Abstract
The lipidic structures, anther cuticle (outer anther surface) and pollen exine (outer pollen wall), play a key protective role for the male gametophyte and pollen grain development. We recently identified ancient cytochrome P450 family member CYP704B2 in rice and proposed a common fatty acid ω-hydroxylation pathway for synthesizing anther cuticle and pollen exine during plant male reproductive development. Here, we propose developmental model of pollen exine formation and discuss key genes required for pollen exine synthesis in the important crop plant rice.
Key words: anther cuticle, pollen exine, CYP704B2, anther, rice
Male reproductive development is a complex biological process in flowering plants and biosynthesis of anther cuticle and pollen exine, the two major protective layers of microspores/pollens, is an essential prerequisite for pollen formation, maturation, pollen disperse, spreading and pollination.1 Cuticle as a skin of plants is consisted of two types of lipophilic biopolymers, cutin and wax.2–4 The insoluble polymer cutin comprises hydroxylated and epoxy C16 and C18 fatty acids, defining the framework of the plant cuticle.5–7 Wax is composed of long-chain fatty acids, fatty alcohols, alkanes and alkenes, etc.3 Our recent investigation on cyp704B2 suggests that the lipidic components of rice anther cuticle are very similar to those of the epidermal cuticle of the leaf and stem.1
Pollen wall comprises three parts: exine (outer pollen wall), intine (inner pollen wall) and pollen coat.8 Pollen exine is mainly consisted of the biopolymer, sporopollenin, which was deduced to have aliphatic polyhydroxy compounds and phenolic OH groups,9 and the polymerization of these chemical molecules likely offers the exine highly resistance to physical and environmental factors. Current knowledge on the molecular basis underlying pollen exine development is mainly from the investigations in the model dicot plant Arabidopsis thaliana.9–15 The innermost sporophytic anther tissue, tapetum, plays a crucial role in synthesizing pollen exine. The lipidic molecules synthesized in the tapetum are putatively transported to outer pollen surface through the specialized organelles such as tapetosomes in Arabidopsis, elaioplasts in Brassicaceae16 and ubisch body in rice and wheat.17,18
Arabidopsis pollen exine contains two layers: the tectum and the foot layer, and the baculum forms between the two layers.19 At later stage, the gap of pollen exine is filled by typsine materials.19 Genetic analyses have revealed several genes critical for Arabidopsis pollen wall development, such as MS1 (MALE STERILITY1), MS2 (MALE STERILITY2), NEF1 (NO EXINE FORMATION 1), DEX1 (DEFECTIVE in EXINE PATTERN FORMATION), FLP1 (FACELESS POLLEN1), CYP703A2, ACOS5 (Acyl-CoA Synthetase 5) and CYP704B1.9–15,20,21
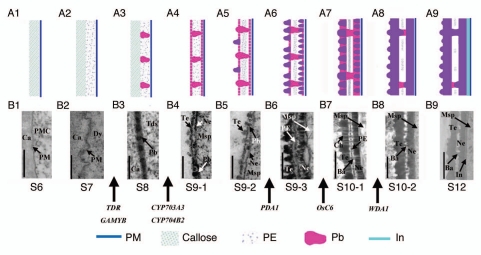
As a model monocot plant, rice has a distinct pollen wall ontology from that of Arabidopsis.1,14 Rice mature pollen exine has two layers with more inter-layer space (Figs. 1A9 and 1B9). Based on the observation of rice pollen wall development at various stages, we proposed a developmental model for rice pollen wall (Fig. 1). At stage 6 before meiosis, the callose is shown to be initially synthesized and deposited onto the pollen mother cell (Figs. 1A1 and 1B1). From stage 7, pollen mother cell undergoes meiosis and forms the tetrad, the primexine with the low electron density appears on the outer surface of the microspore (Figs. 1A2 and 1B2). Primexine is a microfibrillar matrix consisting mainly of cellulose synthesized by the miscrospore and serves as an elaborate template patterning the deposition of sporopollenin precursors and their following polymerization.22 At stage 8 during the tetrad formation, the probaculum is seen to be deposited onto the primexine (Figs. 1A3 and 1B3). At stage 9, microspores are released from the tetrad after callose degeneration; a very thin tectum (sexine) appears on the primexine due to the deposition of sporopollenin precursors putatively secreted from the tapetum. Meanwhile, the formation of nexine (foot layer) is seen on the surface of microspores (Figs. 1A4 and 1B4). Then the deposition of sporopollenin onto tectum is obvious (Figs. 1A5 and 1B5). With the development of microspore, the sporopollenin is gradually deposited to thicken and consolidate the tectum, nexine and probaculum while the primexine becomes condensed between two layers (Figs. 1A6 and 1B6). At stage 10, the microspore becomes vacuolated, and the pollen exine displays a two-layer structure with low electron density intervals or channels across the pollen wall (Figs. 1A7 and 1B7). During later stage, as the increase of the microspore vacuole in volume, the high density materials deposit into the probaculum, and the primexine gradually degenerates and disappears (Figs. 1A8 and 1B8). Subsequently, the microspore initiates the synthesis of intine under the exine (data not shown). Till stage 12 during the mature pollen formation, the pollen exine ontology remains less change(Figs. 1A9 and 1B9).
Figure 1.
Scheme of Rice Pollen Exine Development. (A1–9) The model of pollen exine development from stage 6 to stage 12. The definition of anther developmental stage refers Zhang and Wilson (2009).20 (B1–9) The pictures of TEM (Transmission Electron Micrographs) of pollen exine at corresponding anther development stages. The detailed procedure of performing TEM refers to the description by Li et al. (2010).1 Bars = 500 nm in B1, B6 and B7; =250 nm in B3 to B5; =200 nm in B2; =1 µm in B8 and B9. Some key genes for the formation of pollen exine are indicated in Figures. S6 (stage 6), callose is around with the pollen mother cell; S7, stage 7; S8, stage 8; S9-1, early stage 9; S9-2, middle stage 9; S9-3, later stage 9; S10-1, early stage 10; S10-2, late stage 10; S12, stage 12. Purpel color indicated sporopollenin. Ba, baculum; Ca, callose; Ch, channel; Dy, dyad; In, intine; Msp, microspore; Ne, nexine; Pb, probaculum; PE, primexine; PMC, pollen mother cell; PM, plasma membrane; Tds, tetrads; Te, tectum.
With the advent of rice functional genomics, several regulators critical for pollen exine development have been identified. TDR (Tapetum Degeneration Retardation) and GAMYB were shown to be required for lipid biosynthesis and metabolism during early pollen wall development,1,18,23 and mutants of tdr and gamyb display no obvious pollen exines, and their microspores only form the primexine-like layer.1,18,23 GAMYB encodes a R2R3 MYB transcription factor, which was shown as a positive gibberellin acid (GA)-signaling component. Mutations of GAMYB cause delayed tapetal programmed cell death (PCD) and abnormal formation of exine and Ubisch bodies, leading to male sterility in rice (Fig. 1A).23,24 TDR encodes a putative basic helix-loop-helix transcription factor, and controls rice tapetal development and degeneration by positively triggering PCD.18 Moreover, the expression of about 30 genes associated with pollen wall synthesis and pollen coat deposition is altered in tdr.25 Interestingly, AMS (Abroted Microspores), the ortholog of TDR in Arabidopsis,26 was also shown to be critical for pollen development by directly regulating the genes related to lipid transport and metabolism.27 Moreover, the Post-meiotic Deficicent Anther1 (PDA1) gene was shown to be essential for post-meiotic anther cuticle and pollen exine development in rice.28 The pda1 mutant anther displays obvious defects in postmeiotic tapetal development with abnormal lipidic Ubisch bodies. At stage 9, defective pollen exine only with a single thin layer was observed in pda1 (Fig. 1A). Additionally, RT-PCR analysis indicated that the expression of genes involved in anther development including GAMYB, OsC4 and Wax-deficient anther1 (WDA1) is greatly reduced in the pda1 mutant anther.28
Furthermore, several genes predicted to encode enzymes involved in lipidic synthesis were shown to be required for anther cuticle and/or pollen exine development in rice. A cytochrome family member, CYP703A3, directly regulated by GAMYB, encodes a fatty acid hydroxylase, and the loss function of CYP703A3 causes the defective of ubicsh body and pollen exine development (Fig. 1A).23 Rice CYP704B2 belongs to an ancient and conserved P450 subfamily among terrestrial plants, and is preferentially expressed in tapetal cells. Recombinant CYP704B2 protein has the ability to catalyze the hydroxylation of palmitic acid and unsaturated C18 fatty acids in the ω position of the carbon chain. The cyp704B2 mutant displays undeveloped anther epidermal cuticle and aborted pollen grains without obvious exine (Fig. 1A), suggesting that CYP704B2 controls a common and conserved biosynthetic step of the two biopolymers sporopollenin and cutin.1 In addition, rice WDA1 is a close homolog of Arabidopsis CER1, which is putatively associated with anther cuticle and pollen exine development (Fig. 1A).29 The wda1 plants display abnormal pollen exine and anther surface with significant reduction of very-long-chain fatty acids in anthers. The Wda1 gene is mainly expressed in the epidermal cells of anthers. Recently we revealed the crucial role of OsC6 encoding a binding transfer protein (LTP), which is directly regulated by TDR, in determining postmeiotic anther development.18,30 The OsC6 expression is detectable in tapetal cells, while the wide distribution of OsC6 was observed in anther tissues such as in the extracellular space between the tapetum and middle layer, as well as in the anther locule and anther cuticle by immunological assays. Also biochemical analysis indicated that recombinant OsC6 has lipid transfer activity, and OsC6 silencing plants display defective development of Ubisch bodies and pollen exine, causing reduced pollen fertility.30 Our finding may explain the observation that taptetum-expressed gene CYP704B2 or epidermis-expressed gene WDA1 is able to affect the whole anther development, suggesting that LTPs are crucial for coordination and the distribution of lipidic molecules within anther tissues/cells.
In conclusion, we proposed a scheme to describe the events of rice pollen exine development, and summarized several key regulators controlling anther cuticle and pollen exine development in rice. Also some genes were shown to have counterparts such as CYP703A2 and CYP704B2 in model eudicot plant Arabidopsis, suggesting the conserved and diversified biosynthetic pathways for anther cuticle and pollen exine development in plants.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12562
References
- 1.Li H, Pinot F, Sauveplane V, Werck-Reichhart D, Diehl P, Schreiber L, et al. Cytochrome P450 family member CYP704B2 catalyzes the omega-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell. 2010;22:173–190. doi: 10.1105/tpc.109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riederer M, Muller C. Biology of the plant cuticle. Oxford, UK: Blackwell; 2006. [Google Scholar]
- 3.Samuels L, Kunst L, Jetter R. Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu Rev Plant Biol. 2008;59:683–707. doi: 10.1146/annurev.arplant.59.103006.093219. [DOI] [PubMed] [Google Scholar]
- 4.Kerstiens GE. Plant cuticles: an integrated functional approach. Oxford UK: Bios Sci; 1996. [Google Scholar]
- 5.Kolattukudy PE. Polyesters in higher plants. Adv Biochem Eng Biotechnol. 2001;71:1–49. doi: 10.1007/3-540-40021-4_1. [DOI] [PubMed] [Google Scholar]
- 6.Heredia A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochem Biophys Acta. 2003;1620:1–7. doi: 10.1016/s0304-4165(02)00510-x. [DOI] [PubMed] [Google Scholar]
- 7.Nawrath C. Unraveling the complex network of cuticular structure and function. Curr Opin Plant Biol. 2006;9:281–287. doi: 10.1016/j.pbi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Blackmore S, Wortley AH, Skvarla JJ, Rowley JR. Pollen wall development in flowering plants. New Phytol. 2007;174:483–498. doi: 10.1111/j.1469-8137.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- 9.Morant M, Jorgensen K, Schaller H, Pinot F, Moller BL, Werck-Reichhart D, et al. CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell. 2007;19:1473–1487. doi: 10.1105/tpc.106.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA. DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol. 2001;127:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- 11.Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, et al. Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J. 2004;39:170–181. doi: 10.1111/j.1365-313X.2004.02118.x. [DOI] [PubMed] [Google Scholar]
- 12.Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, et al. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J. 1997;12:615–623. doi: 10.1046/j.1365-313x.1997.00615.x. [DOI] [PubMed] [Google Scholar]
- 13.Souza CD, Kim SS, Koch S, Kienow L, Schneider K, McKim SM, et al. A novel fatty acyl-CoA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell. 2009;21:507–525. doi: 10.1105/tpc.108.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, et al. A novel male-sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis-sensitive exine. Plant Mol Biol. 2003;53:107–116. doi: 10.1023/B:PLAN.0000009269.97773.70. [DOI] [PubMed] [Google Scholar]
- 15.Dobritsa AA, Shrestha J, Morant M, Pinot F, Matsuno M, Swanson R, et al. CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol. 2009;151:574–589. doi: 10.1104/pp.109.144469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh K, Huang AHC. Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell. 2007;19:582–596. doi: 10.1105/tpc.106.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A, Xia Q, Xie W, Datla R, Selvaraj G. The classical Ubisch bodies carry a sporophytically produced structural protein (RAFTIN) that is essential for pollen development. Proc Natl Acad Sci USA. 2003;100:14487–14492. doi: 10.1073/pnas.2231254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Zhang DS, Liu HS, Yin CS, Li XX, Liang WQ, et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell. 2006;18:2999–3014. doi: 10.1105/tpc.106.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005;42:315–328. doi: 10.1111/j.1365-313X.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang DB, Wilson ZA. Stamen specification and anther development in rice. Chin Sci Bull. 2009;54:2342–2353. [Google Scholar]
- 21.Yang C, Vizcay-Barrena G, Conner K, Wilson ZA. MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell. 2007;19:3530–3548. doi: 10.1105/tpc.107.054981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heslop-Harrison J. Pollen wall development. Science. 1968;161:230–237. doi: 10.1126/science.161.3838.230. [DOI] [PubMed] [Google Scholar]
- 23.Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K, Nishimura M, et al. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell. 2009;21:1453–1472. doi: 10.1105/tpc.108.062935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko M, Inukai Y, Ueguchi-Tanaka M, Itoh H, Izawa T, Kobayashi Y, et al. Loss-of-function mutations of the rice GAMYB gene impair alpha-amylase expression in aleurone and flower development. Plant Cell. 2004;16:33–44. doi: 10.1105/tpc.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang DS, Liang WQ, Yuan Z, Li N, Shi J, Wang J, et al. Tapetum Degeneration Retardation is critical for aliphatic metabolism and gene regulation during rice pollen development. Mol Plant. 2008;1:599–610. doi: 10.1093/mp/ssn028. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen AM, Krober S, Unte US, Huijser P, Dekker K, Saedler H. The Arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J. 2003;33:413–423. doi: 10.1046/j.1365-313x.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Yang CY, Yuan Z, Zhang DS, Gondwe MY, Ding ZW, et al. The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell. 2010;22:97–107. doi: 10.1105/tpc.109.071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu LF, Tan HX, Liang WQ, Zhang DB. The Post-meiotic Deficicent Anther1 (PDA1) gene is required for post-meiotic anther development in rice. J Genet Genomics. 2010;37:37–46. doi: 10.1016/S1673-8527(09)60023-0. [DOI] [PubMed] [Google Scholar]
- 29.Jung KH, Han MJ, Lee DY, Lee YS, Schreiber L, Franke R, et al. Wax-deficient anther1 is involved in cuticle and wax production in rice anther walls and is required for pollen development. Plant Cell. 2006;18:3015–3032. doi: 10.1105/tpc.106.042044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang DS, Liang WQ, Yin CS, Zong J, Gu FW, Zhang DB. OsC6, encoding a lipid transfer protein (LTP), is required for postmeiotic anther development in rice. Plant Physiol. 2010;154:149–162. doi: 10.1104/pp.110.158865. [DOI] [PMC free article] [PubMed] [Google Scholar]