Abstract
When applied to the roots of Arabidopsis thaliana, the phytotoxin (±)-catechin triggers a wave of reactive oxygen species (ROS), leading to a cascade of genome-wide changes in gene expression and, ultimately, death of the root system. Biochemical links describing the root secreted phytotoxin, (±)-catechin, represent one of most well studied systems to describe biochemically based negative plant-plant interactions, but of late have also sparked controversies on phytotoxicity and pro-oxidant behavior of (±)-catechin. The studies originating from two labs1–3 maintained that (±)-catechin is not at all phytotoxic but has strong antioxidant activity. The step-wise experiments performed and the highly correlative results reported in the present study clearly indicate that (±)-catechin indeed is phytotoxic against A. thaliana and Festuca idahoensis. Our results show that catechin dissolved in both organic and aqueous phase inflicts phytotoxic activity against both A. thaliana and F. idahoensis. We show that the deviation in results highlighted by the two labs1–3 could be due to different media conditions and a group effect in catechin treated seedlings. We also determined the presence of catechin in the growth medium of C. stoebe to support the previous studies. One of the largest functional categories observed for catechin-responsive genes corresponded to gene families known to participate in cell death and oxidative stress. Our results showed that (±)-catechin treatment to A. thaliana plants resulted in activation of signature cell death genes such as accelerated cell death (acd2) and constitutively activated cell death 1 (cad1). Further, we confirmed our earlier observation of (±)-catechin induced ROS mediated phytotoxicity in A. thaliana. We also provide evidence that (±)-catechin induced ROS could be aggravated in the presence of divalent transition metals. These observations have significant impact on our understanding regarding catechin phytotoxicity and pro-oxidant activity. Our data also illustrates that precise conditions are needed to evaluate the effect of catechin phytotoxicity.
Key words: Arabidopsis thaliana, cell death genes, (±)-catechin, phytotoxin, reactive oxygen species (ROS)
Introduction
Plant survival in natural rhizosphere environments depends on the outcome of various positive and negative interactions with neighboring microbes, animals and other plants.4,5 Plant-plant communication in the soil has often been depicted as a negative form of chemical communication. For example, in an allelopathic interaction, one dominant species releases a toxin, which acts upon a susceptible plant, leading to a competitive advantage for the toxin producer.6 One example of such allelopathy is seen in the Eurasian invasive plant species Centaurea stoebe (spotted knapweed). This plant uses root exudation of the phytotoxin (±)-catechin as one of several suggested mechanisms to gain advantage over native North American plants.1,7–10 In susceptible species such as Arabidopsis thaliana, this compound has been found to induce a wave of reactive oxygen species (ROS) initiated at the root meristem, followed by genome-wide changes in gene expression and ultimately death of the root system.7
The novel weapons hypothesis conceives that an exotic plant species co-evolves its biochemical constituents to invade and succeed in a given environment. It also suggests that exotic species bring these biochemical changes to its invaded range. The exotic forb Centaurea stoebe represents one of the noxious weeds that is widely distributed across North America, and has also served as a great model system to exhibit the novel weapons hypothesis. It has been shown that C. stoebe exudes a racemic mixture of a polyphenol, (±)-catechin, from its roots.1,7,10–12 Although (±)-catechin secretions from C. stoebe represent one of the most widely studied systems to understand biochemical potential for plant invasion, it has also remained highly controversial on the ecological front. Studies conducted by two labs1–3 questioned the overall ecological significance, phytotoxicity, and mechanism of action reported in the literature. They showed that (±)-catechin secreted by C. stoebe is a weak phytotoxin and possesses strong antioxidant activity.1–3 However, other researchers1,7–10,13–15 showed that (±)-catechin in general induces potent phytotoxic activity against various target plant species.
Reactive oxygen species (ROS) are thought to be involved in a wide range of stress signaling and response systems. ROS, including hydrogen peroxide (H2O2), superoxide radical (O2.-) and hydroxyl radical (HO.) are continuously generated during all physiological processes. It is known that (±)-catechin triggers a ROS response as a signaling cascade to induce phytotoxicity in target species, such as Arabidopsis thaliana.5,7,10 Catechins are plant phenolic compounds with a variety of activities, whilst the general convention directs that catechins are potent anti-oxidant compounds; it is known in the literature that under specific conditions catechins show potent cytotoxic and pro-oxidant effects.17 These available data indicate that catechins have stronger ROS production abilities.17–19 In contrast, Duke et al.3 showed that (±)-catechin bears a strong antioxidant activity and concluded that (±)-catechin may not generate oxidative stress. Duke et al.3 also commented that the reason why they concluded that (±)-catechin fails to act as a pro-oxidant is because there exist 1,500 papers in literature (SciFinder search as October 2008) describing antioxidant activity of (±)-catechin.
Arabidopsis thaliana serves as a powerful tool to evaluate the mode of action and molecular targets of the subjected allelochemicals, as its genome has been fully sequenced. Our previous studies have shown that catechin treatment against A. thaliana resulted in upregulation of genes corresponding to cell death and oxidative stress signaling pathways.7 The availability and discovery of some of the lesion mimic mutants that result in constitutive mis-regulation of cell death are powerful tools to reveal the involvement and expression of cell death associated genes in A. thaliana.20 Some of these cell death associated genes have been identified such as accelerated cell death (acd2), lesion stimulating disease (lsd), constitutively activated cell death 1 (cad1) and copin (cin).21 In the present work, we have focused on the reexamination of the phytotoxic response of (±)-catechin at the both signaling and molecular level in A. thaliana. We show that (±)-catechin bears a strong phytotoxic property against the target species A. thaliana and F. idahoensis. Our results showed that higher concentrations of catechin caused a root burnout in F. idahoensis indicating the specific rhizotoxicity of catechin. Our results also revealed the presence of catechin in the in vitro grown cultures of C. stoebe, refuting that the catechin reported in previous studies was from microbial source. Our results revealed that (±)-catechin treatment to A. thaliana plants resulted in activation of signature cell death genes such as accelerated cell death (acd2) and constitutively activated cell death 1(cad1). We also show that (±)-catechin induces a potent pro-oxidant activity which could be triggered in presence of divalent transition metal ions.
Results
(±)-Catechin inhibits the growth of A. thaliana.
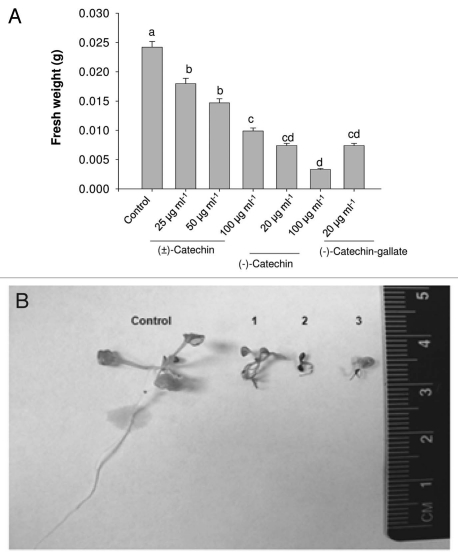
As previously shown, the studies originating from two labs maintained1–3 that (±)-catechin does not act as a potent phytotoxin against various plant species including A. thaliana.2,3 In contrast, other labs, in different continents, were able to show the phytotoxicity of (±)-catechin.7–10,13–15,22 In this report, we aimed to replicate the original observation shown in Bais et al.,7 that (±)-catechin indeed is phytotoxic against the model system A. thaliana. The results (Fig. 1) showed that (±)-catechin had a severe phytotoxic effect on A. thaliana. We picked different concentrations of (±)-catechin (25–100 µg ml−1) reported in the original paper7 to test against A. thaliana seedlings. Racemic catechin treatment resulted in a concentration dependent rhizotoxicity in A. thaliana plants (Fig. 1A and B). As reported in the original paper7 the (−)-catechin isomer also revealed a severe rhizotoxic response at 20 µg ml−1 level (Fig. 1A and B). Treatment of A. thaliana plants with a (−)-catechin gallate also resulted in inhibition of root growth leading to mortality (Fig. 1A and B). Root mortality patterns were also checked by time lapse movies, wherein seedlings treated with (±)-catechin (100 µg ml−1), (−)-catechin (10 µg ml−1) and (+)-catechin (200–250 µg ml−1) were transferred on day 3 to MS plates without any catechins. The Supplementary Videos 1–4 show that seedlings treated with (−)-catechin show strong mortality (as documented by no or reduced root growth) compared to (+)-catechin isomer treated roots. Seedlings treated with lower than MIC (−)-catechin (10 µg ml−1) concentration showed suspended root growth compared to the untreated seedlings (Sup. Vid. 2). The mortality of the plant was mostly due to the complete suppression of plant growth, because of the death of the primary root, similar to the data shown in the original reports.7 In contrast to this recent data and the data shown in the original reports of Bais et al.,7 Blair et al.1,2 and Duke et al.3 failed to replicate any phytotoxic activity of (±)-catechin. In these contrasting reports, authors specifically Duke et al.,3 claimed that they followed an exact procedure from Bais et al.,7 to treat A. thaliana with (±)-catechin. A careful reading of their results suggests that the procedure of Duke et al.,3 was different from that of Bais et al.,7 in the following aspects: they used acetone as a solvent control to dissolve catechin compared to methanol; half strength MS media was used in place of full strength MS media; and most importantly, they used three seedlings with (±)-catechin compared to single seedling per treatment in the original reports.7 These differences may appear too trivial to have caused the deviations in results, but our recent data indicate that media constituents and number of seedlings (group effect) can trigger different (±)-catechin toxicity response against A. thaliana.
Figure 1.
Phytotoxic response of catechin isomers on A. thaliana seedlings. (A) A. thaliana plants growing in liquid MS full strength media were supplemented with different concentrations of racemic catechin, (−)-catechin and (−)-catechin gallate. Post seven days of treatment plants were evaluated for catechin phytotoxicity by representation of total biomass (Fresh weight basis). All data presented are the mean values of five replicates, and the data have been presented as means with standard errors of the means. Means with different letters are significantly different at p ≤ 0.05, according to Duncan's multiple-range test. (B) Pictorial evidence to show the catechin's phytotoxicity on A. thaliana plants. The numbers in the (B) refers to racemic catechin [100 µg ml−1] (1), (−)-catechin [20 µg ml−1] (2) and (−)-catechin gallate [20 µg ml−1] (3) and untreated control (Control).
Liquid phytotoxicity assays.
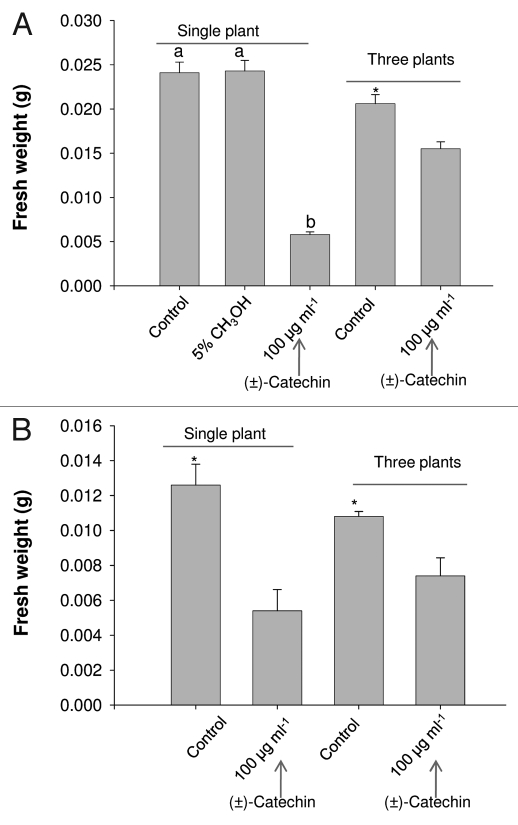
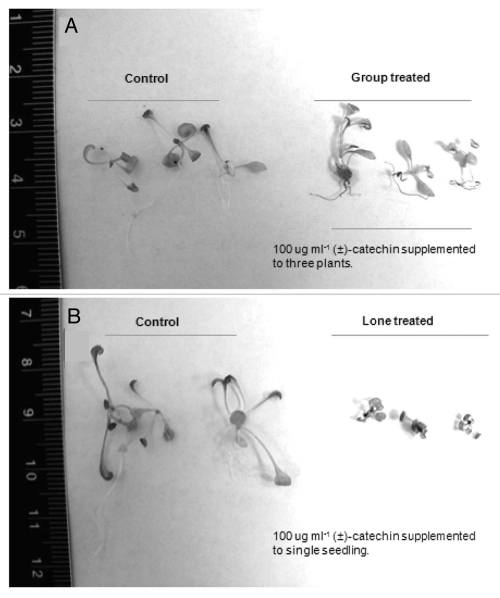
As indicated before, that there were differences in the protocols that were followed in Duke et al.3 leading to making direct comparisons with the original (±)-catechin phytotoxicity reports.7 As mentioned above that Duke et al.3 used three seedlings per treatment and half strength MS media in their analysis compared to one seedling per (±)-catechin treatment and full strength MS media in the original reports.7 In addition, these differences appear too trivial to have caused the change in results, but the recent data shows that (±)-catechin toxicity varies from one seedling to three seedling treatments. The results showed a severe decrease in overall fresh weight in the single seedling compared to three seedlings treated with (±)-catechin under full strength MS media conditions (Figs. 2A; 3A and B). Interestingly, the trend remained unchanged under the half strength MS media conditions, although the difference in single seedling versus three seedlings—(±)-catechin treatment was reduced (Figs. 2B; 3A and B). Recently Duke et al.3 showed that there is no enantiomeric dependent phytotoxic effect in catechin, meaning both + and − catechin isomers bear no or weak phytotoxic response against A. thaliana. We followed the similar recipe to grow plants as shown in the conflicting report,3 but instead of adding three plants per well we treated one plant per well with different concentrations of (+)-catechin (0–384 µg ml−1). Our data shows that the plants treated with (+)-catechin over the MIC levels (>200 µg ml−1) showed inhibited growth (Sup. Fig. 1A and B).
Figure 2.
Effect of media strength and plant group effect on phytotoxic response of (±)-catechin on A. thaliana seedlings. (A) A. thaliana plants growing as single seedling and three seedlings (group) per well in liquid MS full strength media were supplemented with racemic catechin. Post seven days of treatment plants were evaluated for catechin phytotoxicity by representation of total biomass (Fresh weight basis). All data presented are the mean values of five replicates, and the data have been presented as means with standard errors of the means. Means with different letters are significantly different at p ≤ 0.05, according to Duncan's multiple-range test. (B) A. thaliana plants growing as single seedling and three seedlings (group) per well in liquid MS half strength media were supplemented with racemic catechin. *in the panels indicates the p ≤ 0.05. Post seven days of treatment plants were evaluated for catechin phytotoxicity by representation of total biomass (Fresh weight basis).
Figure 3.
Effect of media strength and plant group effect on phytotoxic response of (±)-catechin on A. thaliana seedlings. (A) A. thaliana plants growing as three seedlings (group) per well in liquid MS half strength media were supplemented with racemic catechin. (B) A. thaliana plants growing as single seedling per well in liquid MS full strength media were supplemented with racemic catechin. Post seven days of treatment, plants were evaluated for catechin phytotoxicity by representation of total biomass (Fresh weight basis).
We also checked the phytotoxic efficacy of (±)-catechin dissolved in organic and aqueous phase against A. thaliana and F. idahoensis. We dissolved (±)-catechin in water to rule out the toxic effect of methanol on the target species. Our results clearly show that (±)-catechin dissolved in both aqueous and organic phase exhibited potent toxicity against A. thaliana, resulting in reduced root biomass (Sup. Fig. 2A and B). Interestingly, (±)-catechin dissolved in both water and organic phase severed the root system of F. idahoensis, clearly indicating the toxic activity of catechin against it (Sup. Fig. 3).
HPLC-MS analysis.
Roots of C. stoebe appear to secret catechin under in vitro and in soil conditions,1,2,7–10,12,23 but early reports from one of the studies have not been reproducible.24 In contrast to Stermitz et al.,24 and support of numerous other studies,1–3,8–10,12,23 our data shows that C. stoebe secretes catechin from the in vitro grown cultures. The presence of catechin in the growing in vitro C. stoebe cultures was positively determined based on LC retention and mass spectra fragmentation patterns. Catechin was identified based on following the fragmentation pattern in a negative ion mode (data not shown): 289 [M-H]−. We also quantified the overall catechin production in C. stoebe by performing a time course experiment. Samples were collected from in vitro growth medium at 5, 7 and 10 hours after exposure to light. Catechin concentration was found to be the highest in samples collected 7 hours after exposure (Sup. Fig. 4). This supports the studies of Tharayil and Triebwasser, et al.,12 which report that catechin exudation in growth medium follows a diurnal rhythm.
Effect of (±)-catechin on cell death associated genes in A. thaliana.
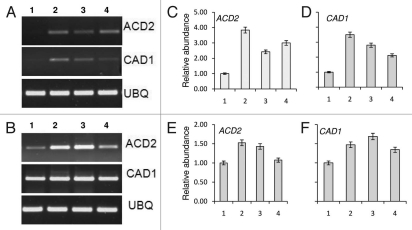
We have shown previously that the largest functional categories observed for catechin-responsive genes corresponded to gene families known to participate in cell death and oxidative stress.7 The transcriptional changes associated with cell death associated genes post (±)-catechin treatment were inferred previously on the basis of commercially prepared oligonucleotide arrays (Affymetrix, Inc., Santa Clara, CA) representing ∼29 K predicted A. thaliana genes.7 Next, we checked for two-cell death associated marker gene responses post (±)-catechin treatment. Our results showed that (±)-catechin treatment on A. thaliana plants resulted in activation of signature cell death genes such as acd2 and cad1 (Fig. 4A–F). The transcriptional upregulation of cell death associated genes post (±)-catechin treatment confirms the phytotoxic response of (±)-catechin as an allelochemical.
Figure 4.
Expression of cell death associated genes in A. thaliana seedlings post (±)-catechin treatment. The seedlings were treated with different concentrations of (±)-catechin (50–200 µg ml−1) and the total RNA was isolated from the seedlings on day 3 and 5 post (±)-catechin treatment. (A) Expression analyses of ACD2, CAD1 genes, 3 days after (±)-catechin treatments. (B) Expression analyses of ACD2, CAD1 genes, 5 days after (±)-catechin treatments. 1 in both panels refers to control (without catechin) treatments. 2–4 in both panels refers to (±)-catechin treatments in an increasing order of 50–200 µg ml−1. RT-PCR was performed with 500 ng of total RNA. The PCR products were analyzed by agarose gel electrophoresis. The band intensity of each gene was adjusted with the band intensity of housekeeping gene ubiquitin. (C–F) show the relative expression levels post normalization. Data shown are mean ± SD of three independent experiments expressed as the fold increase in ACD2 and CAD1 genes of different concentrations of (±)-catechin (50–200 µg ml−1) added to Arabidopsis seedlings normalized with the value for respective control plants.
(±)-Catechin generates elevated levels of ROS in A. thaliana roots.
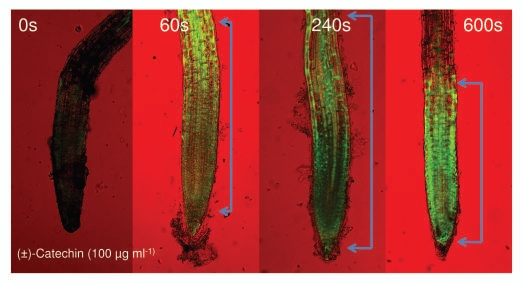
Since (±)-catechin is a phenolic compound and such compounds are known to generate reactive oxygen species (ROS), in our original reports,7 we showed that (±)-catechin generates ROS in A. thaliana roots. Recently, Duke et al.3 challenged our original report of pro-oxidant activity of (±)-catechin. In their argument, Duke et al.3 showed anti-oxidant activity of catechin; furthermore, they referred to the conventional literature showing the antioxidant activity of catechins. Nowhere in their recent report did Duke et al.3 show the pro-oxidant activity of (±)-catechin. To test the argument of Duke et al.,3 we imaged the roots of the wild-type A. thaliana (Col-0) plants by using a fluorescent H2DCFDA staining method under two different treatments: a control and plus (±)-catechin (100 µg ml−1). The time course (0–600 seconds) imaging data exhibited the production of elevated levels of ROS on the roots treated with (±)-catechin (100 µg ml−1) (Fig. 5). This data along with the other original reports confirms that (±)-catechin generates ROS on A. thaliana roots.7,10,16
Figure 5.
Induction of reactive oxygen species (ROS) by racemic catechin (100 µg ml−1) on A. thaliana roots. ROS generation was analyzed for over a 10-min time period. The images are confocal scanning laser micrographs and each image is representative of the roots of at least six independent plants analyzed and imaged. (Scale bars: 100 µM for ROS images). Brackets in the panel indicate ROS generation in the CEZ region of roots. See Materials and Methods for details on microscopy and staining methodology.
Is (±)-catechin a proxidant or an antioxidant?
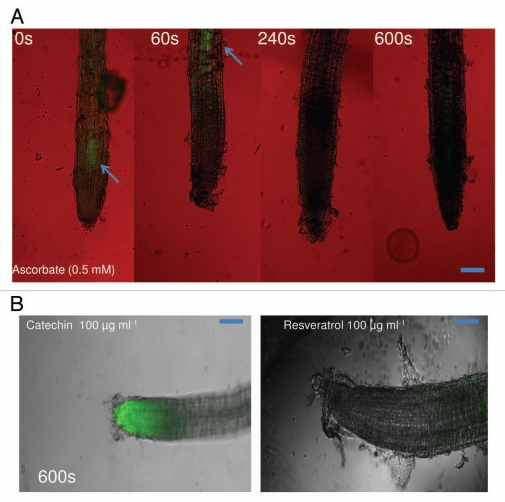
Duke et al.3 argued that (±)-catechin is a strong antioxidant and will always quench singlet oxygen species rather than generating one. To this end, they showed strong anti-oxidant activity in the catechin isomers. Furthermore, they argued that there exist no reports apart from ours,7,10,16 that showed pro-oxidant activity of catechins. In the above-mentioned results, we confirmed our original observation,7 that (±)-catechin induces ROS response in A. thaliana roots. We also wanted to pursue the Duke et al.3 arguments; can all anti-oxidants bear pro-oxidant activity? To this end, we tested known anti-oxidant compounds such as ascorbic acid (0.5 mM) and resveratrol (100 µg ml−1) for pro-oxidant activity on A. thaliana roots under the similar time course (0–600 s) as shown for (±)-catechin treatments (Fig. 5). Interestingly, both ascorbic acid and resveratrol treatment negated pro-oxidant activity on A. thaliana roots (Fig. 6), confirming the notion that not every antioxidant compound elevates singlet oxygen species.
Figure 6.
Induction of root surface reactive oxygen species (ROS) by ascorbate (0.5 mM) and resveratrol at (100 µg ml−1) on A. thaliana seedlings. ROS generation was analyzed for over a 10-min time period. The representative scans for (B) for resveratrol are shown for 600 s. The green fluorescence (arrows in A) in the panel indicates generation of ROS. The images are confocal scanning laser micrographs and each image is representative of the roots of at least six independent plants analyzed and imaged. (Scale bars: 100 µM for ROS images). See Materials and Methods for details on microscopy and staining methodology.
(±)-Catechin mediated ROS response is triggered by divalent transition ions.
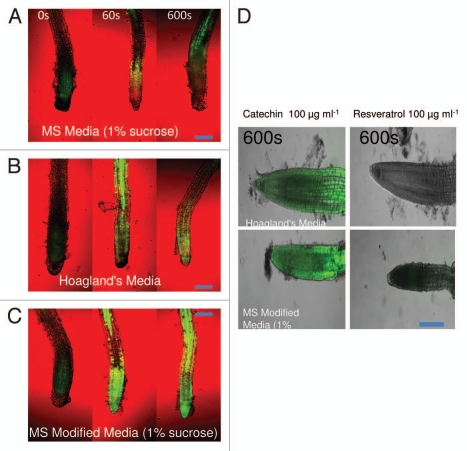
As originally reported,7 we confirmed the ROS generation response of (±)-catechin on A. thaliana roots. It is also known that some of the known antioxidants such as catecholamines (CA) and related compounds bear both antioxidant and pro-oxidant activity.25 It is also verified that this shift to release singlet oxygen species instead of quenching is dependent on exposure to divalent metal ions.17–19,25 To evaluate if (±)-catechin followed a similar trend, we modified the MS media by doubling the concentrations of two trace divalent transition metal ions (Cu2+ and Mn2+). We also compared the modified MS media with a known plant growth media Hoagland's (a carbon-free and low-nitrogen medium, pH 5.8), which constitutes elevated levels of both Cu2+ and Mn2+ ions. A. thaliana seedlings grown in modified MS media (MMS) and Hoagland's treated with (±)-catechin revealed elevated levels of ROS compared to full strength MS media grown and (±)-catechin supplemented seedlings (Fig. 7A–C). We checked if other anti-oxidants such as resveratrol follow a similar pattern of shifting to a pro-oxidant in the presence of divalent transition metal ions. To this end, A. thaliana seedlings grown in MMS and Hoagland's media were supplemented with resveratrol (100 µg ml−1). Surprisingly, A. thaliana plants treated with resveratrol and grown in MMS and Hoagland's did not show any ROS response post 600 s of treatment (Fig. 7D). This data confirms earlier observations that divalent transition metal ions can elicit ROS response of catechin and also suggests that not every antioxidant could turn pro-oxidant in the presence of divalent transition metal ions.18,19,25
Figure 7.
Induction of root surface reactive oxygen species (ROS) by racemic catechin (100 µg ml−1) on A. thaliana grown in different media compositions. (A–D) Represents ROS generation in A. thaliana roots grown in different media and subsequently treated with racemic catechin (100 µg ml−1). (D) shows comparative ROS imaging for plants grown in different media and treated with catechin and resveratrol, the scans for (D) were taken at 600 s post treatment. ROS was analyzed for over a 10-min time period. The images are confocal scanning laser micrographs and each image is representative of the roots of at least six independent plants analyzed and imaged. (Scale bars: 100 µM for ROS images). See Materials and Methods for details on microscopy and staining methodology.
Effect of divalent transition metal ions on (±)-catechin phytotoxicity response on A. thaliana plants.
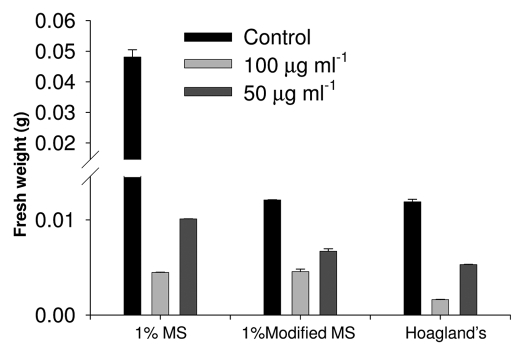
In the above section, we showed that divalent transition metal ions influence the ROS response of (±)-catechin on A. thaliana roots. Previously, we have shown that phytotoxicity inflicted by (±)-catechin on A. thaliana roots is mediated by a ROS and Ca2+ response.7 In here, we evaluated if the elevated ROS response of (±)-catechin in the presence of divalent transition metal ions relays any phytotoxic effect on A. thaliana plants. Briefly, A. thaliana seedlings grown in MMS, MS and Hoagland's media supplemented with (±)-catechin (50–100 µg ml−1) were analyzed for total biomass post-7-days of incubation. The results shown in Figure 8 revealed that MMS and Hoagland's media supplemented with (±)-catechin resulted in severe decline of biomass in A. thaliana plants. Specifically, plants grown in MMS/Hoagland's and treated with lower than MIC concentrations of (±)-catechin (50 µg ml−1), showed decreased biomass compared to MS media-catechin supplemented seedlings (Fig. 8).
Figure 8.
Phytotoxic response of racemic catechin (50–100 µg ml−1) on A. thaliana seedlings grown under different media compositions. Post seven days of treatment, plants were evaluated for catechin phytotoxicity by representation of total biomass (Fresh weight basis). Data is mean ± S.D., n = 6.
Discussion
Numerous studies have reported (±)-catechin phytotoxcity.7,9,10,13,14,22,23,26–30 In contrast, Blair et al.1,2 and recently Duke et al.3 reported that (±)-catechin does not have any phytotoxic activity against A. thaliana and other tested plants. Direct comparisons were made in these contrasting studies,1–3 where in, it was reported that (±)-catechin bears no phytotoxicity but has strong anti-oxidant activity thereby refuting earlier claims of its phytotoxicity and pro-oxidant activity.7,9,10,13,14,22,26–30 Based on the current study, we categorically pin point the specific differences in these conflicting studies to the ones conducted in the original reports.7 In particular, we report that (±)-catechin and its bound forms (catechin gallate) are indeed phytotoxic against A. thaliana, the induced phytotoxic response is triggered by a ROS signal and expression of cell death associated genes. We also show that the (±)-catechin mediated ROS response is triggered in the presence of divalent transition metal ions.
We showed that (±)-catechin is phytotoxic against A. thaliana plants and native F. idahoensis and the (−)-catechin isomer bears a potent phytotoxic activity. The lone (−)-catechin isomer treated A. thaliana plants showed decreased biomass compared to the racemic catechin and (+)-isomer treated plants. We also conducted experiments using catechin stocks made in water to rule out the possibility that methanol could be responsible for the phytotoxic effects observed. (±) Catechin with or without methanol was found to have phytotoxic effects in A. thaliana. On the other hand, Duke et al.3 showed no phytotoxic activity of either the racemic or the (−)/(+)-isomer on A. thaliana plants. Our results showed that (±)-catechin dissolved in both organic and aqueous phase imparts similar toxicity against target plant species. Duke et al.3 claimed that they followed the exact protocol shown in Bais et al.7 papers to obtain their results. A careful reading of Duke et al.3 revealed that the exact protocol taken from the original reports7 was not followed. The difference in phytotoxic assays shown in the original reports7 and that of Duke et al.3 remain in the number of seedlings per treatment. In the original reports,7 (±)-catechin was administered to single A. thaliana plants grown in full strength MS media. Duke et al.3 treated three seedlings per well, grown in half strength MS media with catechin and reported the average biomass post incubation. Though, these differences appear very trivial to cause the differences in between reports, the recent data shows that the chemical administration to single seedling inflicts far worse damage to plants compared to three seedling treatments. The effect was much more pronounced when comparisons were made in between full and half strength MS media, with half strength MS media grown seedlings (3 plants per well) showing less damage inflicted by catechin compared to full strength MS media grown seedlings (1 plants per well). The results shown in these reports could be discussed on the basis of dilution effect of a known phytotoxin. It is known that important factors such as growth kinetics, target tissue, organ and cellular stages must be considered in assessing phytotoxicity.31 It is argued that plants respond to abiotic stresses differently when they are in monocultures verses isolated or mixed stands.29 It is shown that plants in groups can tackle both abiotic and biotic stress much more efficiently by triggering their innate defense and signaling responses.32–34 To our knowledge, except for studies on donor allelopathic plants and their interactions with the recipients under biotic and abiotic stress, not a single study shows how a stress experiencing recipient plant community responds to the allelopathic species.34,35 Of this finding to the group effect suggests that plants in groups may perceive the phytotoxin differently compared to isolated single plants. This explains the seemingly contradictory findings of Duke et al.,3 who suggested that there was no evidence for catechin phytotoxicity in their study since the group treated plants showed no difference in biomass post catechin treatments.
Recent reports indicate the irreproducibility of catechin exudation and phytotoxicity.24 In contrast, several other lines of work,9,10,22 some originating from the same research group showed catechin secretion to an order of mg g−1 samples.8–10 A recent paper from Tharayil and Triebwasser,12 showed catechin secretion from C. stoebe hydroponic cultures, wherein authors showed higher catechin recovery compared to the levels reported in Blair et al.1 Interestingly, Tharayil and Triebwasser,12 reported that catechin secretion in C. stoebe is diurnally regulated; wherein catechin degrades to catechol. We also detected catechin in the in vitro grown cultures of C. stoebe supporting the original and recent reports of catechin secretion.1,2,7–10,12,23,26,27 It is valid to argue that the irreproducibility in catechin secretion observed by Stermitz et al.24 could be related to factors such as instability of catechin in medium and sampling time. Since Stermitz et al.24 did not dwell on the methodological difficulties that were encountered negating the detection of catechin in their repetition trials, it is hard to explain how the same group8–10 reported catechin in the secretions of C. steobe to a level of mg g−1 sample.
In our original reports we showed that the phytotoxicity of catechin treated roots is triggered by elevated levels of ROS.7,16 This conclusion was further supported by the observation that the catechin treatment results in the production of elevated levels of ROS. Contrasting to our published reports, Duke et al.3 recently showed a strong anti-oxidant activity with catechins. Although, the data shown by Duke et al.3 is not very surprising, as catechins are reportedly known anti-oxidants (SciFinder search as October 2008), the conclusions made by them that because catechins are anti-oxidants they could not bear pro-oxidant activity is premature. Our recent results and those of others have shown that catechins have strong ROS production abilities.17–19,25 It is known that catechins and other catecholamines (CA) are antioxidants against ROO. and ·OH, but when transition divalent metal ions are present, they can turn into prooxidants.25 Catechins and other CAs have been recognized to act as electron donors to quench singlet oxygen species and subsequently interrupt Ferroreducatse (FR) chain reactions.25 Interestingly, Cao et al.35 have also demonstrated that anti- and pro-oxidant capacity of catechins and CA was structurally correlated to the number of hydroxyl groups. Furthermore, Sofic et al.25 concluded that anti to proxidant shifts of CAs might be attributed to its hydroxyl and amine groups. Based on our data, it can be further concluded that divalent transition metals such Cu and Mn could induce the pro-oxidant properties of catechins. Although, (+)-catechin has a redox potential low enough to act as an electron donor,36 other reports and that of Duke et al.3 agree that the ROS generating ability of the (+)-isomer is markedly lower than of other catechins, such as (−)-epigallocatechins and (−)-epigallocatechin gallate.17 Interestingly, Duke et al.3 also showed that the (−)-catechin isomer bears a strong antioxidant property, but the assumption that because of its antioxidant nature, catechins negate pro-oxidant property was not supported by the data. In literature, it is known that some catechins under certain conditions and especially in presence of transition metals, cause DNA disruption and lipid peroxidation, a prime signature of ROS mediated damage.17–19 The in vitro culturing in MS and Hoagland's media to grow most of the plants under sterile conditions constitutes trace levels of both Cu and Mn17–19 and there is a strong possibility that the ROS generating abilities of catechins are triggered due to the media constituents. Our modified media experiments support the assumptions that media constituents and especially transition elements could play a major role in aggravating phytotoxicity and ROS response. An important question here is; how do ROS modify the downstream targets leading to phytotoxicity and do transition elements contribute to that effect? Incidentally, it is known that catechin could bind to transition elements to enhance its phytotoxicity and exhibit a conditional allelopathic response.37 The response reported in the present study may be occurring through catechin-metal complex mediated ROS leading to modification of the downstream signaling proteins leading to altered gene expression and cell death.
Overall, the step-wise experiments performed and the highly correlative results reported in the present study clearly indicate that (±)-catechin is indeed phytotoxic against both A. thaliana and F. idahoensis. We also show that the deviation in the results highlighted in earlier reports1–3 could be due to different media conditions and a group effect in catechin treated seedlings. The expression of cell death associated genes post catechin treatments validates the phytotoxic response of the allelochemical at the molecular level. Further, we confirmed our earlier observation of (±)-catechin induced ROS mediated phytotoxicity in A. thaliana. We also provide the evidence that (±)-catechin induced ROS could be aggravated in the presence of divalent transition metals. These observations have significant impact on our understanding regarding catechin phytotoxicity and pro-oxidant activity. While our study backs the original reports of phytotoxicity and pro-oxidant nature of catechin, but it raises an important question regarding the percipience of the allelochemical in the recipient plant community. Our data suggests that the recipient plant communities may perceive the allelochemical differently when they are in monocultures versus isolated stands. Our data also directs that precise conditions are needed to evaluate the overall effect of catechin secretion and phytotoxicity.
Materials and Methods
Plant material and chemicals.
Arabidopsis thaliana wild-type cultivar Columbia 0 (Col-0) seeds were procured from Lehle Seeds (Round Rock, TX, USA). Festuca idahoensis seeds were obtained from University of Kentucky, Lexington, KY. The aseptic cultures were used for the in vitro studies. Catechins (+, −, − gallate and ±) were all obtained from Sigma-Aldrich, USA.
Culture conditions.
Seeds were washed in double-distilled water thrice and surface-sterilized using 50% commercial bleach (sodium hypochlorite) for 3–5 min for A. thaliana and 5–10 min for all the other seeds followed by 3–4 washes in sterile distilled water. The seeds were cultured on Murashige and Skoog's (MS)38 solid medium with 3% sucrose and allowed to germinate for 5 days until the roots and shoots emerged. The seedlings were then incubated at 25 ± 2°C under 16 h light and 8 h dark. The plates were illuminated with cool fluorescent light with an intensity of 24 µmol m−2 s−1.
In vitro phytotoxicity assays.
Five-day-old A. thaliana Col-0 seedlings that had been germinated on MS solid medium with 3% sucrose were transferred to 24 well plates containing 1% (sucrose) MS liquid medium. The treatments included various concentrations of different catechin isomers and racemic catechin. The control cultures did not receive any catechin. Another control was set up with methanol (highest concentration of 5% v/v) to account for the dilution effect, if any. The cultures were incubated on a rotary shaker set at 90 rpm maintained at 25 ± 2°C. The plants were illuminated with cool white fluorescent light for a 16 h light and 8 h dark photoperiod. All of the treatments had a minimum of 5 replicates and the experiment was repeated at two independent times. The experiment was terminated at the end of the seventh day and fresh weight in grams of the plants was recorded. Some of the plants treated with racemic catechin, (+) and (−)-isomers were removed from the liquid media post 3 days of treatment and were placed on solid MS (3% sucrose) media without catechin to record the root mortality patterns. A HR-Proscope (www.lsw.com/stores/luxusstore) with a M50 lens was used to record movies. Seedlings in the MS media (without catechins) were recorded for 72 h post-transfer from catechin plates.
Comparative liquid phytotoxicity assays.
To compare the liquid phytotoxicity results shown in Duke et al.,3 we followed a similar protocol as shown in the cited paper. Five-day-old A. thaliana seedlings were transferred to both full and half strength MS media (1% sucrose), supplemented with (±)-catechin (100 µg ml−1). As per the methodology of Duke et al.,3 we also used one seedling versus three seedlings per well assay. Briefly, one or three five day old Arabidopsis seedlings were transferred per well in both full and half strength MS media (1% sucrose), supplemented with (±)-catechin (100 µg ml−1). Post-seven days of catechin treatments, plants were harvested and weighed to evaluate catechin toxicity. For the three seedlings per treatment, the fresh weights of three seedlings were averaged to evaluate the catechin toxicity response.
Media modifications.
To monitor the effect of divalent transition metal ions on catechin toxicity, aqueous stock solutions of MnCl2.4H2O and CuSO4.5H2O were prepared. Both of the stock solutions were added in the MS liquid media to reach the final concentration of 34 mg l−1 and 0.05 mg l−1 for MnCl2 and CuSO4 respectively. We also compared the modified MS media (hereafter MMS) supplemented with 1% sucrose to the routinely used Hoagland's media (a carbon-free and low-nitrogen medium, pH 5.8). Hoagland's media constitutes high levels of both MnCl2.4H2O (1.8 g/l) and CuSO4.5H2O (0.1 g l−1). All of the three media (MS, MMS and Hoagland's) were adjusted to pH 5.8 before sterilization. Five-day-old Arabidopsis plants (one seedling per well) were transferred to 24 well plates with 1 ml of media (MS, MMS and Hoagland's). Racemic catechin (100 µg ml−1) was supplemented in the media with five day old Arabidopsis seedlings. The experiment was terminated at the end of the seventh day and the observations were recorded as fresh weight in grams.
Effect of different concentrations of catechin on five-day-old Arabidopsis and Festuca seedlings.
Five-day-old seedlings of Arabidopsis plants were transferred to 24-well plates with 1 ml of 1X MS media. Racemic catechin (100–250 µg ml−1) was supplemented in the plants growing in the liquid cultures. Another set of 5-day-old Arabidopsis was repeated with the same concentrations of catechin, however for this batch, the catechin stocks were made with double-distilled water to rule out the toxic effects of low concentrations of methanol used in the previous experiments. As before, the experiment was terminated after 7 days and plant weight was recorded as fresh weight in grams. The experiments were repeated twice with 5 replicates per treatment with Festuca.
Determination of catechin in C. stoebe growth medium.
Centaurea stoebe seeds were surface sterilized and plated on 1% MS using the protocol described by Bais et al.7 Ten-day-old seedlings were transferred to 6-well tissue culture plates containing 1% MS liquid medium containing MES salts. The plates were maintained on a 25°C shaker under 16 h lights for 5 days. The growth media were concentrated using lyophillization and extracted in methanol. The samples were filter sterilized before injecting 20 µl on a reverse phase 5-µm, C18 column (4.6 × 150 mm; Dionex Corp.). The chromatographic system (Dionex) consisted of P680 pumps (Dionex) connected to an ASI-100 Automated Sample Injector (Dionex). The absorbance was measured by a PDA-100 Photodiode variable array detector (Dionex). The mobile phase consisted of 100% acetonitrile (Fisher Co.). A sample or a standard was separated by an Agilent 1100 series high performance liquid chromatography (HPLC) system composed of a pump, an autosampler and a diode array detector (Santa Clara, CA). First, it was introduced into an analytical column (Luna 3 µ, C18, 100 × 2.00 mm, Phenomenex, Torrance, CA) and analytes were eluted from the column with gradient of two mobile phases [water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B)] starting at A:B of 100:0 and ramping to 0:100 over 34 minutes at a flow rate of 200 µL/min. Then, the system was returned to 100:0 and re-equilibrated till 50 minutes. For the equal split of the eluent into the diode array detector and a mass spectrometer (Applied Biosystems QTRAP mass spectrometer, Foster City, CA), a micro-tee connector (Upchurch Scientific, Oak Harbor, WA) was used. The diode array detector was set to the observation wavelength at 275 nm (bandwidth of 10 nm) and the reference wavelength at 500 nm (bandwidth of 100 nm). For mass spectrometry, the HPLC system and the mass spectrometer was interfaced by Applied Biosystems Turbospray source (curtain gas of 20.0 psi, high collision gas, ion spray voltage of −4,200 V, temperature of 420°C, ion source gas1 of 20 psi, and ion source gas2 of 20 psi) and analyte ions introduced into the mass spectrometer were analyzed by using full mass scan (mass range between 75 and 325 m/z, scan rate of 1,000 amu/s, and dynamic fill time) in the negative ion mode. At the same time, tandem mass spectrometry (MS/MS scan) of ions observed at 109 m/z and 289 m/z was also carried out (the collision energy of −30.0 V and the mass range between 50 and 325 m/z).
Imaging for reactive oxygen species (ROS).
Reactive oxygen species (ROS) imaging was done following a slightly modified protocol of Foreman et al.39 Briefly, 4–5-day old A. thaliana seedlings germinated on MS solid medium with 3% sucrose were transferred to different media (MS, MMS and Hoagland's) and pre-treated with racemic catechin (100 µg ml−1), 500 µM ascorbic acid (AsA) and resveratrol; controls did not receive chemicals. After a time course (10 s to 600 s), a fluorescent ROS stain, 2.5 µM 2′,7′-dichlorodihydrofluorescein diacetate (HB2-DCFDA), dissolved in DMSO (less than 0.001% final concentration) procured from Invitrogen (USA) was added to the cultures and incubated for 30 min at 4°C. The cultures were then washed with 0.1 mM KCl, 0.1 mM CaCl2 (pH 6.0). The washed roots were incubated in sterilized water for another 30 minutes at 23 ± 2°C. The roots were imaged by using excitation and emission wavelengths of 488 nm and 522 nm respectively. The images were of 512 × 512 pixels with 10 scans of 1 s each in a confocal laser scanning microscope (CLSM) detailed in the following section. Images were captured with either a 10X or 40X (with water correction) objective on a Zeiss LSM 510 NLO attached to an Axiovert 200M with an automated stage microscope equipped with Zeiss LSM 510 software.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR).
Five-day-old A. thaliana seedlings were transferred to MS liquid media (1% sucrose), subsequently, (±)-catechin (50–200 µg ml−1) was added to the liquid media. Total RNA was isolated from the seedlings on day 3 and 5 post (±)-catechin treatment. RNA was extracted using PureLink RNA isolation buffer according to the instruction manual (Invitrogen, CA). Possible contaminant genomic DNA in RNA extracts was removed using turbo DNA-free™ kit (Ambion). The concentration of total RNA was determined spectrophotometrically at 260 nm. The integrity of RNA was checked by electrophoresis in formaldehyde denaturing gels stained with ethidium bromide. The gene-specific primers for the genes ACD2, CAD1 and UBQ, were designed using Primer3 software (Table 1) and synthesized (Invitrogen). First-strand complementary DNAs were synthesized from 500 ng of total RNA in 20-µl final volume, using M-MuLV reverse transcriptase and oligo-dT (18 mer) primer (Fermentas GmbH, Germany). PCR amplifications were performed using PCR mixture (15 µl) that contained 1 µl of RT reaction product as template, 1× PCR buffer, 200-µM dNTPs (Fermentas GmbH), 1 U of Taq DNA polymerase (Promega), and 0.1 µM of each primer depending on the gene. PCR was performed at initial denaturation at 94°C for 4 min, 22 or 26 cycles (30 sec at 94°C; 30 sec at 60°C; 30 sec at 94°C), and final elongation (8 min at 72°C) using a thermal cycler (Bio-rad). The PCR products obtained were separated on 1.4% agarose gel, stained with ethidium bromide (0.001%), and documented in a gel documentation system.
Table 1.
Specific primers, annealing temperatures and total numbers of amplification cycles used for RT-PCR
| Primer | Primer sequence (5′-3′) | Annealing temperature | Total number of amplification cycle | Amplicon size (bp) |
| ACD2-forward | AGTCCATGGAAGACCACGAC | 60°C | 23 | 450 |
| ACD2-reverse | AGCACAAGCGACTTGGAACT | |||
| CAD1-forward | TCAACGCCTAGCTTTGCTCCAG | 60°C | 26 | 480 |
| CAD1-reverse | CTTGAGCAAAGCCATGCTCGTTGG | |||
| UBQ-forward | TCGTAAGTACAATCAGGATAAGATG | 55°C | 22 | 210 |
| UBQ-reverse | CACTGAAACAAGAAAAACAAACCCT |
Data analysis.
All data presented are the mean values of five replicates, and the data have been presented as means with standard errors of the means. The data were analyzed by one-way analysis of variance (ANOVA) using Microsoft Excel 2007® (Microsoft Corporation, Washington), and post-hoc mean separations were performed by Duncan's Multiple Range Test at p ≤ 0.05,40 by using the software SPSS version 12.0 and also by using the Tukey-Kramer test at p ≤ 0.05. Whenever two means were compared, student's t-test was used.
Acknowledgements
Authors acknowledge the help of Bais lab members for germinating and maintaining Arabidopsis thaliana wild-type Col-0 and Festuca idahoensis seeds for the assay described in the manuscript. Authors thank the two anonymous reviewers for helpful reviews. Authors also acknowledge the help of Dr. Yong Seok Choi for the LC-MS characterization.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11823
Supplementary Material
References
- 1.Blair AC, Hanson BD, Brunk GR, et al. New techniques and findings in the study of a candidate allelochemical implicated in invasion success. Ecol Lett. 2005;8:1039–1047. [Google Scholar]
- 2.Blair AC, Nissen SJ, Brunk GR, et al. A lack of evidence for an ecological role of the putative allelochemical (±)-catechin in spotted knapweed invasion success. J Chem Ecol. 2006;32:2327–2331. doi: 10.1007/s10886-006-9168-y. [DOI] [PubMed] [Google Scholar]
- 3.Duke SO, Blair AC, Dayan FE, et al. Is (−)-Catechin a “Novel Weapon” of Spotted Knapweed (Centaurea stoebe)? J Chem Ecol. 2009;35:141–153. doi: 10.1007/s10886-008-9587-z. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AM, Bauer WD, Bird DM, et al. Molecular signals and receptors—controlling rhizosphere interactions between plants and other organisms. Ecology. 2003;84:858–868. [Google Scholar]
- 5.Bais HP, Park SW, Weir TF, et al. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004;9:26–32. doi: 10.1016/j.tplants.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Rice EL. Allelopathy. 2nd ed. Orlando: Academic Press; 1984. [Google Scholar]
- 7.Bais HP, Vepachedu R, Gilroy S, et al. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science. 2003;301:1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- 8.Perry LG, Johnson C, Alford ER, et al. Screening of grassland plants for restoration after spotted knapweed invasion. Restor Ecol. 2005;13:725–735. [Google Scholar]
- 9.Perry LG, Thelen GC, Ridenour WM, et al. Soil concentrations of the allelochemical (±)-catechin. J Chem Ecol. 2007;33:2171–2345. doi: 10.1007/s10886-007-9383-1. [DOI] [PubMed] [Google Scholar]
- 10.Weir TL, Bais HP, Stull VJ, et al. Oxalate contributes to the resistance of Gaillardia grandiflora and Lupinus sericeus to a phytotoxin produced by Centaurea stoebe. Planta. 2006;223:785–795. doi: 10.1007/s00425-005-0192-x. [DOI] [PubMed] [Google Scholar]
- 11.Ridenour WM, Vivanco JM, Feng YL, et al. No evidence for trade-offs: Centaurea plants from America are better competitors and defenders. Ecol Mono. 2008;78:369–386. [Google Scholar]
- 12.Tharayil N, Triebwasser DJ. Elucidation of a diurnal pattern of catechin exudation by Centaurea stoebe. J Chem Ecol. 2010 doi: 10.1007/s10886-100-9749-7. [DOI] [PubMed] [Google Scholar]
- 13.He WM, Feng Y, Ridenour WM, et al. Novel weapons and invasion: biogeographic differences in the competitive effects of Centaurea maculosa and its root exudate (±)-catechin. Oecologia. 2009;159:803–815. doi: 10.1007/s00442-008-1234-4. [DOI] [PubMed] [Google Scholar]
- 14.Inderjit, Pollock JL, Callaway RM, et al. Phytotoxic effects of (±)-catechin in vitro, in soil, and in the field. PLoS ONE. 2008a;3:2536. doi: 10.1371/journal.pone.0002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorpe AS, Thelen GC, Diaconu A, et al. Root exudate is allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol. 2009;97:641–645. [Google Scholar]
- 16.Prithiviraj B, Perry LG, Badri DV, et al. Chemical facilitation and induced pathogen resistance mediated by a root-secreted phytotoxin. New Phytol. 2007;173:852–860. doi: 10.1111/j.1469-8137.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaku M, Nakagawa N. (+)-catechin with Cu2+ induces protein modifications via reactive oxygen species-independent pathway. J Health Sci. 2009;3:441–446. [Google Scholar]
- 18.Oikawa S, Furukawa A, Asada H, et al. Catechins induce oxidative damage to cellular and isolated DNA through the generation of reactive oxygen species. Free Radical Research. 2003;37:881–890. doi: 10.1080/1071576031000150751. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa K, Kaku M, Abukawa T, et al. Copper (II) ions convert catechins from antioxidants to prooxidants in protein carbonyl formation. J Health Sci. 2007;53:591–595. [Google Scholar]
- 20.Morita-Yamamuro C, Tsutsui T, Sato M. The Arabidopsis gene CAD1 controls programmed cell death in the plant immune system and encodes a protein containing a MACPF domain. Plant Cell Physiol. 2005;46:902–912. doi: 10.1093/pcp/pci095. [DOI] [PubMed] [Google Scholar]
- 21.Mach JM, Castillo AR, Hoogstraten R. The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA. 2001;98:771–776. doi: 10.1073/pnas.021465298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudrappa T, Bonsall J, Gallagher JL, et al. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J Chem Ecol. 2007;33:1898–1918. doi: 10.1007/s10886-007-9353-7. [DOI] [PubMed] [Google Scholar]
- 23.Pollock JL, Callaway RM, Thelen GC, Holben WE. Catechin-metal interactions as a mechanism for conditional allelopathy by the invasive plant, Centaurea maculosa. Journal of Ecology. 2009 (in press) [Google Scholar]
- 24.Stermitz FR, Hufbauer RA, Vivanco JM. Enantiomeric-Dependent Phytotoxic and Antimicrobial Activity of (±)-Catechin. A Rhizosecreted Racemic Mixture from Spotted Knapweed. Plant Physiol. 2009;151:967. doi: 10.1104/pp.011019. [DOI] [PubMed] [Google Scholar]
- 25.Sofic E, Denisova N, Youdim K, Vatrenjak-Velagic V, De Filippo C, Mehmedagic A, et al. Antioxidant and pro-oxidant capacity of catecholamines and related compounds. Effects of hydrogen peroxide on glutathione and sphingomyelinase activity in pheochromocytoma PC12 cells: potential relevance to age-related diseases. J Neural Transm. 2001;108:541–557. doi: 10.1007/s007020170055. [DOI] [PubMed] [Google Scholar]
- 26.Thelen GC, Vivanco JM, Newingham B, et al. Insect herbivory stimulates allelopathic exudation by an invasive plant and suppression of natives. Ecol Lett. 2005;8:209–217. [Google Scholar]
- 27.Thorpe AS. Ph.D. dissertation. Missoula, MT: The University of Montana; 2006. Biochemical effects of Centaurea stoebe on soil nutrient cycles and plant communities. [Google Scholar]
- 28.Simoes K, Du J, Kretzschmar FS, et al. Phytotoxic catechin leached by seeds of the tropical weed Sesbania virgata. J Chem Ecol. 2008;34:681–687. doi: 10.1007/s10886-008-9443-1. [DOI] [PubMed] [Google Scholar]
- 29.Inderjit, Seastedt TR, Callaway RM, et al. Allelopathy and plant invasions: traditional, congeneric and biogeographical approaches. Biol Invasions. 2008b;10:875–890. [Google Scholar]
- 30.Veluri R, Weir TL, Bais HP, Stermitz FR, Vivanco JM. Phytotoxic and antimicrobial activities of catechin derivatives. J Agric Food Chem. 2004;10:1077–1082. doi: 10.1021/jf030653+. [DOI] [PubMed] [Google Scholar]
- 31.Zilkah S, Gressel J. Cell cultures vs. whole plants for measuring phytotoxicity III. Correlations between phytotoxicities in cell suspension cultures, calli and seedlings. Plant Cell Physiol. 1977;18:815–820. [Google Scholar]
- 32.Rose USR, Manukian A, Heath RR, et al. Volatile semiochemicals released from undamaged cotton leaves. Plant Physiol. 1996;111:487–495. doi: 10.1104/pp.111.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickett JA, Rasmussen HB, Woodcock CM, et al. Plant stress signaling: understanding and exploiting plant-plant interactions. Biochem Soc Trans. 2003;31:123–127. doi: 10.1042/bst0310123. [DOI] [PubMed] [Google Scholar]
- 34.Catska V. Interrelationships between vesicular-arbuscular mycorrhiza and rhizosphere microflora in apple replant disease. Biol Planatrum. 1994;36:99–104. [Google Scholar]
- 35.Cao J, Xu Y, Chen J, et al. Chemopreventive effects of green and black tea on pulmonary and hepatic carcinogenesis. Toxicol Sci. 1996;29:244–250. doi: 10.1006/faat.1996.0028. [DOI] [PubMed] [Google Scholar]
- 36.Nanjo F, Goto K, Seto R, et al. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Rad Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 37.Callaway RM. COS 45-2: Conditional effects of an allelopathic root exudate: The toxicity of (±)-catechin is affected by interactions with different metals. 93rd ESA meeting. 2008 [Google Scholar]
- 38.Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 39.Foreman J, Demidchik V, Bothwell JHF, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 40.Harter LN. Critical values for Duncan's new multiple range test. Biometrics. 1960;16:671–685. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.