Abstract
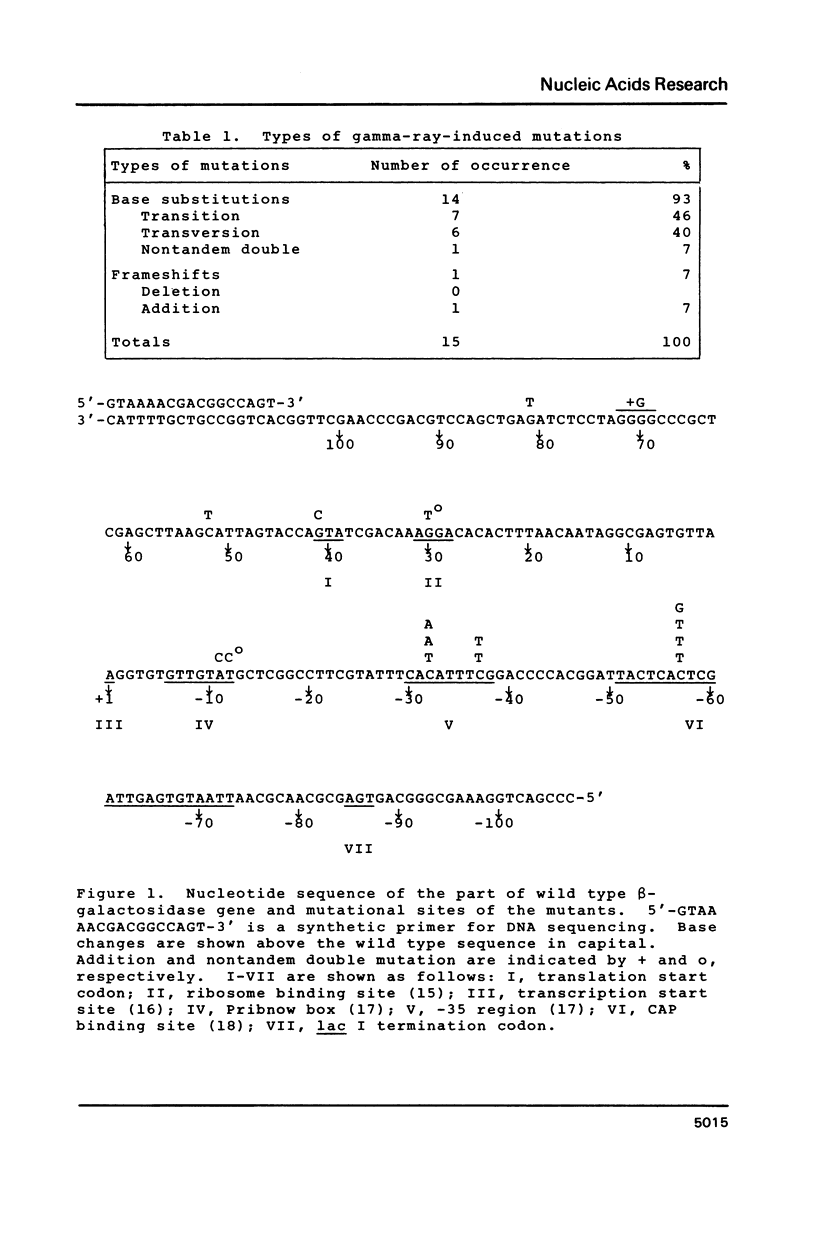
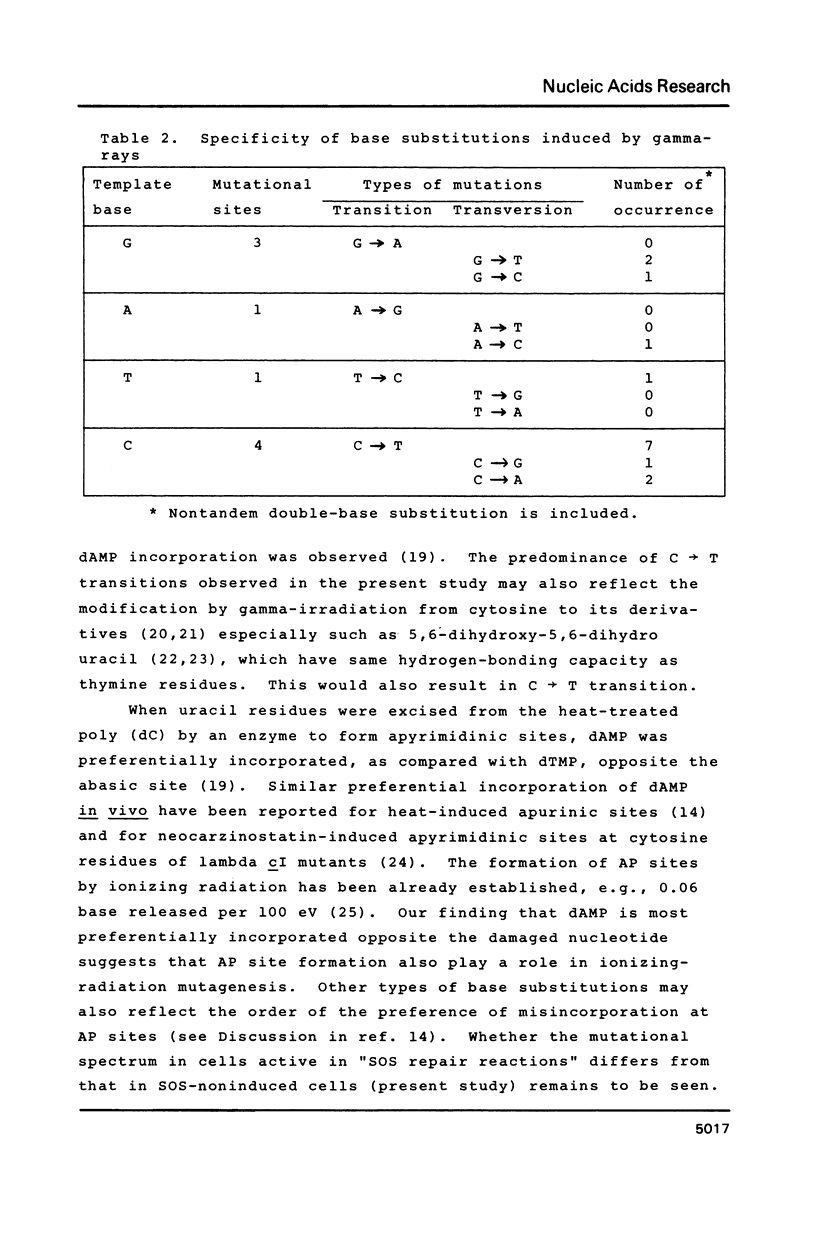
M13 mp10 single-stranded phage DNA was irradiated with 60 Co gamma-rays, and transfected into Escherichia coli. One hundred and sixteen mutant clones having lesions in the lac insert were selected, and mutational sites were examined by DNA sequence analysis. Fourteen out of the 15 nucleotide changes thus detected were base substitutions, and the rest was a base addition. Transitions and transversions were almost equal in number. Mutational events were observed at cytosine residues more frequently than at other residues, and the predominant base change was a C ---- T transition. Possible roles in gamma-ray-induced mutagenesis played by the misincorporation of dAMP owing to radiolytic derivatives of cytosine residues and/or formation of apurinic/apyrimidinic sites are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boiteux S., Laval J. Coding properties of poly(deoxycytidylic acid) templates containing uracil or apyrimidinic sites: in vitro modulation of mutagenesis by deoxyribonucleic acid repair enzymes. Biochemistry. 1982 Dec 21;21(26):6746–6751. doi: 10.1021/bi00269a020. [DOI] [PubMed] [Google Scholar]
- Brandenburger A., Godson G. N., Radman M., Glickman B. W., van Sluis C. A., Doubleday O. P. Radiation-induced base substitution mutagenesis in single-stranded DNA phage M13. Nature. 1981 Nov 12;294(5837):180–182. doi: 10.1038/294180a0. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Rietveld K., Aaron C. S. gamma-Ray induced mutational spectrum in the lacI gene of Escherichia coli: comparison of induced and spontaneous spectra at the molecular level. Mutat Res. 1980 Jan;69(1):1–12. doi: 10.1016/0027-5107(80)90171-2. [DOI] [PubMed] [Google Scholar]
- Kato T., Oda Y., Glickman B. W. Randomness of base substitution mutations induced in the lacI gene of Escherichia coli by ionizing radiation. Radiat Res. 1985 Feb;101(2):402–406. [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L., Saran B. R., Allen R., Jr Sequence analysis of ultraviolet-induced mutations in M13lacZ hybrid phage DNA. J Mol Biol. 1984 Dec 5;180(2):217–237. doi: 10.1016/s0022-2836(84)80001-7. [DOI] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L. Specificity of UV mutagenesis in the lac promoter of M13lac hybrid phage DNA. Nature. 1982 Jun 17;297(5867):596–598. doi: 10.1038/297596a0. [DOI] [PubMed] [Google Scholar]
- Maizels N. E. coli lactose operon ribosome binding site. Nature. 1974 Jun 14;249(458):647–649. doi: 10.1038/249647b0. [DOI] [PubMed] [Google Scholar]
- Majors J. Initiation of in vitro mRNA synthesis from the wild-type lac promoter. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4394–4398. doi: 10.1073/pnas.72.11.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutagenic specificity of ultraviolet light. J Mol Biol. 1985 Mar 5;182(1):45–65. doi: 10.1016/0022-2836(85)90026-9. [DOI] [PubMed] [Google Scholar]
- Piette J., Decuyper-Debergh D., Gamper H. Mutagenesis of the lac promoter region in M13 mp10 phage DNA by 4'-hydroxymethyl-4,5',8-trimethylpsoralen. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7355–7359. doi: 10.1073/pnas.82.21.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Van de Vorst A. Targeted mutagenesis of M13 DNA induced by photosensitized reaction. Photochem Photobiol. 1984 Nov;40(5):635–640. doi: 10.1111/j.1751-1097.1984.tb05352.x. [DOI] [PubMed] [Google Scholar]
- Polverelli M., Bonicel A., Teoule R. Gamma-irradiation of cytosine 14C-2 labelled DNA of Escherichia coli. Identification of the major radiolysis products resulting from rupture of the N-glycosidic bond. J Radiat Res. 1976 Sep;17(3):127–134. doi: 10.1269/jrr.17.127. [DOI] [PubMed] [Google Scholar]
- Polverelli M., Teoule R. Gamma irradiation of cytosine in an aerated aqueous solution. II. Kinetic study of the formation of the radiolysis products of cytosine resulting from the deamination pathway. Z Naturforsch C. 1974 Jan-Feb;29(1):16–18. [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Base substitution mutations induced in the cI gene of lambda phage by neocarzinostatin chromophore: correlation with depyrimidination hotspots at the sequence AGC. Nucleic Acids Res. 1986 Feb 11;14(3):1417–1426. doi: 10.1093/nar/14.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoule R., Cadet J. Radiation-induced degradation of the base component in DNA and related substances--final products. Mol Biol Biochem Biophys. 1978;27:171–203. doi: 10.1007/978-3-642-81196-8_9. [DOI] [PubMed] [Google Scholar]
- Ullrich M., Hagen U. Base liberation and concomitant reactions in irradiated DNA solutions. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(6):507–517. doi: 10.1080/09553007114550701. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Steitz T. A. Model of specific complex between catabolite gene activator protein and B-DNA suggested by electrostatic complementarity. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3973–3977. doi: 10.1073/pnas.81.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D., Skopek T. R., Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage by ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):273–291. doi: 10.1016/0022-2836(84)90121-9. [DOI] [PubMed] [Google Scholar]


