Abstract
As the primary site for photosynthetic carbon fixation and the interface between plants and the environment, plant leaves play a key role in plant growth, biomass production and survival, and global carbon and oxygen cycles. Leaves can be simple with a single blade or compound with multiple units of blades known as leaflets. In a palmate-type compound leaf, leaflets are clustered at the tip of the leaf. In a pinnate-type compound leaf, on the other hand, leaflets are placed on a rachis in distance from each other. Higher orders of complexities such as bipinnate compound leaves of the “sensitive” plant, Mimosa pudica, also occur in nature. However, how different leaf morphologies are determined is still poorly understood. Medicago truncatula is a model legume closely related to alfalfa and soybean with trifoliate compound leaves. Recently, we have shown that Palmate-like Pentafoliata1 (PALM1) encodes a putative Cys(2) His(2) zinc finger transcription factor essential for compound leaf morphogenesis in M. truncatula. Here, we present our phylogenetic relationship analysis of PALM1 homologs from different species and demonstrate that PALM1 has transcriptional activity in the transactivation assay in yeast.
Key words: Compound leaves, Medicago truncatula, PALM1, LFY/UNI/SGL1, transcription factor
Leaf development is divided into three continuous phases, organ initiation, primary morphogenesis and secondary morphogenesis (or histogenesis).1–4 Initiation of leaf primordia occurs along the periphery of the shoot apical meristem (SAM), a pluripotent structure capable of self renewable. Downregulation of the class I Knotted-like homeobox transcription factors (KNOXIs) at sites of incipient leaf primordia (P0, P for Plastochron) is essential for the initiation of leaf primordia.5–7 KNOXI proteins remain downregulated in simple leaf primordia. In contrast, they are reactivated in leaf primordia in most compound-leafed eudicot species studied.8–12 Thus, development of a compound leaf requires a transient phase of indeterminacy along the margin of the leaf primordium, or called marginal blastozones.13
Legume (Fabaceae) represents the third largest family of flowering plants with significant economic importance.14 The diverse array of leaf forms found in legume species presents legume as an ideal system for genetic and evolutionary studies of plant forms.15 In garden pea (Pisum sativum) and Medicago truncatula, both belonging to the inverted repeat lacking clade (IRLC) of legume, the role of KNOXI proteins in compound leaf development is replaced by the FLORICAULA (FLO)/LEAFY (LFY) orthologs UNIFOLIATA (UNI) and SINGLE LEAFLET1 (SGL1), respectively.16–20 This is mainly because (1) KNOXI proteins are not reactivated in compound leaf primordia in these plants and (2) loss-of-function mutants of UNI and SGL1 develop simplified or simple leaves. Interestingly, leaf developmental programs remain responsive to ectopically expressed KNOXI proteins in these species.17 Both UNI and SGL1 are similarly expressed in young leaf primordia.16,20–22 The expression was greatly reduced in older leaf primordia, consistent with their role in promoting a transient phase of indeterminacy required for leaflet initiation in these species. Although both UNI and SGL1 play a similar role in compound leaf development in pea and M. truncatula, leaflet primordia develop acropetally in pea with pinnate compound leaves and tendrils19 but basipetally in M. truncatula with ternate leaves.16 This suggests that differences in compound leaf development in closely related species may explain some differences in compound leaf phenotypes of loss-of-function uni mutants in pea and sgl1 mutants in M. truncatula.
In M. truncatula palmate-like pentafoliata1 (palm1) mutants, each compound leaf is consisted of five leaflets clustered at the tip with two distally oriented lateral leaflets subtended by rachis.23 This is in contrast with the morphology of the WT compound leaf with three leaflets at the tip and only the terminal leaflet is subtended by a rachis.16,23 In palm1 mutants, petiole is slightly longer and the central rachis is slightly shorter than the WT counterparts, indicating that PALM1 also plays a role in the proximal-distal axis development of compound leaves. The proliferation of lateral leaflets in loss-of-function palm1 mutants requires the activity of SGL1 because (1) the expression level of SGL1 is upregulated by 2.7-folds in palm1 mutants and (2) palm1 sgl1 double mutants exhibit the simple leaf morphology similarly as the sgl1 mutants.
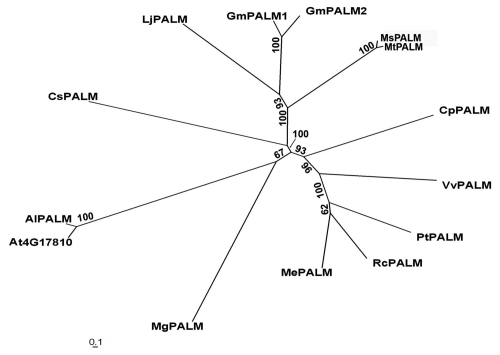
The PALM1 gene encodes a putative transcription factor with a single Cys(2) His(2) zinc finger DNA binding domain at the N-terminus and an EAR transcription repressor domain at the C-terminus.23 Cys(2)His(2) zinc finger transcription factors belong to a large divergent family of transcription factors in eukaryotic organisms. Using synteny analyses in plants with available genome sequences, we uncovered candidate PALM1 orthologs from closely related legume species such as alfalfa (M. sativa), soybean (Glycine max) and Lotus japonicus, and from remotely related species such as Arabidopsis thaliana, A. lyrata, Vitis vinifera, Cucumis sativus, Manihot esculenta, Mimulus guttatus, Populus trichocarpa, Carica papaya and Ricinus comunis (Fig. 1). A duplication event results in two closely related PALM1 orthologs in the soybean genome (Fig. 1). The observation that PALM1 homologous sequences exist in lower land plants (our unpublished results) and in species with simple leaves (Fig. 1) suggests a diverged function or recruitment of the Cys(2) His(2) zinc finger protein in dissected leaf morphogenesis in some compound-leafed lineages.
Figure 1.
Phylogenetic relationships of PALM1 homologs. A neighbor joining phylogenetic tree was reconstructed using PAUP4.0 with 1,000 bootstrap repeats. MtPALM1, Palmate-like pentafoliata1 from Medicago truncatula; Ms, M. sativa; Gm, Glycine max; Lj, Lotus japonicus; At, Arabidopsis thaliana; Al, A. lyrata; Vv, Vitis vinifera; Cs, Cucumis sativus; Me, Manihot esculenta; Mg, Mimulus guttatus; Pt, Populus trichocarpa; Rc, Ricinus comunis; and Cp, Carica papaya.
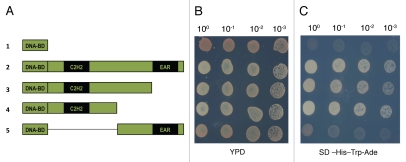
PALM1 is localized to nucleus in onion epidermis cells, consistent with its role as a putative transcription factor.23 To provide evidence that PALM1 encodes a transcription factor, we carried out a transactivation assay in yeast. Figure 2 shows that the full-length PALM1 protein is able to activate transcription of reporter genes in the yeast system, supporting its role as a transcription factor. To delineate domains required for the transactivation activity, we tested several truncated fragments of the PALM1 gene in yeast. The experiment showed that the N-terminal Cys(2) His(2) zinc finger DNA binding domain is essential for the transactivation activity in yeast, whereas the C-terminal EAR domain is not.
Figure 2.
Yeast transactivation assay. The full-length and various truncated fragments of PALM1 were cloned into the pGBKT7 DNA-BD vector (Clonetech). The resulting plasmids were transformed into the yeast (Saccharomyces cerevisiae) Y2HGold strain (Clonetech). (A) pGBKT7 DNA-BD PALM1 fusion constructs. (1) BD, empty vector; (2) BD-PALM1FL, BD-full-length PALM1 fusion; (3) BDPALM1CΔ1, BD-PALM1 C-terminal deletion 1 fusion; (4) BD-PALM1CΔ2, BD-PALM1 C-terminal deletion 2 fusion; (5) BD-PALM1NΔ, BD-PALM1 N-terminal deletion fusion. (B) Yeast cultures at O.D.600 = 0.5 were diluted as specified and grown at 30°C on YPD medium for 48 hrs. (C) Yeast cultures at O.D.600 = 0.5 were diluted as specified and grown at 30°C on SD (synthetically defined) medium without tryptophan, histidine and adenine for 48 hrs. Table 1 lists primers used.
Our study identifies PALM1 as a key regulator of compound leaf development in M. truncatula, an IRLC legume. Our studies show that PALM1 binds specifically to the promoter sequence and regulates the spatial-temporal expression of SGL1 in developing leaf primordia.23 Together, they define the trifoliate morphology of WT leaves. In future, identification of the role of PALM1 orthologs in non-IRLC legumes and elucidating its mode of regulation in compound leaf development promise to provide new insights in the evolution of complex leaf forms in legume.
GenBank Database
The sequences reported in this addendum have been deposited in the GenBank database [accession nos. HM038482 (PALM1); HM038483 (MsPALM1); HM038484 (LjPALM1); HM038485 (GmPALM1); HM038486 (GmPALM2); HM453333 (VvPALM1); HM453334 (AlPALM1); HM453335 (CsPALM1); HM453336 (MePALM1); HM453337 (MgPALM1); HM453338 (PtPALM1); HM453339 (RcPALM1); and HM453340 (CpPALM1)].
Table 1.
Primers used in the study
| PALM1_F1 | aaaGAATTCATGGCTACAGATATTGGCC |
| PALM1_R1 | aaaGGATCCTCAAGTTGGTGTTGGCTTGTTCCC |
| PALM1_R2 | aaaGGATCCTCATTGCTGGTCCACTTTGC |
| PALM1_R3 | aaaGGATCCTCACGGTGGTTGGGTTTGATGG |
| PALM1_F2 | aaaGAATTCTCTCATCCTTCATCACC |
Nucleotides underlined are introduced restriction sites.
Acknowledgements
This work was supported in part by the Samuel Roberts Noble Foundation and the National Science Foundation (DBI 0703285).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12640
References
- 1.Shani E, Burko Y, Ben-Yaakov L, Berger Y, Amsellem Z, Goldshmidt A, et al. Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE HOMEOBOX proteins. Plant Cell. 2009;21:3078–3092. doi: 10.1105/tpc.109.068148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat Genet. 2008;40:1136–1141. doi: 10.1038/ng.189. [DOI] [PubMed] [Google Scholar]
- 3.Poethig RS. Leaf morphogenesis in flowering plants. Plant Cell. 1997;9:1077–1087. doi: 10.1105/tpc.9.7.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efroni I, Blum E, Goldshmidt A, Eshed Y. A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. Plant Cell. 2008;20:2293–2306. doi: 10.1105/tpc.107.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson D, Veit B, Hake S. Expression of maize KNOTTED 1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:405–413. [Google Scholar]
- 6.Sinha NR, Williams RE, Hake S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Genes Dev. 1993;7:787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- 7.Smith LG, Greene B, Veit B, Hake S. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development. 1992;116:21–30. doi: 10.1242/dev.116.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Ori N, Cohen AR, Etzioni A, Brand A, Yanai O, Shleizer S, et al. Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat Genet. 2007;39:787–791. doi: 10.1038/ng2036. [DOI] [PubMed] [Google Scholar]
- 9.Jasinski S, Kaur H, Tattersall A, Tsiantis M. Negative regulation of KNOX expression in tomato leaves. Planta. 2007;226:1255–1263. doi: 10.1007/s00425-007-0572-5. [DOI] [PubMed] [Google Scholar]
- 10.Hay A, Tsiantis M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat Genet. 2006;38:942–947. doi: 10.1038/ng1835. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Pham T, Hamidi A, McCormick S, Kuzoff RK, Sinha N. Reduced leaf complexity in tomato wiry mutants suggests a role for PHAN and KNOX genes in generating compound leaves. Development. 2003;130:4405–4415. doi: 10.1242/dev.00655. [DOI] [PubMed] [Google Scholar]
- 12.Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR. Homologies in leaf form inferred from KNOXI gene expression during development. Science. 2002;296:1858–1860. doi: 10.1126/science.1070343. [DOI] [PubMed] [Google Scholar]
- 13.Hagemann W, Gleissberg S. Organogenetic capacity of leaves: The significance of marginal blastozones in angiosperms. Plant Syst Evol. 1996;199:121–152. [Google Scholar]
- 14.Graham PH, Vance CP. Legumes: importance and constraints to greater use. Plant Physiol. 2003;131:872–877. doi: 10.1104/pp.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle JJ, Luckow MA. The rest of the iceberg. Legume diversity and evolution in a phylogenetic context. Plant Physiol. 2003;131:900–910. doi: 10.1104/pp.102.018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Chen J, Wen J, Tadege M, Li G, Liu Y, et al. Control of compound leaf development by FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago truncatula. Plant Physiol. 2008;146:1759–1772. doi: 10.1104/pp.108.117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champagne CE, Goliber TE, Wojciechowski MF, Mei RW, Townsley BT, Wang K, et al. Compound leaf development and evolution in the legumes. Plant Cell. 2007;19:3369–3378. doi: 10.1105/tpc.107.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofer J, Gourlay C, Michael A, Ellis TH. Expression of a class 1 knotted1-like homeobox gene is downregulated in pea compound leaf primordia. Plant Mol Biol. 2001;45:387–398. doi: 10.1023/a:1010739812836. [DOI] [PubMed] [Google Scholar]
- 19.Hofer J, Ellis TH. The genetic control of patterning in pea leaves. Trends Plant Sci. 1998;3:439–444. [Google Scholar]
- 20.Hofer J, Turner L, Hellens R, Ambrose M, Matthews P, Michael A, et al. UNIFOLIATA regulates leaf and flower morphogenesis in pea. Curr Biol. 1997;7:581–587. doi: 10.1016/s0960-9822(06)00257-0. [DOI] [PubMed] [Google Scholar]
- 21.DeMason DA, Schmidt RJ. Roles of the Uni gene in shoot and leaf development of pea (Pisum sativum); phenotypic characterization and leaf development in the uni and uni-tac mutants. Int J Plant Sci. 2001;162:1033–1051. [Google Scholar]
- 22.Gourlay CW, Hofer JM, Ellis TH. Pea compound leaf architecture is regulated by interactions among the genes UNIFOLIATA, cochleata, afila and tendrilless. Plant Cell. 2000;12:1279–1294. doi: 10.1105/tpc.12.8.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Yu J, Ge L, Wang H, Berbel A, Yu L, et al. Control of dissected leaf morphology by a Cys(2)His(2) zinc finger transcription factor in the model legume Medicago truncatula. Proc Natl Acad Sci USA. 2010;107:10754–10759. doi: 10.1073/pnas.1003954107. [DOI] [PMC free article] [PubMed] [Google Scholar]