Abstract
Since the photosynthetic apparatus of plants contains a massive amount of nitrogen, the regulation of its development by sugar signals is important to the maintenance of the carbon-nitrogen balance. Recently, we isolated a new Arabidopsis mutant, sicy (sugar-inducible cotyledon yellow)-192, whose cotyledons were prevented from greening by treatment with sucrose. On treatment with sucrose, the expression of photosynthesis- and nitrogen assimilation-related genes was respectively weaker and stronger in the mutant seedlings than the wild-type seedlings. In the mutants, the gene encoding plastidic alkaline/neutral (A/N) invertase (INV-E) was point-mutated at codon 294, with Tyr substituted for Cys (C294Y). These findings provide new insights into the regulation of greening and carbon-nitrogen balance by sugar metabolism through INV-E in plastids. In this addendum, we describe the phenotypes of sicy-192 on treatment with sucrose in more detail, and propose a possible relationship among sugar metabolism through INV-E, plastid-to-nucleus retrograde signaling, and ethylene, a plant hormone, in the regulation of plant development and metabolism.
Key words: invertase, carbon-nitrogen balance, greening, plastid gene expression, plastid signaling, ethylene
The photosynthetic apparatus contains a massive amount of nitrogen and so its development closely depends on the distribution of nitrogen in plants. The system for regulating the distribution of nitrogen via sugar signaling appears to be important for the adjustment of primary metabolism to ensure growth, survival and completion of the life cycle in plants. Therefore, the isolation of sugar-sensitive and -insensitive mutants should provide important clues to how the regulatory mechanism for the carbon-nitrogen balance in plants works. Recently, we isolated a new Arabidopsis mutant, sicy (sugar-inducible cotyledon yellow)-192, whose cotyledons were prevented from greening by treatment with sucrose.1 On treatment with sucrose, the expression of photosynthesis-related genes was weaker in the mutant than wild-type seedlings, while the activity of nitrate reductase was stronger in the mutant. In the sicy-192 plants, the gene encoding plastidic alkaline/neutral (A/N) invertase (INV-E) was point-mutated at codon 294, with Tyr substituted for Cys (C294Y), but a recombinant INV-E:C294Y protein had the same enzymatic activity and substrate specificity as a recombinant INV-E protein. Interestingly, sicy-192 is a recessive but gain-of-function mutant and the protein level and activity, but not the transcript level, of INV-E were increased in the mutant, suggesting that the point mutation enhances the stability of INV-E. These findings provide new insights into the regulation of greening and carbon-nitrogen balance by sugar metabolism through INV-E in plastids.1 In this addendum, we describe the effects of treatment with sucrose and plant hormones on the greening of sicy-192 mutants in detail, and discuss how INV-E is involved in the regulation of plant development and metabolism.
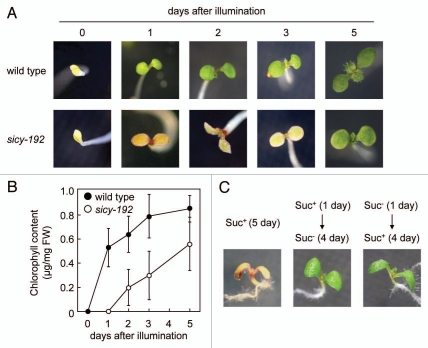
One interesting feature of sicy-192 was that the greening was inhibited only in early seedlings in the Murashige & Skoog (MS) medium with sucrose (Suc+ medium). As shown in Figure 2, 2-week-old sicy-192 plants were phenotypically similar to wild-type plants in the Suc+ medium. To investigate this in more detail, the plants were grown in the Suc+ medium in the dark for 5 days, and the timing of the greening of the etiolated cotyledons was checked. One day after illumination, the cotyledons of wild-type plants turned green, while the cotyledons of sicy-192 plants were still yellow (Fig. 1A). Some 30 to 50% of the sicy-192 cotyledons turned slightly green 2 or 3 days after illumination. De-etiolation of almost all the cotyledons of sicy-192 was observed at 5 days. The chlorophyll content of the cotyledons of wild-type and sicy-192 plants reflected the phenotypes of these plants (Fig. 1B). Next, we investigated the short-term effect of sucrose treatment on the greening inhibition in the sicy-192 mutant. When 1-day-old sicy-192 seedlings grown in the Suc+ medium were transferred to the Suc− medium, the greening of cotyledons was not inhibited (Fig. 1C). Moreover, when 1-day-old mutants grown in the Suc− medium were transferred to the Suc+ medium, the greening was not inhibited (Fig. 1C). These findings suggest that a mutation of sicy-192 inhibits greening at a very early stage, and that continuous treatment with sucrose is required for the phenotypes of sicy-192.
Figure 2.
Effect of co-treatment with sucrose and ACC on the greening of wild-type and sicy-192 seedlings. The wild-type and sicy-192 plants were grown in the Suc+ medium with or without 0.1 µM ACC for 2 weeks.
Figure 1.
Effect of sucrose on the greening of wild-type, sicy-192 and KO-INV-E seedlings. (A and B) The wild-type and sicy-192 plants were grown in the Suc+ medium in the dark for 5 days. After illumination, the seedlings were photographed (A) and levels of chlorophyll were measured according to Tamoi et al. 2010.1 (C) One-day-old sicy-192 plants grown in the Suc+ or Suc− (without sucrose) medium were transferred to Suc− medium from Suc+ medium (right) to Suc+ medium from Suc− medium (middle). The sicy-192 plants were also grown in the Suc+ medium for 5 days (left).
Until recently, several mutants whose greening was inhibited at an early stage had been isolated. Arabidopsis mutants lacking sigma factor 6 (AtSIG6) exhibited a cotyledon-specific pale green phenotype.2,3 A nuclear-encoded AtSIG6 is thought to be a major general sigma factor, a subunit of the plastidic RNA polymerase, essential for transcription of photosynthesis-related genes encoded by the plastid genome.2,3 Moreover, plants with snowy cotyledon1-1 (sco1-1), a weak allele of sco1, exhibited greening inhibition as young seedlings, while the null mutants of sco1 died in the embryonic stage.4,5 SCO1 encodes a plastidic elongation factor G (EF-G), and is involved in the translation of photosynthesis-related genes encoded in the plastid genome. The phenotypes of these mutants are similar to those of sicy-192 mutants, except that the greening inhibition in sicy-192 mutants occurred only on treatment with sugars. AtSIG6 and SCO1 are involved in plastid gene expression (PGE), suggesting that normal PGE is essential for greening among early seedlings and thus a component of the plastid-to-nucleus retrograde signaling (plastid signaling) pathway.6–9 When PGE is prevented by an inhibitor, such as lincomycin, the expression of photosynthesis-related genes encoded by the nuclear genome is suppressed by plastid signaling. In the sicy-192 mutants, the expression of rbcL (the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase) encoded by the plastid genome as well as enzymes related to photosynthesis encoded by the nuclear genome were suppressed in the Suc+ medium.1 Therefore, previous reports and our findings suggest that sugar metabolism through INV-E in plastids is associated with PGE and plastid signaling.
Plant hormones such as abscisic acid (ABA) and ethylene are associated with sugar signaling.10,11 Additionally, ABA may be involved in plastid signaling.6,7 Therefore, we studied the effects of such hormones on the greening inhibition in the sucrose-treated sicy-192 mutants. However, ABA had no effect on the greening of sicy-192 mutants grown in the Suc+ medium (data not shown). Moreover, the sicy-192 and abi4 (abscisic acid insensitive 4) double mutants, in which the transcription factor ABI4 involved in ABA signaling as well as sugar and plastid signaling is disrupted,6 showed the same sugar-sensitivity as the sicy-192 mutants,1 indicating that the greening inhibition in sicy-192 mutants was ABA-independent. Interestingly, co-treatment with sucrose and 1-amino cyclopropane-1-carbonic acid (ACC), a precursor of ethylene, markedly enhanced the greening inhibition in sicy-192 mutants compared to sucrose alone (Fig. 2), suggesting the sugar signaling derived from plastids was related to ethylene signaling in sicy-192 mutants to regulate greening and carbon-nitrogen balance.
At present, we cannot explain how the sugar metabolism in plastids is associated with greening and the carbon-nitrogen balance in the sicy-192 mutants. However, it seems that sugar metabolism through INV-E promotes plastid and/or ethylene signaling to regulate both greening and the carbon-nitrogen balance.
Acknowledgements
We thank Ms. Kana Mizuuchi for technical assistance. This work was supported by CREST, JST (S.S. 2005–2010).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12568
References
- 1.Tamoi M, Tabuchi T, Demuratani M, Otori K, Tanabe N, Maruta T, et al. Point mutation of a plastidic invertase inhibits development of the photosynthetic apparatus and enhances nitrate assimilation in sugar-treated Arabidopsis seedlings. J Biol Chem. 2010;285:15399–15407. doi: 10.1074/jbc.M109.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishizaki Y, Tsunoyama Y, Hatano K, Ando K, Kato K, Shinmyo A, et al. A nuclear-encoded sigma factor, Arabidopsis SIG6, recognizes sigma-70 type chloroplast promoters and regulates early chloroplast development in cotyledons. Plant J. 2005;42:133–144. doi: 10.1111/j.1365-313X.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 3.Loschelder H, Schweer J, Link B, Link G. Dual temporal role of plastid sigma factor 6 in Arabidopsis development. Plant Physiol. 2006;142:642–650. doi: 10.1104/pp.106.085878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrecht V, Ingenfeld A, Apel K. Characterization of the snowy cotyledon 1 mutant of Arabidopsis thaliana: the impact of chloroplast elongation factor G on chloroplast development and plant vitality. Plant Mol Biol. 2006;60:507–518. doi: 10.1007/s11103-005-4921-0. [DOI] [PubMed] [Google Scholar]
- 5.Ruppel NJ, Hangarter RP. Mutations in a plastidlocalized elongation factor G alter early stages of plastid development in Arabidopsis thaliana. BMC Plant Biol. 2007;13:7–37. doi: 10.1186/1471-2229-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:715–719. [PubMed] [Google Scholar]
- 7.Kleine T, Voigt C, Leister D. Plastid signalling to the nucleus: messengers still lost in the mists? Trends Genet. 2009;25:185–192. doi: 10.1016/j.tig.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Waters MT, Langdale JA. The making of a chloroplast. EMBO J. 2009;28:2861–2873. doi: 10.1038/emboj.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba T. Bilateral communication between plastid and the nucleus: plastid protein import and plastid-to-nucleus retrograde signaling. Biosci Biotechnol Biochem. 2010;74:471–476. doi: 10.1271/bbb.90842. [DOI] [PubMed] [Google Scholar]
- 10.Smeekens S. SUGAR-INDUCED SIGNAL TRANSDUCTION IN PLANTS. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Gazzarrini S, McCourt P. Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr Opin Plant Biol. 2001;4:387–391. doi: 10.1016/s1369-5266(00)00190-4. [DOI] [PubMed] [Google Scholar]