Abstract
Photosynthetic organisms display adaptations to changes in light and nutrient availability. Iron, which is required for the function of photosynthetic photosystems and other important biochemical processes, is an essential mineral that consequently impacts not only overall photosynthetic efficiency, but also the physiology of organisms in general. Our recent study represents the first functional characterization of a cyanobacterial TonB protein. TonB proteins classically are membrane proteins that support the transport of iron and vitamin B12 into cells. TonB proteins thus generally serve a critical role in organismal iron acclimation. We recently identified FdTonB, a TonB-family protein, in the filamentous freshwater cyanobacterium Fremyella diplosiphon. FdTonB contains conserved TonB residues and domains, as well as novel protein domains. Our recent study, however, supports a novel function for this protein in the photoregulation of morphology, rather than iron acclimation, in F. diplosiphon. Our detailed investigations into the responses of SF33 wild-type and ΔtonB mutant strains did not support a role for FdTonB in organismal responses to iron limitation. However, close examination of our recent results did highlight a novel interaction between light and iron acclimation in F. diplosiphon.
Key words: cellular morphology, complementary chromatic adaptation, cyanobacteria, iron, phycobiliprotein, photomorphogenesis, photoregulation, siderophores
Introduction
Cyanobacteria and other photosynthetic organisms utilize absorbed light for the production of stored chemical energy through the process of photosynthesis. To maximize photosynthetic efficiency for development and reproduction, these organisms that exhibit limited mobility tune their pattern of growth and development to their ambient photoenvironment. The regulatory molecules driving this photoadaptive process are sensory photoreceptors. Noted acclimatory responses to changes in the prevalent wavelengths of light in the environment include the regulation of cellular morphology and the regulation of accumulation of distinct photosynthetic pigments. The acclimation of the filamentous cyanobacterium Fremyella diplosiphon to red light (RL) and green light (GL) has long been studied. F. diplosiphon regulates the biliprotein composition of the photosynthetic light harvesting complexes, i.e., phycobilisomes, and cellular morphology and filament length in response to changes in the prevalent wavelength of light in a process known as complementary chromatic adaptation (CCA).1–3 In addition to the photoenvironment, nutrient availability is also an essential factor that impacts photosynthesis. As the nutrient iron is required as a core component of the photosystems, iron availability in particular has strong impacts on photosynthetic efficiency.
New Insights into the Photoregulation of Cellular Morphology in F. diplosiphon
Whereas the regulation of pigmentation changes during CCA has been investigated broadly,2,3 research is only beginning to unravel the molecular basis of the light- and RcaE-dependent regulation of cellular morphology and filament length.4–7 Our group has demonstrated that RcaE is the photoreceptor responsible for directing light-dependent changes in cellular morphology and filament length during CCA.4,5 We also demonstrated that response regulators RcaF and RcaC, which function downstream of RcaE in the pigmentation response,8,9 are necessary for RL-dependent regulation of cellular morphology.5 Most recently, we reported on the characterization of a novel factor, FdTonB, which is required for regulation of cellular morphology in GL.6 FdTonB is a member of the TonB family of proteins that classically have been implicated in organismal responses to iron limitation.3,6,10 The existence of TonB proteins in cyanobacterial systems has only recently been confirmed.6,11 Our analysis of FdTonB function is the first report of the characterization of the physiological role of a TonB protein in cyanobacteria.6 Detailed analyses of a ΔtonB mutant strain, including assessment of cellular morphology, growth responses, and pigment accumulation in iron-replete and iron-limited media under both RL and GL, demonstrated that in F. diplosiphon FdTonB has a role in the photoregulation of cellular morphology in GL, but does not appear to exhibit the classic TonB function of contributing to organismal responses to iron limitation.6 These findings establish the first GL-specific component associated with the regulation of cellular morphology in F. diplosiphon.
Light-Dependent Regulation of Cellular Responses to Iron Limitation in F. diplosiphon
Although FdTonB was not associated with adaptation under iron-limited growth conditions, we did identify a novel interaction between light and iron acclimation in F. diplosiphon.6,12 Whereas SF33 cells grown under RL display classic responses including retardation of growth when iron limitation is imposed, cells growing in iron-depleted medium under GL showed no responses to iron limitation.6,12 The growth rate of SF33 cells in GL was nearly indistinguishable for cells grown in BG11 compared to those grown in BG11-Fe medium.6,12 In addition, pigment accumulation of the cells was observed to be lower in iron limitation particularly under RL conditions, whereas similar pigment accumulation was detected for GL-grown iron-deficient cells when compared to the pigment content of the cells grown under an iron-replete condition.6,12
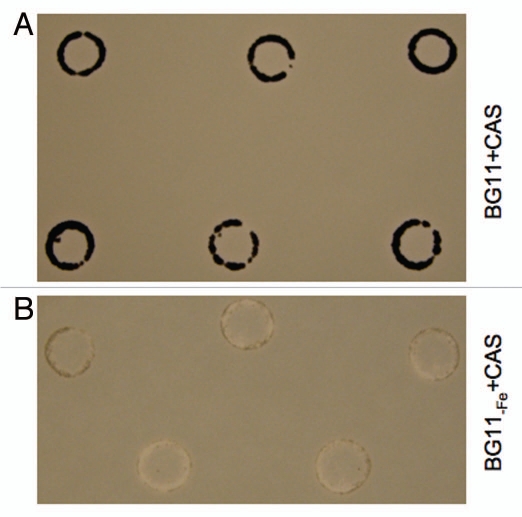
Siderophore production is often associated with growth under iron limitation.10,13 Siderophores are compounds that are excreted extracellularly, chelate iron, and result in increased intracellular iron concentration when the siderophore-iron complexes are transported into cells and the bound iron is released.10,13 To investigate the production of siderophores in F. diplosiphon, we analyzed siderophore secretion using a plate-based diffusion assay. Chromeazurol S (CAS)-containing agar plates were prepared as described.13,14 A 1-ml aliquot of SF33,15 or ΔtonB mutant cultures that had been grown to an A750 of ∼0.1 was pelleted and the pellet washed with BG11 or BG11-Fe medium and resuspended in 100 µl of corresponding growth medium. Ten µl of the resuspended cells were spotted on a white surfactant-free filter (Millipore, Billerca, MA) placed on top of medium in the BG11 + CAS and BG11-Fe + CAS plates. Plates were kept under white illumination of 20 µmol m−2 s−1 for 15 days. SF33 cells produced siderophores in the presence of CAS on BG11-Fe medium plates, resulting in the production of a yellow/white halo around the spotted cells (Fig. 1). The halo was lacking for cells grown on BG11 + CAS plates (Fig. 1). We observed a similar response for ΔtonB mutant on the CAS plates for both the nutrient conditions (data not shown). The exact chemical nature of the siderophores produced by F. diplosiphon requires additional investigation.
Figure 1.
Siderophore production by Fremyella diplosiphon in response to iron limitation. SF33 cells were spotted on (A) BG11 plates containing Chromeazurol S (CAS), i.e., BG11 + CAS or (B) BG11-Fe + CAS, grown for 15 days under white illumination of 20 µmol m−2 s−1, and imaged.
Perspectives on TonB Function and the Photoregulation of Iron Acclimation in F. diplosiphon
A TonB-dependent receptor transport protein is known to interact with TonB and cell-shape periplasmic protein MreC in the bacterium Caulobacter crescentus.16 Cells depleted of MreC protein acquired gross changes in cell shape and defects in the integrity of the peptidoglycan layer.16 This result, taken together with our identification of a role for FdTonB in the photoregulation of cell shape in GL in F. diplosiphon,6 strongly supports a model in which FdTonB could be interacting with cell wall biosynthetic complexes during the photoregulation of cellular morphology that occurs during CCA. Upon mutation of tonB, FdTonB could fail to interact with the cell wall complexes, thereby interfering with the integrity of the peptidoglycan layer and impacting cellular morphology. Previous reports that the expression of tonB is upregulated under GL,6,17 the conditions under which F. diplosiphon cells are elongated1,4 and would presumably require additional synthesis of cell wall components, as well as the GL-specific defect in cellular morphology for a ΔtonB mutant fit this proposed model. In the Gram-negative bacterium Escherichia coli, TonB is a cytoplasmic membrane protein that spans the periplasmic space and functions to transduce energy to outer membrane receptors using the cytoplasmic membrane-derived proton motive force.18 Notably, one current model of TonB function during energy transduction suggests that TonB shuttles between the cytoplasmic and outer membranes during the course of energy transduction.18 In future studies, it would be interesting to investigate the localization of FdTonB, which could provide strong evidence of the functional role of TonB in F. diplosiphon.
Although our results support a role for FdTonB in the GL-dependent regulation of morphology in F. diplosiphon, canonical TonB proteins impact the regulation and transport of ferric-iron complexes and vitamin B12 across the outer membrane in Gram-negative bacterial cells.18 However, our recent study demonstrates that the growth of SF33 and a tonB truncation mutant strain are similar in the presence and absence of iron in the BG11 growth media.6 Thus, although we show here that F. diplosiphon secretes siderophores under iron-limited growth conditions (Fig. 1), we do not expect that FdTonB impacts siderophore production or iron scavenging in F. diplosiphon.
As we demonstrated that F. diplosiphon secretes siderophores under iron-limited growth conditions when grown in white light (Fig. 1), it would be interesting to assess whether siderophore production is differentially impacted by RL vs. GL, a distinct possibility given the light-dependent differences in iron acclimation observed for F. diplosiphon. In summary, in addition to representing the first functional analysis of a cyanobacterial TonB protein, our recent study implicates novel TonB functions for FdTonB in the photoregulation of cellular morphology rather than in iron acclimation. Our studies also led to identification of a novel, intriguing interaction between light and iron acclimation in F. diplosiphon. Further studies of iron acclimation in this organism are likely to provide insight into unique organismal responses to iron limitation.
Acknowledgements
Work on light sensing and photomorphogenesis in cyanobacteria in the Montgomery lab is supported by a CAREER award from the National Science Foundation (Grant MCB-0643516 to B.L.M.) and the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (DE-FG02-91ER20021 to B.L.M.). The authors thank Melissa Whitaker and Sankalpi Warnasooriya for reading and commenting on the manuscript.
Abbreviations
- CCA
complementary chromatic adaptation
- chla
chlorophyll a
- GL
green light
- PBP
phycobiliprotein
- PC
phycocyanin
- PE
phycoerythrin
- RL
red light
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11827
References
- 1.Bennett A, Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973;58:1–3. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kehoe DM, Gutu A. Responding to color: The regulation of complementary chromatic adaptation. Annu Rev Plant Biol. 2006;57:127–150. doi: 10.1146/annurev.arplant.57.032905.105215. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery BL. Shedding new light on the regulation of complementary chromatic adaptation. Cent Eur J Biol. 2008;3:351–358. [Google Scholar]
- 4.Bordowitz JR, Montgomery BL. Photoregulation of cellular morphology during complementary chromatic adaptation requires sensor-kinase-class protein RcaE in Fremyella diplosiphon. J Bacteriol. 2008;190:4069–4074. doi: 10.1128/JB.00018-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordowitz JR, Whitaker MJ, Montgomery BL. Independence and interdependence of the photoregulation of pigmentation and development in Fremyella diplosiphon. Commun Integr Biol. 2010:3. doi: 10.4161/cib.3.2.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pattanaik B, Montgomery BL. FdTonB is involved in the photoregulation of cellular morphology during complementary chromatic adaptation in Fremyella diplosiphon. Microbiology. 2010;156:731–741. doi: 10.1099/mic.0.035410-0. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker MJ, Bordowitz JR, Montgomery BL. CpcF-dependent regulation of pigmentation and development in Fremyella diplosiphon. Biochem Biophys Res Commun. 2009;389:602–606. doi: 10.1016/j.bbrc.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Kehoe DM, Grossman AR. New classes of mutants in complementary chromatic adaptation provide evidence for a novel four-step phosphorelay system. J Bacteriol. 1997;179:3914–3921. doi: 10.1128/jb.179.12.3914-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang GG, Schaefer MR, Grossman AR. Complementation of a red-light-indifferent cyanobacterial mutant. Proc Natl Acad Sci USA. 1992;89:9415–9419. doi: 10.1073/pnas.89.20.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery BL, Pattanaik B. Regulation during adaptation of cyanobacteria to changes in iron availability: A case study of responses to iron limitation in Fremyella diplosiphon. In: Blanc G, Moreau D, editors. Biometals: Molecular Structures, Binding Properties and Applications. Hauppauge, NY: Nova Science Publishers, Inc; 2010. pp. 215–226. [Google Scholar]
- 11.Mirus O, Strauss S, Nicolaisen K, von Haeseler A, Schleiff E. TonB-dependent transporters and their occurrence in cyanobacteria. BMC Biol. 2009;7:68. doi: 10.1186/1741-7007-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattanaik B, Sawyer BN, Montgomery BL. Acclimation responses to iron limitation are impacted by light quality in Fremyella diplosiphon. submitted. [DOI] [PubMed]
- 13.Neilands JB. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 14.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 15.Cobley JG, Zerweck E, Reyes R, Mody A, Seludo-Unson JR, Jaeger H, et al. Construction of shuttle plasmids which can be efficiently mobilized from Escherichia coli into the chromatically adapting cyanobacterium, Fremyella diplosiphon. Plasmid. 1993;30:90–105. doi: 10.1006/plas.1993.1037. [DOI] [PubMed] [Google Scholar]
- 16.Divakaruni AV, Loo RRO, Xie Y, Loo JA, Gober JW. The cell-shape protein MreC interacts with extra-cytoplasmic proteins including cell wall assembly complexes in Caulobacter crescentus. Proc Natl Acad Sci USA. 2005;102:18602–18607. doi: 10.1073/pnas.0507937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stowe-Evans EL, Ford J, Kehoe DM. Genomic DNA microarray analysis: identification of new genes regulated by light color in the cyanobacterium Fremyella diplosiphon. J Bacteriol. 2004;186:4338–4349. doi: 10.1128/JB.186.13.4338-4349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postle K, Kadner RJ. Touch and go: tying TonB to transport. Mol Microbiol. 2003;49:869–882. doi: 10.1046/j.1365-2958.2003.03629.x. [DOI] [PubMed] [Google Scholar]