Abstract
Monoterpenes at high atmospheric concentrations are strong growth inhibitors in allelopathic interactions. Effects depend on dose, molecular structure of the monoterpene and on the species of the receiver plant. Stomata are among the first targets affected by camphor and menthol. Previously, it could be demonstrated that the compounds induce swelling of the protoplasts, prevent stomatal closure and enhance transpiration. In this study, we show that the block of stomatal closure is accompanied by changes to the cytoskeleton, which has a direct role in stomatal movements. Although MPK3 (MAP3 kinase) and ABF4 gene expressions are induced within six hours, stomatal closure is prevented. In contrast to ABF4, ABF2 (both transcription factors) is not induced. MPK3 and ABF4 both encode for proteins involved in the process of stomatal closure. The expression of PEPCase, an enzyme important for stomatal opening, is downregulated. The leaves develop stress symptoms, mirrored by transient changes in the expression profile of additional genes: lipoxygenase 2 (LOX2), CER5, CER6 (both important for wax production) and RD29B (an ABA inducible stress protein). Non-invasive methods showed a fast response of the plant to camphor fumigations both in a rapid decrease of the quantum yield and in the relative growth rate. Repeated exposures to the monoterpenes resulted finally in growth reduction and a stress related change in the phenotype. It is proposed that high concentrations or repeated exposure to monoterpenes led to irreversible damages, whereas low concentrations or short-term fumigations may have the potential to strengthen the plant fitness.
Key words: monoterpene, cytoskeleton, gene expression, phenotype modulation
Introduction
Monoterpenoids are constituents of the VOC-(volatile organic compound) bouquets emitted by plants. Many of these compounds have been identified as mediator molecules in plantherbivore, plant-microorganism and plant-plant communication. Following adsorption of VOCs at the leaf surface, uptake into the leaf can occur via the stomata or by cuticle diffusion.1 Analysis of the molecular backgrounds of the defense mechanisms that take part in plant-insect, plant-microbe, plant-herbivore interactions has become an important topic during the last two decades.1–5 VOCs trigger the release of volatile terpenoids in tomato plants and induce defence genes in Arabidopsis.6 Lima beans, exposed to terpenoids, responded in a similar manner.7 Other roles in plant-plant interactions have received less attention, although a high atmospheric abundance of monoterpenoids is known to have essential influences on structuring distribution, density and diversity of species in different plant communities, such as the High Chaparral in south California or the sand pine scrub communities in Florida. In these communities, the compounds suppress the growth of individuals of other or of their own species.8–11
On the other hand, monoterpenes have been shown to protect leaf membranes from oxidation and to increase heat stress resistance by modification of the leaf thermal tolerance.12,13 It was assumed that the release of monoterpenes present in low atmospheric concentration in Mediterranean canopies enhanced heat stress resistance of other plants of the community.
Thus, depending on their chemical structure and the dose, monoterpenes can have different effects. Whereas α-pinene protects the photosynthetic apparatus, α-terpinol is even toxic under non-heat stress conditions. Exogenous monoterpenes in high concentrations of 0.5 g/l are toxic to plant cell cultures.14 Zunino and Zygadlo15 reported on oxidative stress induction and lipid oxidation induced by the monoterpenes 1,8-cineole, thymol, geraniol, menthol and camphor in maize roots. Essential oil of Artemisia scoparia with β-myrcene, limonene, β-ocimene and γ-terpinene as major compounds generates ROS and oxidative damage in receiver plants.16 Peppermint essential oil caused a decrease in membrane potential hyperpolarization at 5 to 50 ppm, but 100 to 500 ppm increased depolarization. The induced membrane depolarizations are known to change ion fluxes across the plasma membrane. The increase in membrane polarization was also found to be correlated to a decline in water solubility of several monoterpenes tested. In cucumber roots, menthol induced an increase of cytosolic free calcium ions, an event that may trigger many signal transduction pathways.17 Monoterpenes increase the production of phenolic compounds in cell cultures of Pelargonium fragrans.14 A calcium influx and protein phosphorylation/dephosphorylation dependent induction of the expression of PAL (phenylalanine ammonia-lyase: a key enzyme in phenylpropane assembly), PR-2 (pathogenesis related protein-2: β-1,3-glucanase), PR-3 (pathogenesis related protein-3: chitinase) and farnesyl pyrophosphate synthase (FPS) was reported by Arimura et al.7 The acyclic monoterpenes ocimene and myrcene induced substantial changes in the transcription of several hundred genes in Arabidopsis, many of them are annotated as transcription factors, stress and defence genes.18 Allo-ocimene is known to prime defence reactions in Arabidopsis against Botrytis cinerea, for example by accumulation of antifungal substances and enhanced lignification.19
In allelopathic interactions, detailed studies are published for camphor and 1,8-cineol. Both compounds, which are strong growth inhibitors, leading to growth abnormality, inhibition of respiration of isolated mitochondria, aspartate synthase activity and mitosis.20–23 The present state of knowledge clearly points to a strong dose- and structure-dependency that trigger positive or negative effects of monoterpenoids on defined plant species, cells, tissues and organs. Meanwhile, many studies demonstrate that plant volatiles can have a great future in sustainable development of agriculture. They may be used for pest control, to monitor plant health and to suppress weeds or to modulate plant fitness.
In a previous study, it was demonstrated that the waxy leaf surface and the stomata are among the first targets affected by the cyclic monoterpenes camphor and menthol. The compounds induced stomata opening and swelling of the protoplasts accompanied by the inability of stomata to close as long as the compounds were present. Long term exposures to high concentrations resulted in irreversible desiccation and plant death.24 Here we show that the block in stomatal closure is accompanied via a change in the cytoskeleton, especially in the actin filaments, which have direct roles in stomata movements.25–32 Consequently, the leaves develop stress symptoms, mirrored by changes in the expression profile of several selected genes. Repeated exposures to the monoterpenes resulted finally in growth reduction and a changed, stress-related Arabidopsis phenotype.
Results
Modulation of gene expression.
Since cyclic monoterpene applications led to opening of stomata, an enhanced transpiration and dehydration, we set out to characterize the expression of several selected genes involved in stomata movement, abiotic stress response (drought, osmotic stress) and wax synthesis by real time PCR.
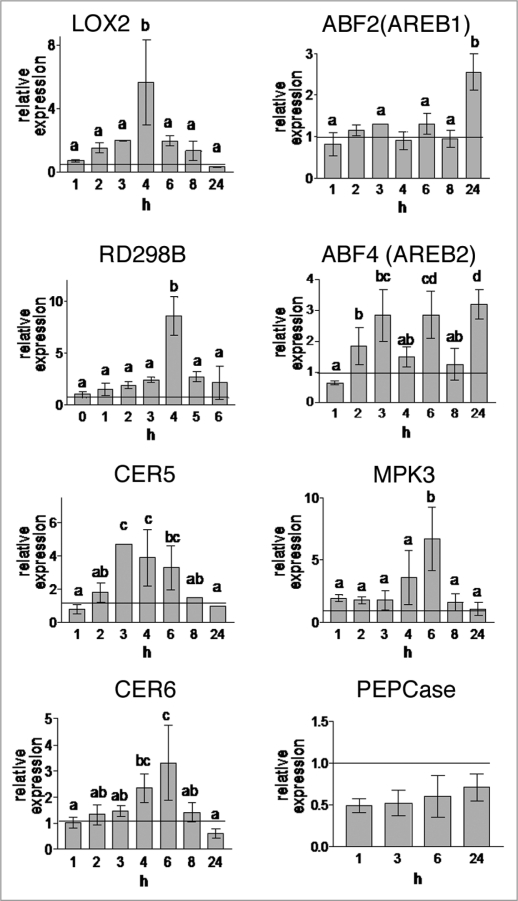
MAP kinase 3 (MPK3), known to be activated in response to H2O2 and ABA, has an important role in guard cell signaling and promotion of stomatal closure.33 The kinase activity was found to be induced by H2O2 and ABA, which both play a key role in adaption to drought and other stresses.34,35 In our study, the expression of the kinase gene was upregulated 3–6-fold after 4 to 6 h of fumigation (Fig. 1). On the other hand, transcription of phosphoenolpyruvate carboxylase (PEPCase), which fixes CO2 into oxaloacetic acid for malate production, was downregulated. Malate, however, is important for stomatal opening. During the whole period of treatment, PEPCase transcript levels were always below the controls. Expression of the ABA-induced genes ABF2 (AREB1) and ABF4 (AREB2), encoding basic leucine zipper transcription factors, were included in this study.36,37 ABF4 is suggested to influence directly the regulation of gene expression in guard cells and is known to be involved in drought tolerance and reducing water loss.38 Abundance of ABF4 transcripts oscillated, with increases (about 3 fold) after 3, 6 and 24 h. ABF2 (AREB1) was not induced during the first 8 h, but after 24 h, transcript levels were about 2.5 fold. An 8 fold induction of the ABA-inducible and, dehydration responsive RD29B gene was observed after 6 h, but the induction was transient. The expression profiles of these few genes indicate a moderate water deficiency in the leaves due to monoterpene fumigation. This is consistent with the induction of CER5 after 3–6 h and CER6 after 4 h. Both genes encode proteins essential for the production of the wax layer of plant aerial surfaces.39,40
Figure 1.
Effects of the monoterpenes on gene expressions analyzed by qRT-PCR. The abundance of mRNA (x fold) is shown relative to the control values referred as to 1 (line). Mean values ± SD of three independent experiments. Letters above the histogram bars refer to statistically significant differences (p < 0.05) within groups as determined by Duncan test. Values marked by the same or no letter are not significantly different within bar values.
Lipoxygenase 2 catalyzes the hydroperoxidation of fatty acids with cis, cis-1,4-pentadiene structures such as linolenic and linoleic acid.6,41,42 The products are precursors of C6-volatiles, whereas hydroperoxylinolenic acid presents an intermediate of jasmonic acid (JA) biosynthesis, a plant growth regulator. LOX2 expression is regulated in response to stress conditions such as wounding or water deficiency. In our study, this gene was upregulated 2–7-fold after 3–4 h of fumigation. Long term exposure resulted in a downregulation.
Effects on the cytoskeleton.
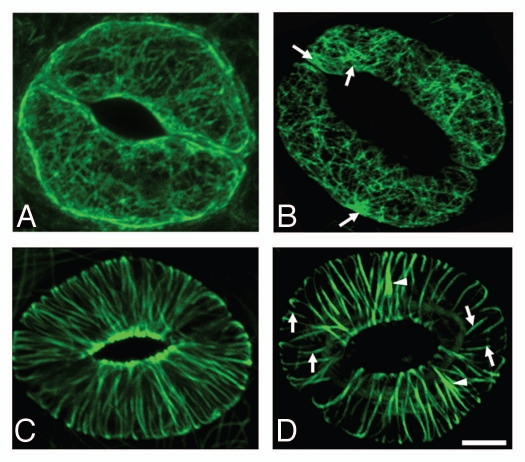
Monoterpenes caused reorganisation and partial disruption of F-actin filaments leading to aberrantly over-polymerized actin cytoskeleton (Fig. 2A and B). Typical actin filaments in control stomata showed radial orientation and the cell periphery showed strong signal. However, in monoterpene-treated plants actin arrays were replaced by less-organized F-actin networks and over-polymerized patches. In addition, F-actin was not assembled abundantly at the cell periphery in monoterpene-treated plants. Also microtubules were sensitive to the monoterpenes showing over-polymerized radial arrays whereas they were depleted at the open stomatal pore (Fig. 2C and D). Overall, these cytoskeletal reorganizations were fully consistent with the morphological changes observed, such as the swelling of guard cells and opening of stomata. The cytoskeleton is known to integrate sensory signals with ionic activities and metabolic processes.29,30 The aberrantly organized and perhaps less dynamic cytoskeleton is proposed to be a major reason for the failure of endogenous signals to induce stomatal closure after monoterpene fumigation. Disruption of F-actin filaments had no effect on actin 2 expression.43
Figure 2.
Effect of camphor and menthol on the cytoskeleton in stomata guard cells. Effect of camphor (10 mg/l) and menthol (5 mg/l) on the cytoskeleton in stomata of Arabidopsis thaliana cotyledons. Actin cytoskeleton visualized with the 35S::GFP:FABD2 construct as in vivo marker for filamentous actin in control conditions (A) and after treatment with monoterpenoids for 48 h (B). Arrows point to the patches of bundled F-actin. Microtubular cytoskeleton visualized with the 35S::GFP:MBD construct as in vivo marker for microtubules in control conditions (C) and after treatment with monoterpenoids for 48 h (D). Note pronounced bundling of microtubules (arrowheads) and appearance of short disrupted microtubular bundles (arrows). Bar: 10 µm.
Modulations of the phenotype.
Stress induced modulations occur at multiple spatial and temporal levels and require sensitive phenotyping techniques.44 Therefore, phenotypic modulations were assayed with GROWSCREEN FLUORO, which captures plant size as projected leaf area (APT) and integrates chlorophyll fluorescence over the APT.45
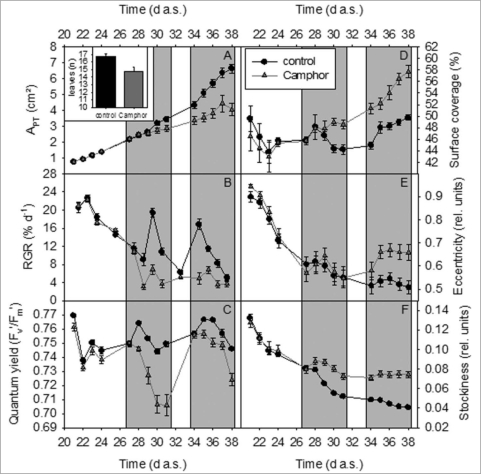
Camphor treatment led to a rapid decrease of relative growth rate of the plants (Fig. 3B), which was followed by noticeable differences in APT (Fig. 3A, Table 1) of the treated plants compared to the untreated plants. Smaller plant size was accompanied by a lower number of leaves (insert in Fig. 3A) Concomitant with the decrease of growth, quantum yield of the leaves was lowered (Fig. 3C). Whereas quantum yield recovered after the end of the camphor fumigation, growth rate remained low. A second period of camphor treatment amplified the differences in plant sizes and lowered quantum yield again. Camphor treatment modified plant morphology in terms of higher surface coverage (Fig. 3D) and stockiness (Fig. 3F) compared to untreated plants. This hints at a more compact growth, i.e., the leaves of the rosette were closer together than in the untreated plants. Especially during the second period of camphor treatment, plants became eccentric (Fig. 3E) while the untreated plants were more round-shaped. The eccentricity was due to an unequal distribution of leaves around the centr of the rosette, associated with the formation of lesions during prolonged camphor treatment (Fig. 4).
Figure 3.
Modification of A. thaliana phenotypes analyzed with GROWSCREEN FLUORO. Black lines/bars: control treated plants, grey lines/bars camphor treated plants. (A) projected leaf area (APT), insert in (A), amount of leaves at the final data acquisition; (B) relative growth rate (RGR); (C) quantum yield; (D) surface coverage; (E) eccentricity; (F) stockiness. Dark background indicates periods of fumigation.
Table 1.
Phenotypes influenced by camphor treatment
| growth | ↓ |
| amount of leaves | ↓ |
| surface coverage | ↑ |
| stockiness | ↑ |
| eccentricity | ↑ |
| photosynthetic performance | ↓ |
Figure 4.
Phenotypes of untreated (left) and camphor-treated plant (right) at the end of the measuring period.
Discussion
Abscisic acid is known as a key inducer of stomatal closure in response to the plant water status. It also mediates responses to various stresses by modifying expression of an assortment of genes.34,45 Fumigation with camphor and menthol causes water stress due to the inability of stomata to close, with the consequence of high transpiration.24 Guard cell linked MPK3, required for stomatal closing, is activated by ABA and H2O2.33,47,48 The promotor of RD29B, a marker gene of ABA-induced gene expression, contains an ABRE (abscisic acid responsive element). A number of basic leucine zipper transcription factors can bind to ABRE, such as ABF4 (AREB2) and ABF2 (AREB1).36 In response to camphor and menthol, the expression of MPK3, ABF4 and RD29 B was induced within six hours, in contrast to ABF2. It has been shown that ABF2 is important in the regulation of seedling growth and responses to glucose. ABF4, among others, is required for ABA, stress responses and stomatal closure.49,50 PEPCase is a major enzyme in malate synthesis. Malate is known to be an important counterion for potassium, which drives stomatal opening.51 A downregulation of the PEPCase transcript level in response to monoterpene induced high transpiration rates seems reasonable. An osmotic and salt repressed PEPCase expression was reported by Ueda et al.52
Water stress in monoterpene fumigated Arabidopsis leaves is also indicated by the upregulation of CER6 and CER5 transcription. Whereas CER6 is the most important condensing enzyme involved in VLCFA (very long chain fatty acid) production—an early step in wax biosynthesis, CER5 is responsible for the transport of wax monomers out of epidermal cells to the surface. CER6 transcript accumulation is known to be enhanced in response to osmotic stress. The presence of ABREs in the CER6 promotor led to the suggestion that the gene is ABA inducible. Hooker et al.39 demonstrated a 2.5- to 3-fold enhancement of CER6 transcripts after treatment with 0.1 mM ABA. Similarly, CER5 was found to be inducible by 50 µM ABA and osmotic stress (Panikashvili et al. 2007).53 Both genes were upregulated only 3–5 h after the start of fumigation. A 3.5- to 5-fold increase in LOX2 transcript abundance within 24 h was recently demonstrated by Godard et al.18 after treatment of Arabidopsis with the acyclic monoterpene ocimene. LOX2 is a key enzyme in jasmonate biosynthesis and C6-volatiles production, proposed precursor molecules for the syntheses are the glycolipids of chloroplasts.6,54 Induced expression and an increase in LOX2 activity occurred in lima beans exposed to volatiles of emitter leaves infested with Tetranychus urticae. Thus, induction of LOX2 gene is possible with a large variety of monoterpenoids. The resulting increase in jasmonate production is known to modulate the expression profiles of many genes, including defense genes, and to prime defense reactions.7,19 A microarray-based screening of jasmonate-responsive Arabidopsis genes by Jung et al.55 revealed an upregulation of 74 genes including LOX2 (6.8-fold after 24 h) whereas 63 genes were repressed.
In this study, the upregulation of all genes investigated was transient. Possibly, a single fumigation period of 24 h was not sufficient to manifest stress physiology and plants recovered or the consequences of the disturbed actin cytoskeleton, together with drought stress reactions, inhibited continued gene inductions. It is rather likely that camphor and menthol led to modulations of many other gene activities, in addition to the few investigated in this study. Antagonistic interactions between ABA and jasmonate signaling pathways have been described by Anderson et al.56 On the other hand, methyljasmonate is assumed to stimulate ABA production.57 Hassanein et al.58 describe ameliorating effects of jasmonic acid in plants under drought stress. Jitratham et al.59 reported on stomatal closure in citrus leaves by jasmonate applications. We propose therefore that the monoterpenes camphor and menthol directly affect the actin cytoskeleton as well as microtubules, abolishes ABA and JA mediated stomatal closing and prevent actions which enable leaves to cope with drought stress. As a consequence, drought stress symptoms set in some hours after starting the treatment with the monoterpenes. According to first studies, Arabidopsis treated with Arthemisia camphorata volatiles shows, for instance, a similar increase of MPK3 expression (data not shown).
Stabilization of actin filaments by phalloidin treatment is known to inhibit stomatal closing in a concentration-dependent manner, whereas depolymerization and fragmentation of actin filaments by cytochalasin D increases stomata opening.25 These older data have been confirmed later.26,27,32 Our data reveal that the actin cytoskeleton is very sensitive to monoterpenes not only in guard cells but also in root cells (data not shown). Presumably, the monoterpenes cause depolarizations of the plasma membrane too, affecting activities of ion channels.26,29,30 Changed ion fluxes across the plasma membrane and dysfunctional ion channels would enhance the effects on the cytoskeleton, interfering with the processes of stomatal closure and movements. As a consequence, water flux into the leaves does not compensate water loss by transpiration and leaves start to suffer from drought stress, even where plants were well watered. Effects on the actin cytoskeleton in mammals seem to be a common mode of action of several monoterpenes, which inhibit bone resorption. This was observed with osteoclasts from rats.60 Besides the actin cytoskeleton, microtubules are affected by monoterpenes and they are also implicated in stomatal movements.31 Dependency of the biological activity of monoterpenes on their chemical structure is ascertained by the work of Chaimovitish et al.61 who show an immediate reaction of plant cells to citral which disrupts microtubules, whereas actin filaments remain intact. These features are highly consistent with the voluminous reports that the cytoskeleton is both regulator and target of biotic interactions (reviewed in ref. 62).
Camphor has the potential to modulate the phenotype of plants. In this study, we showed that repeated fumigation with camphor led to stress-related phenotypic changes of Arabidopsis plants. Lowering of growth and photosynthetic performance demonstrated an adverse effect of the fumigation on plant performance (Table 1). The camphor-induced growth phenotypes were similar to those observed under drought stress.45 Finally, camphor-mediated lesion formation destroyed leaf material and thereby led to eccentric rosette shapes. The increase in compactness and surface coverage of the fumigated plants (Table 1) counteracts water loss and can be seen as a response to the high transpiration. Thus, high concentrations, prolonged or repeated exposure to monoterpenes led to irreversible damage of the whole plant, whereas low concentration or short term fumigations with bioactive monoterpenes can lead to reversible responses and may strengthen the plant.
Materials and Methods
Real time PCR.
For real time PCR studies 3 week old Arabidopsis thaliana ecotype Col-0 plants were placed in plastic boxes and fumigated as described in Schulz et al.24 for 1, 2, 3, 4, 6, 8 and 24 h with 10 mg camphor/L and 5 mg menthol/L.
Leaves were harvested and used for RNA isolation with the RNeasy Plant Mini Kit (Qiagen) according to the instructions of the manufacturers. Reverse transcription was performed with the Fermentas or with the Quantitec Rev. Transcription kit (Qiagen). cDNA synthesis was performed with DNAse treated RNA (0.2 µg). For real time PCR the POWER SYBR Green PCR Master Kit, microplates LSH 96 well and 3G optical adhesive covers (all from Applied Biosystem) were used. The following primers were designed (synthesis by MWG): LOX2 (lipoxygenase 2: At3g5140): forward 5′-TAC TTG CCT TCC CAA ACA CC-3′; reverse 5′-AGT GCC CTT GGC TGT AGA GA-3′; ABF2 (AREB1) (At1g45249): forward TGG AGG TGG AGG GTT GAC TA-3′, reverse 5′-CAT CCT TGT TCA TTG ACC CA-3′; ABF4 (AREB2) (At3g19290): forward GTA GTG TCA TGC CCT TGG CT-3′, reverse 5′-ATC GAC CCG AAA TCT TTT CC, CER5 (At5g1500): forward 5′-GTC CGA CTC GAA GAT TGC TC-3′, reverse 5′-TCG TTG ACT TCT TCT TTG GTC A-3′; CER6 (At5g43760): 5′-ATC GAC GAG CTC CAA AAG AA-3′, reverse 5′-TTA CAT TTC CAC ACG GCA GA-3′; RD29B, (At5g52300): forward 5′-GCA CCA CCG TTG GGA CTA TG, reverse 5′-CCA CTG CCT CCA ACT CAC TT-3′; 18SrRNA forward 5′-CGT CCC TGC CCT TTG TAC AC-3′, reverse 5′-AAC ACT TCA CCG GAC CAT TCA; MPK3 (At3g45640): forward 5′-GAC AGA GTT GCT TGG CAC AC-3′, reverse 5′-CCT CAT CCA GAG GCT GTT GT-3′; PEPC (At2g42600): forward 5′-TTG AGG GTA ACG GTT CAA GG-3′; reverse 5′-CAC GGG TAA GTG AAC CTC GT-3′; actin 2 (At3g18780): forward 5′-TGC CAA TCT ACG AGG GTT TC-3′, reverse 5′-TTC TCG ATG GAA GAG CTG GT-3′. The cDNA was diluted 10-fold with water and used for real time PCR in a final volume of 10 µl. The PCR conditions consisted of denaturation at 95°C, for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min.63 At the end of each run, a dissociation curve was generated to ascertain single product amplification (Applied Biosystem software, Applied Biosystems fast real time PCR 7500).
All transcript levels were measured with at least four independent biological replicates, each biological replicate was analyzed in three technical replicates. 18s RNA was used to normalize expression data between samples. Negative control reactions without template were routinely performed.
In vivo cytoskeleton visualization.
For in vivo actin visualization an Arabidopsis thaliana line stably transformed with 35S::GFP:FABD construct was used as reliable marker for filamentous actin.64 For in vivo visualization of microtubules an Arabidopsis thaliana line stably transformed with 35S::GFP:MBD construct was used as reliable marker for microtubular cytoskeleton.65 Seedlings were treated with 10 mg camphor/L and 5 mg menthol/L for 48 h as a maximum (within 24 h, 10 mg menthol evaporate at room temperature). Stomata cells of the treated cotyledons and those of control plants were analyzed for effects of the cytoskeleton by using Olympus FV1000 confocal laser scanning microscopy system equipped with Argon Laser using 488 nm wavelength, and operated with the FV10-ASW1.7 software (Olympus, Hamburg, Germany). We investigated at least 30 stomata complexes in cotyledons of 3 seedlings. This experiment was repeated 3 times. The variability in cytoskeletal arrangements was low and the presented images are the most representative ones.
Plant cultivation and camphor treatment for non-invasive studies.
Plants of Arabidopsis thaliana ecotype Col-0 were grown under controlled conditions at 22°C/18°C, 170 µmol m−2 s−1 PAR, and an 8 h/16 h day/night regime. After cotyledon unfolding, single plants were transferred into pots filled with a mixture of potting soil and sand 67 vol.-% potting soil (De Ceuster Meststoffen SA/NV, Grobbendonk, Belgium; 33 vol.-% sand [quartz, grain size 0.7 to 1.4 mm, Rheinische Baustoffwerke, Weilerswist, Germany]). 40 pots (7 cm × 7 cm × 8 cm) were arranged on a tray and were watered thoroughly immediately after pricking out the plants.
For non-invasive studies, plants were fumigated with camphor by placing the pots together with a camphor-containing dish into a 76.8 L plastic box covered with transparent foil. Ten plants were placed in one box. For treatment, the dish contained 768 mg of camphor (i.e., 10 mg for each liter air volume, 39 mg will evaporate within 24 h at room temperature) and for control plants the dish was empty. Fumigation took place for 96 h followed by 72 h without treatment and then a second 96 h-fumigation period.
Data acquisition for non-invasive studies.
Repeated measurements of plant size, morphology and chlorophyll fluorescence were carried out with GROWSCREEN-FLUORO. The tray with 40 plant pots was placed under the measuring unit, which moved from one pot to the next and acquired images according to a predefined protocol.45 Image processing yielded in information on plant size, number of leaves, surface coverage, eccentricity, stockiness and quantum yield for each plant. Plant sizes were acquired as projected leaf area (APT), which is a good proxy for plant biomass.65 Relative growth rates (RGR in %d−1) were calculated from subsequently measured APT values (A1, A2) according to RGR = 100 × 1/t × ln(A2/A1) with “t” indicating the time between the acquisitions of A1 and A2.
Acknowledgements
The authors thank Thomas Eichert, University of Bonn, INRES, for help with statistics and Michael Thorpe, Forschungszentrum Julich GmbH, ICG-3 for checking the language and critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12032
References
- 1.Baldwin IT, Halitsche R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant nteractions: “Talking trees” in the genomics era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 2.Dudareva N, Pichersky E, Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer EE. Surface to air signals. Nature. 2001;411:854–856. doi: 10.1038/35081189. [DOI] [PubMed] [Google Scholar]
- 4.Heil M, Silva Bueno JC. Within plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. PNAS. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershonzon J. Plant volatiles carry both public and private messages. Proc Natl Acad Sci USA. 2007;104:5257–5258. doi: 10.1073/pnas.0700906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bate NJ, Rothstein SJ. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense related genes. Plant J. 1998;16:561–569. doi: 10.1046/j.1365-313x.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 7.Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 8.Muller CH, Muller WH, Haines BL. Volatile growth inhibitors produced by shrubs. Science. 1964;143:471–473. doi: 10.1126/science.143.3605.471. [DOI] [PubMed] [Google Scholar]
- 9.Muller CH. Inhibitory terpenes volatilized from Salvia shrubs. Bull Torrey Bot Club. 1965;92:38–45. [Google Scholar]
- 10.Williamson GB, Fischer NH, Richardson DR, De LaPena A. Chemical inhibition of fire-prone grasses by fire sensitive Conradina canescens. J Chem Ecol. 1989;15:1567–1577. doi: 10.1007/BF01012384. [DOI] [PubMed] [Google Scholar]
- 11.Fischer NH, Tanrisever N, Williamson GB. Allelopathy in the Florida scrub community as a model for natural herbicide actions. In: Cutler HG, editor. Biological active natural products: Potential use in Agriculture. ACS Symposium Series No. 380. Washington DC: American Chemical Society; 1988. pp. 232–249. [Google Scholar]
- 12.Penuelas J, Munne-Bosch S. Isoprenoids: an evolutionary pool for photoprotection. Trend Plant Sci. 2005;10:166–169. doi: 10.1016/j.tplants.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Copolovici LO, Filella I, Llusia J, Niinemets Ü, Penuelas J. The capacity for thermal protection of photosynthetic electron transport varies for different monoterpenes in Quecus ilex. Plant Physiol. 2005;139:485–496. doi: 10.1104/pp.105.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JT, Hegarty PK, Charlwood BV. The toxicity of monoterpenes to plant cell cultures. Plant Sci. 1987;48:195–201. [Google Scholar]
- 15.Zunino MP, Zygadlo JA. Effects of monoterpenes on lipid oxidation in maize. Planta. 2004;219:303–309. doi: 10.1007/s00425-004-1216-7. [DOI] [PubMed] [Google Scholar]
- 16.Singh HP, Kaur S, Mittal S, Batish DR, Kohli RK. Essential oil of Artemisia scoparia inhibits plant growth by generating reactive oxygen species and causing oxidative damage. J Chem Ecol. 2009;35:154–162. doi: 10.1007/s10886-009-9595-7. [DOI] [PubMed] [Google Scholar]
- 17.Maffei M, Camusso W, Sacco S. Effect of Mentha × piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry. 2001;8:703–707. doi: 10.1016/s0031-9422(01)00313-2. [DOI] [PubMed] [Google Scholar]
- 18.Godard KA, White R, Bohlmann J. Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry. 2008;69:1838–1849. doi: 10.1016/j.phytochem.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Analysis of defensive responses activated by volatile allo-ocimene treatment in Arabidopsis thaliana. Phytochemistry. 2006;67:1520–1529. doi: 10.1016/j.phytochem.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Duke SO, Oliva A. Mode of action of phytotoxic terpenoids. In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG, editors. Allelopathy, Chemistry and Mode of action of allelochemicals. ED 1 Boca Raton: Florida CRC Press; 2004. pp. 201–216. [Google Scholar]
- 21.Dayan FE, Romagni JC, Duke SO. Investigation of the mode of action of natural phytotoxins. J Chem Ecol. 2000;26:2079–2094. [Google Scholar]
- 22.Romagni JG, Allen SN, Dayan FE. Allelopathic effects of volatile cineols on two weedy plant species. J Chem Ecol. 2000;26:303–313. [Google Scholar]
- 23.Romagni JG, Duke SO, Dayan FE. Inhibition of plant asparagine synthese by monoterpene cineols. Plant Physiol. 2000;123:725–732. doi: 10.1104/pp.123.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Schulz M, Kussmann P, Knop M, Kriegs B, Gresens F, Eichert T, et al. Allelopathic monoterpenes interfere with Arabidopsis thaliana cuticular waxes and enhance transpiration. Plant Signal Behav. 2007;24:231–239. doi: 10.4161/psb.2.4.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M, Hepler PK, Eun S-O, Ha KS, Lee Y. Actin filaments in mature guard cells are radially distributed and involved in stomatal movement. Plant Physiol. 1995;109:1077–1084. doi: 10.1104/pp.109.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eun SO, Lee Y. Actin filaments of guard cells are reorganized in response to light and abscisic acid. Plant Physiol. 2007;115:1491–1498. doi: 10.1104/pp.115.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang JU, Suh S, Yi H, Kim J, Lee Y. Actin filaments modulate both stomatal opening and inward K+-channel activities in guard cells of Vicia faba L. Plant Physiol. 1997;115:335–342. doi: 10.1104/pp.115.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang JU, Eun SO, Lee Y. Structure and function of actin filaments in mature guard cells. In: Steiger C, Baluška F, Volkmann D, Barlow PW, editors. Actin—a dynamic framework for multiple plant cell functions. Kluwer Academic Publishers; 2000. pp. 427–436. [Google Scholar]
- 29.Volkmann D, Baluška F. Actin cytoskeleton in plants: from transport networks to signaling networks. Micr Res Techn. 1999;47:135–154. doi: 10.1002/(SICI)1097-0029(19991015)47:2<135::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Staiger CJ. Signaling to the actin cytoskeleton in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:257–288. doi: 10.1146/annurev.arplant.51.1.257. [DOI] [PubMed] [Google Scholar]
- 31.Lahav M, Abu-Abied M, Belausov E, Schwartz A, Sadot E. Microtubules of guard cells are light sensitive. Plant Cell Physiol. 2004;45:573–582. doi: 10.1093/pcp/pch067. [DOI] [PubMed] [Google Scholar]
- 32.Higaki T, Kutsuna N, Sano T, Kondo N, Hasezawa S. Quantification and cluster analysis of actin cytoskeletal structures in plant cells: Role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. Plant J. 2009;61:156–165. doi: 10.1111/j.1365-313x.2009.04032.x. [DOI] [PubMed] [Google Scholar]
- 33.Gudesblat GE, Iusem ND, Morris PC. Guard cell—specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol. 2007;173:713–721. doi: 10.1111/j.1469-8137.2006.01953.x. [DOI] [PubMed] [Google Scholar]
- 34.Huang D, Wu W, Abrams SR, Cutler AJ. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J Exp Bot. 2008;59:2991–3007. doi: 10.1093/jxb/ern155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C-P. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–1448. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang J-Y, Choi H, Im M, Kim SY. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell. 2002;14:343–357. doi: 10.1105/tpc.010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA. 2002;97:11632–11637. doi: 10.1073/pnas.190309197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilson SE, Assmann SM. The control of transpiration. Insights from Arabidopsis. Plant Physiol. 2007;143:19–27. doi: 10.1104/pp.106.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hooker TS, Millar AA, Kunst L. Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiol. 2002;129:1568–1580. doi: 10.1104/pp.003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pighin JA,, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, et al. Plant cuticular lipid export requires an ABC transporter. Science. 2005;306:702–704. doi: 10.1126/science.1102331. [DOI] [PubMed] [Google Scholar]
- 41.Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA. 1995;92:8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell E, Mullet JE. Lipoxygenase gene expression is modulated in plants by water deficit, wounding and methyl jasmonate. Mol Gen Genet. 1991;230:456–462. doi: 10.1007/BF00280303. [DOI] [PubMed] [Google Scholar]
- 43.Kandasamy MK, McKinney EC, Meagher RB. Functional nonequivalency of actin isovariants in Arabidopsis. Mol Biol Cell. 2002;12:251–261. doi: 10.1091/mbc.01-07-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter A, Silk WK, Schurr U. Environmental Effects on spatial and temporal patterns of leaf and root growth. Annu Rev Plant Biol. 2009;60:279–304. doi: 10.1146/annurev.arplant.59.032607.092819. [DOI] [PubMed] [Google Scholar]
- 45.Jansen M, Gilmer F, Biskup B, Nagel KA, Rascher U, Fischbach A, et al. Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN-FLUORO allows detection of drought and chilling tolerance in Arabidopsis thaliana and other rosette plants. Funct Plant Biol. 2009;36:902–914. doi: 10.1071/FP09095. [DOI] [PubMed] [Google Scholar]
- 46.Seki M, Kamei A, Yamaguchi-Shinozaki K, Shinozaki K. Molecular responses to drought, salinity and frost: common and different paths for plant protection. Curr Opin Biotechnol. 2003;14:194–199. doi: 10.1016/s0958-1669(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 47.Lu C, Han MH, Guevara-Garcia A, Fedoroff NV. Mitogen-activated protein kinase signaling in postgermination arrest of development by abscisic acid. Proc Natl Acad Sci USA. 2002;99:15812–15817. doi: 10.1073/pnas.242607499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J, An GY, Wang PT, Wang PT, Han JF, Jin YB, Song CP. MAP kinase specifically mediates the ABA-induced H2O2 generation in guard cells of Vicia faba L. Chinese Sci Bull. 2003;48:1919–1926. [Google Scholar]
- 49.Kim S, Kang J, Cho D, Park JH, Kim SY. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J. 2004;40:75–87. doi: 10.1111/j.1365-313X.2004.02192.x. [DOI] [PubMed] [Google Scholar]
- 50.Choi H, Hong J, Ha J, Kang J, Kim SY. ABFs, a familiy of ABA-responsive element binding factors. J Biol Chem. 2000;275:1723–1730. doi: 10.1074/jbc.275.3.1723. [DOI] [PubMed] [Google Scholar]
- 51.Cotelle V, Pierre JN, Vavasseur A. Potential strong regulation of guard cell phosphoenolpyruvate carboxylase through phosphorylation. J Exp Bot. 1999;50:777–783. [Google Scholar]
- 52.Ueda A, Kathiresan A, Inada M, Narita Y, Nakamura T, Shi W, et al. Osmotic stress in barley regulates expression of a different set of genes than salt stress does. J Exp Bot. 2004;55:2213–2218. doi: 10.1093/jxb/erh242. [DOI] [PubMed] [Google Scholar]
- 53.Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Höfer R, et al. The Arabidopsis DERPERADO/AtWBC11 Transporter is required for cutin and wax secretion. Plant Physiol. 2007;145:1345–1360. doi: 10.1104/pp.107.105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhuang H, Hamilton-Kemp TR, Anderson RA, Hildebrand DF. The impact of alteration of polysaturated fatty acid levels on C6-aldehyde formation of Arabidopsis thaliana leaves. Plant Physiol. 1996;111:805–812. doi: 10.1104/pp.111.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung C, Lyou SH, Yeu SY, Kim MA, Rhee S, Kim M, et al. Microarray-based screening of jasmonate-responsive genes in Arabidopsis thaliana. Plant Cell Rep. 2007;22:1053–1063. doi: 10.1007/s00299-007-0311-1. [DOI] [PubMed] [Google Scholar]
- 56.Anderson JP, Baruzsaufari E, Schenk PM, Manners JM, Desmold OJ, Ehlert C, et al. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;6:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim EH, Park SH, KIM JK. Methyljasmonate triggers loss of grain yield under drought stress. Plant Signal Behav. 2009;4:348–349. doi: 10.4161/psb.4.4.8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassanein RA, Hassnein A, El-Din AB, Salama M, Hashem HA. Role of jasmonic acid and abscisic acid treatments in alleviating the adverse effects of drought-stress and regulating trypsin inhibitor production in soybean plant. Aust J Basic Appl Sci. 2009;3:904–919. [Google Scholar]
- 59.Jitratham A, Yazama F, Kondo S. Effects of drought stress on abscisic acid and jasmonate metabolism in citrus. Environ Contr Biol. 2006;44:41–49. [Google Scholar]
- 60.Dolder S, Hofstetter W, Wetterwald A, Mühlbauer RC, Felix R. Effect of monoterpenes on the formation and activation of osteoclasts in vitro. J Bone Min Res. 2006;21:647–655. doi: 10.1359/jbmr.060111. [DOI] [PubMed] [Google Scholar]
- 61.Chaimovitsh D, Abu-Abied M, Belausov E, Rubin B, Dudai N, Sadot E. Microtubules are an itracellular target of the plant terpene citral. Plant J. 2009;61:399–408. doi: 10.1111/j.1365-313X.2009.04063.x. [DOI] [PubMed] [Google Scholar]
- 62.Takemoto D, Hardham AR. The cytoskeleton as a regulator and target of biotic interactions in plants. Plant Physiol. 2006;136:3864–3876. doi: 10.1104/pp.104.052159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baerson SR, Sanchez-Moreiras A, Pedrol-Bonjoch N, Schulz M, Kagan IA, Agarwal AK, et al. Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J Biol Chem. 2005;280:21867–21881. doi: 10.1074/jbc.M500694200. [DOI] [PubMed] [Google Scholar]
- 64.Voigt B, Timmers AC, Samaj J, Müller J, Baluška F, Menzel D. GFP-FABD2 fusion construct allows in vivo visualization of the dynamic actin cytoskeleton in all cells of Arabidopsis seedlings. Eur J Cell Biol. 2005;84:595–608. doi: 10.1016/j.ejcb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Marc J, Granger CL, Brincat J, Fisher DD, Kao T-H, McCubbin AG, Cyr RJ. A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell. 1998;10:1927–1939. doi: 10.1105/tpc.10.11.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walter A, Scharr H, Gilmer F, Zierer R, Nagel KA, Ernst M, et al. Dynamics of seedlings growth acclimation towards altered light conditions can be quantified via GROWSCREEN: a setup and procedure designed for rapid optical phenotyping of different plant species. New Phytol. 2007;174:447–455. doi: 10.1111/j.1469-8137.2007.02002.x. [DOI] [PubMed] [Google Scholar]