roundness were extracted for each worm object across frames. From these parameters, speed, omega initiation rate, and reversal initiation rate were calculated using a custom-written program in MATLAB (The MathWorks). Omega turns were detected by circular object topologies. This method gave 90.9% success using the stringent criterion that worm head touches worm tail. Reversal events were defined as forward movement (F), followed by backward movement (B), followed by return to forward movement (F). Using the criterion of an F-B-F event and optimized parameters minimum allowable reversal angle (150°), maximum reversal duration (7.5 s), and minimum reversal distance (0.3 mm, life size), reversal detection success rate ran at 81.25%. Detection parameters were optimized by minimizing the sum of the squared differences between detection outputs of computer and a human observer for Movie S1. Behavior occurring during merger of worm objects was discarded. Temporal gradient assay data represent the average of 16 or more movies for off food and nine or more for on food.
In all experiments, percent (%) CO2 was balanced by percent (%) N2 while 21% O2 was maintained. In rescue experiments, transgenic animals were preselected by following coinjection markers. In all figures, statistical significance was determined using the two-tailed Student's t test.
Calcium Imaging
Ca2+ imaging was on an inverted microscope (Axiovert; Zeiss), using a 40× C-Apochromat lens and MetaMorph acquisition software (Molecular Devices). Agarose pads were made in M9 Buffer (pH 6.8) and 1 mM CaCl2, mimicking an NGM substrate. Worms expressing the Ca2+ sensor YC3.60 showed wild-type avoidance in 5%-0% CO2 gradients (Figure S1). Worms were glued to pads using Nexaband glue (WPI Inc.) and placed under the stem of the Y-chamber microfluidic device. Photobleaching was minimized using a 2.0 optical density filter and a shutter to limit exposure time to 100 ms per frame. An excitation filter (Chroma) restricted illumination to the cyan channel. A beam splitter (Optical Insights) was used to separate the cyan and yellow emission light. The ratio of the background-subtracted fluorescence in the YFP and CFP channels was calculated with Jmalyze (Kerr and Schafer, 2006). Fluorescence ratio (YFP/CFP) plots were made in MATLAB. Movies were captured at 2 fps. Average Ca2+ traces were compiled from at least six recordings made on 2 or more days.
Acknowledgments
We thank the Caenorhabditis Genetics Centre, the C. elegans Knockout Consortium, Piali Sengupta, Bill Schafer, Ikue Mori, and Oliver Hobert for strains; the Dana-Farber Cancer Institute and Source Bioscience for reagents; Robyn Branicky for comments on the manuscript; and all the de Bono and Schafer lab members for insight, help, and advice. K.E.B. was funded by the Swiss National Science Foundation, P.L. was funded by EMBO, and otherwise research was funded by either the Medical Research Council, UK, or private funds.
Published: March 23, 2011
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, one table, and two movies and can be found with this article online at doi:10.1016/j.neuron.2011.02.023.
Supplemental Information
Animals placed within the square chamber microfluidic device are exposed to 5% CO2 stimuli off food. Movie speeded up 5×. Timing of CO2 stimulus is indicated.
As in Movie S1, but animals are exposed to 5% CO2 while on a lawn of E. coli food. Movie speeded up 5×. Timing of CO2 stimulus is indicated.
References
- Bargmann C.I., Horvitz H.R. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- Boon E.M., Marletta M.A. Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins. Curr. Opin. Chem. Biol. 2005;9:441–446. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A.J., Busch K.E., de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan G.F., Richerson G.B. Central serotonin neurons are required for arousal to CO2. Proc. Natl. Acad. Sci. USA. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K.J., Williams B.A., Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustami H.P., Harrison J.F., Hustert R. Evidence for oxygen and carbon dioxide receptors in insect CNS influencing ventilation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;133:595–604. doi: 10.1016/s1095-6433(02)00155-1. [DOI] [PubMed] [Google Scholar]
- Cammer W.B., Brion L.P. Carbonic anhydrase in the nervous system. EXS. 2000;90:475–489. doi: 10.1007/978-3-0348-8446-4_24. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Yarmolinsky D., von Buchholtz L., Oka Y., Sly W., Ryba N.J., Zuker C.S. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Biron D., Sengupta P., Samuel A.D. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J. Neurosci. 2006;26:7444–7451. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Gabel C.V., Gabel H., Samuel A.D. Temporal activity patterns in thermosensory neurons of freely moving Caenorhabditis elegans encode spatial thermal gradients. J. Neurosci. 2007;27:6083–6090. doi: 10.1523/JNEUROSCI.1032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates E.L., Wells C.M., Smith R.P. Identification of carbonic anhydrase activity in bullfrog olfactory receptor neurons: histochemical localization and role in CO2 chemoreception. J. Comp. Physiol. A. 1998;182:163–174. doi: 10.1007/s003590050167. [DOI] [PubMed] [Google Scholar]
- Coates J.C., de Bono M. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature. 2002;419:925–929. doi: 10.1038/nature01170. [DOI] [PubMed] [Google Scholar]
- Coburn C.M., Bargmann C.I. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- Colosimo M.E., Brown A., Mukhopadhyay S., Gabel C., Lanjuin A.E., Samuel A.D., Sengupta P. Identification of thermosensory and olfactory neuron-specific genes via expression profiling of single neuron types. Curr. Biol. 2004;14:2245–2251. doi: 10.1016/j.cub.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Conradt B., Horvitz H.R. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- de Bono M., Bargmann C.I. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- Dusenbery D.B., Sheridan R.E., Russell R.L. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics. 1975;80:297–309. doi: 10.1093/genetics/80.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasseas M.K., Tsikou D., Flemetakis E., Katinakis P. Molecular and biochemical analysis of the beta class carbonic anhydrases in Caenorhabditis elegans. Mol. Biol. Rep. 2010;37:2941–2950. doi: 10.1007/s11033-009-9857-z. [DOI] [PubMed] [Google Scholar]
- Faucher C., Forstreuter M., Hilker M., de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J. Exp. Biol. 2006;209:2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- Feldman J.L., Mitchell G.S., Nattie E.E. Breathing: rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischler W., Kong P., Marella S., Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- Gray J.M., Karow D.S., Lu H., Chang A.J., Chang J.S., Ellis R.E., Marletta M.A., Bargmann C.I. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Hallem E.A., Sternberg P.W. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E.M., Russell R.L. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz S.K., Bradley T.J. Insects breathe discontinuously to avoid oxygen toxicity. Nature. 2005;433:516–519. doi: 10.1038/nature03106. [DOI] [PubMed] [Google Scholar]
- Hilliard M.A., Apicella A.J., Kerr R., Suzuki H., Bazzicalupo P., Schafer W.R. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., Johnston R.J.J., Chang S. Left-right asymmetry in the nervous system: the Caenorhabditis elegans model. Nat. Rev. Neurosci. 2002;3:629–640. doi: 10.1038/nrn897. [DOI] [PubMed] [Google Scholar]
- Hu J., Zhong C., Ding C., Chi Q., Walz A., Mombaerts P., Matsunami H., Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Inada H., Ito H., Satterlee J., Sengupta P., Matsumoto K., Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Rojas A., Wang R., Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir. Physiol. Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Jones W.D., Cayirlioglu P., Kadow I.G., Vosshall L.B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Kerr R.A., Schafer W.R. Intracellular Ca2+ imaging in C. elegans. Methods Mol. Biol. 2006;351:253–264. doi: 10.1385/1-59745-151-7:253. [DOI] [PubMed] [Google Scholar]
- Kimura K.D., Miyawaki A., Matsumoto K., Mori I. The C. elegans thermosensory neuron AFD responds to warming. Curr. Biol. 2004;14:1291–1295. doi: 10.1016/j.cub.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Kindt K.S., Quast K.B., Giles A.C., De S., Hendrey D., Nicastro I., Rankin C.H., Schafer W.R. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007;55:662–676. doi: 10.1016/j.neuron.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Mori I., Rhee J.S., Akaike N., Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- Kwon J.Y., Dahanukar A., Weiss L.A., Carlson J.R. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S., Forster R.E., 2nd CO2/H+ sensing: peripheral and central chemoreception. Int. J. Biochem. Cell Biol. 2003;35:1413–1435. doi: 10.1016/s1357-2725(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Lehmann F.O., Heymann N. Unconventional mechanisms control cyclic respiratory gas release in flying Drosophila. J. Exp. Biol. 2005;208:3645–3654. doi: 10.1242/jeb.01788. [DOI] [PubMed] [Google Scholar]
- L'Etoile N.D., Coburn C.M., Eastham J., Kistler A., Gallegos G., Bargmann C.I. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron. 2002;36:1079–1089. doi: 10.1016/s0896-6273(02)01066-8. [DOI] [PubMed] [Google Scholar]
- Mori I., Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Ortiz C.O., Etchberger J.F., Posy S.L., Frokjaer-Jensen C., Lockery S., Honig B., Hobert O. Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics. 2006;173:131–149. doi: 10.1534/genetics.106.055749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C.O., Faumont S., Takayama J., Ahmed H.K., Goldsmith A.D., Pocock R., McCormick K.E., Kunimoto H., Iino Y., Lockery S., Hobert O. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr. Biol. 2009;19:996–1004. doi: 10.1016/j.cub.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson A., Gross E., Laurent P., Busch K.E., Bretes H., de Bono M. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature. 2009;458:1030–1033. doi: 10.1038/nature07820. [DOI] [PubMed] [Google Scholar]
- Richerson G.B. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson G.B., Wang W., Hodges M.R., Dohle C.I., Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp. Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Richmond J.E., Davis W.S., Jorgensen E.M. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat. Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderstrale Y., Hanson M. Histochemical study of the distribution of carbonic anhydrase in the cat brain. Acta Physiol. Scand. 1985;124:557–564. doi: 10.1111/j.1748-1716.1985.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Satterlee J.S., Ryu W.S., Sengupta P. The CMK-1 CaMKI and the TAX-4 cyclic nucleotide-gated channel regulate thermosensory neuron gene expression and function in C. elegans. Curr. Biol. 2004;14:62–68. doi: 10.1016/j.cub.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Satterlee J.S., Sasakura H., Kuhara A., Berkeley M., Mori I., Sengupta P. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron. 2001;31:943–956. doi: 10.1016/s0896-6273(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Sawin E.R., Ranganathan R., Horvitz H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Sharabi K., Hurwitz A., Simon A.J., Beitel G.J., Morimoto R.I., Rechavi G., Sznajder J.I., Gruenbaum Y. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2009;106:4024–4029. doi: 10.1073/pnas.0900309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese S., Petrie M., Schuske K., Ailion M., Ann K., Iwasaki K., Jorgensen E.M., Martin T.F. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 2007;27:6150–6162. doi: 10.1523/JNEUROSCI.1466-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh G.S., Wong A.M., Hergarden A.C., Wang J.W., Simon A.F., Benzer S., Axel R., Anderson D.J. A single population of olfactory sensory neurons mediates an innate avoidance behavior in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Suh G.S., Ben-Tabou de Leon S., Tanimoto H., Fiala A., Benzer S., Anderson D.J. Light activation of an innate olfactory avoidance response in Drosophila. Curr. Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- Sun L., Wang H., Hu J., Han J., Matsunami H., Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc. Natl. Acad. Sci. USA. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Thiele T.R., Faumont S., Ezcurra M., Lockery S.R., Schafer W.R. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature. 2008;454:114–117. doi: 10.1038/nature06927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjänen L., Tolvanen M., Hilvo M., Olatubosun A., Innocenti A., Scozzafava A., Leppiniemi J., Niederhauser B., Hytonen V.P., Gorr T.A. Characterization of the first beta-class carbonic anhydrase from an arthropod (Drosophila melanogaster) and phylogenetic analysis of beta-class carbonic anhydrases in invertebrates. BMC Biochem. 2010;11:28. doi: 10.1186/1471-2091-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S.L., Ray A. Modification of CO2 avoidance behavior in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- Vosshall L.B., Stocker R.F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wang W., Bradley S.R., Richerson G.B. Quantification of the response of rat medullary raphe neurones to independent changes in pHo and pCO2. J. Physiol. 2002;540:951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Thomson N., White J.G., Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- White J.G., Southgate E., Thomson J.N., Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Williams R.H., Jensen L.T., Verkhratsky A., Fugger L., Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc. Natl. Acad. Sci. USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.M., Waters H., Dong C., Fulle H.J., Liman E.R. Degeneration of the olfactory guanylyl cyclase D gene during primate evolution. PLoS ONE. 2007;2:e884. doi: 10.1371/journal.pone.0000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Avery L., Baude E., Garbers D.L. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc. Natl. Acad. Sci. USA. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann A.E., Allen J.E., Dahdaleh N.S., Drebot I.I., Coryell M.W., Wunsch A.M., Lynch C.M., Faraci F.M., Howard M.A.R., Welsh M.J., Wemmie J.A. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M., Gray J.M., Pokala N., Chang A.J., Karow D.S., Marletta M.A., Hudson M.L., Morton D.B., Chronis N., Bargmann C.I. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animals placed within the square chamber microfluidic device are exposed to 5% CO2 stimuli off food. Movie speeded up 5×. Timing of CO2 stimulus is indicated.
As in Movie S1, but animals are exposed to 5% CO2 while on a lawn of E. coli food. Movie speeded up 5×. Timing of CO2 stimulus is indicated.