Abstract
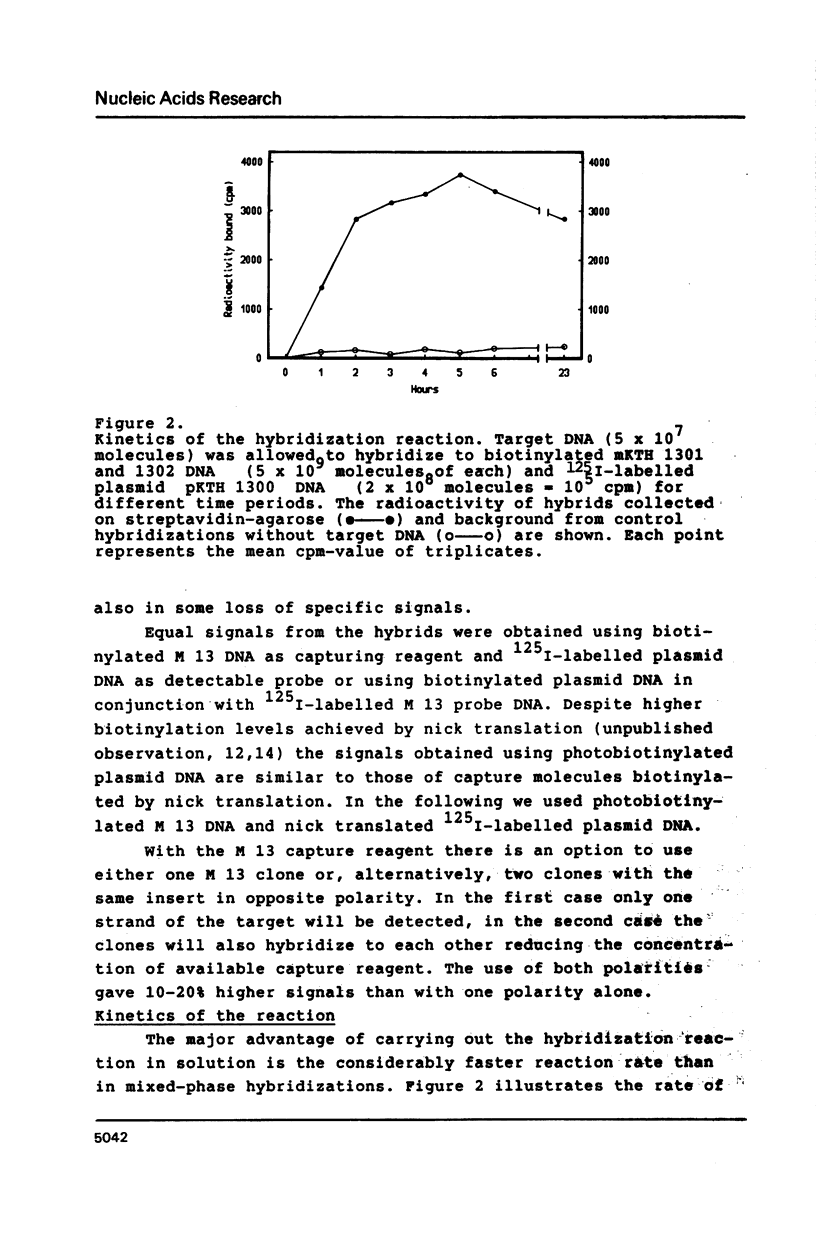
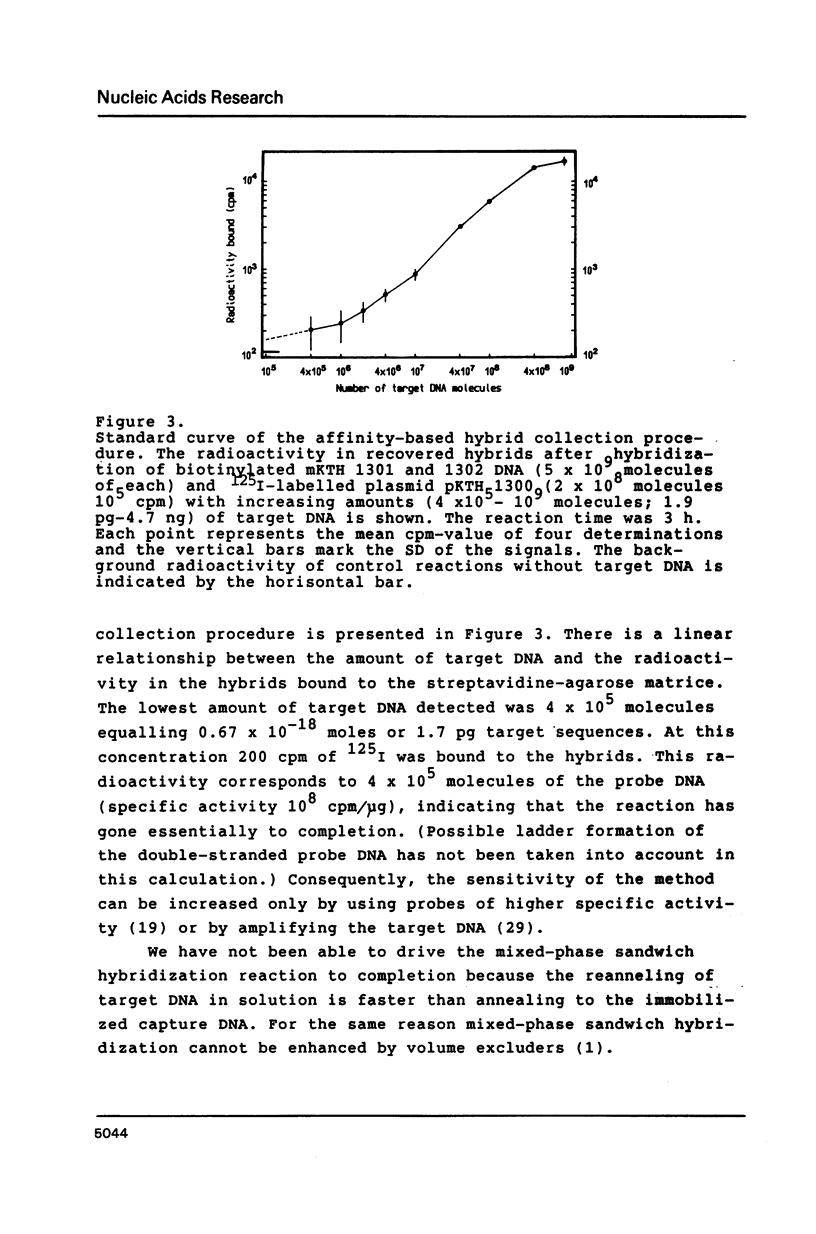
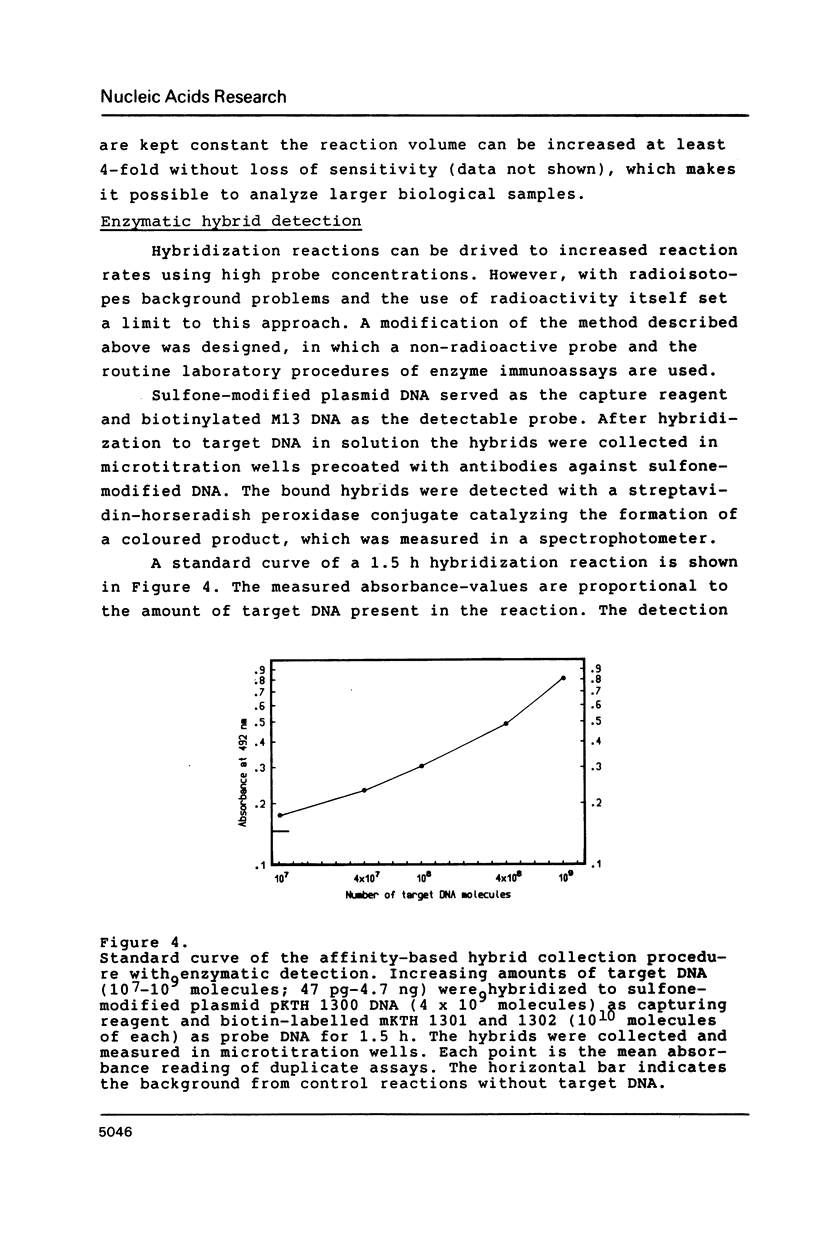
A hybridization technique for the quantification of nucleic acids is described. In the method a probe pair is allowed to form hybrids with the target nucleic acid in solution. One of the probes has been modified with an affinity label, by which the formed hybrids can be isolated after the reaction. Streptavidin-agarose was used to capture hybrids containing biotinylated DNA. The hybrids were measured using radioiodine as label on the second probe. The rate of the hybridization reaction in solution is fast, allowing the whole procedure to be carried out in 3 h. The method is quantitative with a detection limit of 4 X 10(5) molecules (0.67 attomoles) target DNA. The test is insensitive to impurities in biological samples, which are analyzed without purification of the target DNA. Non-isotopic measurement of the hybrids can also be applied. In this case the hybrids are bound to microtitration wells and detected spectrophotometrically by peroxidase-catalyzed colour development.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arsenyan S. G., Avdonina T. A., Laving A., Saarma M., Kisselev L. L. Isolation of rat liver 5S RNA genes. Gene. 1980 Oct;11(1-2):97–108. doi: 10.1016/0378-1119(80)90090-6. [DOI] [PubMed] [Google Scholar]
- Banfalvi G., Bhattacharya S., Sarkar N. Selective isolation of mercurated DNA by affinity chromatography on thiol matrices. Anal Biochem. 1985 Apr;146(1):64–70. doi: 10.1016/0003-2697(85)90396-3. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Delius H., van Heerikhuizen H., Clarke J., Koller B. Separation of complementary strands of plasmid DNA using the biotin-avidin system and its application to heteroduplex formation and RNA/DNA hybridizations in electron microscopy. Nucleic Acids Res. 1985 Aug 12;13(15):5457–5469. doi: 10.1093/nar/13.15.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Birfelder E. J., Sanders J. P., Borst P. DNA-DNA hybridization on nitrocellulose filters. 1. General considerations and non-ideal kinetics. Eur J Biochem. 1974 Sep 16;47(3):535–543. doi: 10.1111/j.1432-1033.1974.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Forster A. C., McInnes J. L., Skingle D. C., Symons R. H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985 Feb 11;13(3):745–761. doi: 10.1093/nar/13.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegent J. E., Jasen in de Wal N., Baan R. A., Hoeijmakers J. H., Van der Ploeg M. 2-Acetylaminofluorene-modified probes for the indirect hybridocytochemical detection of specific nucleic acid sequences. Exp Cell Res. 1984 Jul;153(1):61–72. doi: 10.1016/0014-4827(84)90448-8. [DOI] [PubMed] [Google Scholar]
- Langdale J. A., Malcolm A. D. A rapid method of gene detection using DNA bound to Sephacryl. Gene. 1985;36(3):201–210. doi: 10.1016/0378-1119(85)90175-1. [DOI] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J., Pellegrini M., Davidson N. A method for gene enrichment based on the avidin-biotin interaction. Application to the Drosophila ribosomal RNA genes. Biochemistry. 1977 Apr 5;16(7):1364–1370. doi: 10.1021/bi00626a020. [DOI] [PubMed] [Google Scholar]
- Polsky-Cynkin R., Parsons G. H., Allerdt L., Landes G., Davis G., Rashtchian A. Use of DNA immobilized on plastic and agarose supports to detect DNA by sandwich hybridization. Clin Chem. 1985 Sep;31(9):1438–1443. [PubMed] [Google Scholar]
- Ranki M., Palva A., Virtanen M., Laaksonen M., Söderlund H. Sandwich hybridization as a convenient method for the detection of nucleic acids in crude samples. Gene. 1983 Jan-Feb;21(1-2):77–85. doi: 10.1016/0378-1119(83)90149-x. [DOI] [PubMed] [Google Scholar]
- Renz M. Polynucleotide-histone H1 complexes as probes for blot hybridization. EMBO J. 1983;2(6):817–822. doi: 10.1002/j.1460-2075.1983.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Alanen M., Söderlund H. A complex of single-strand binding protein and M13 DNA as hybridization probe. Nucleic Acids Res. 1985 Apr 25;13(8):2789–2802. doi: 10.1093/nar/13.8.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Tchen P., Ranki M., Söderlund H. Time-resolved fluorometry: a sensitive method to quantify DNA-hybrids. Nucleic Acids Res. 1986 Jan 24;14(2):1017–1028. doi: 10.1093/nar/14.2.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchen P., Fuchs R. P., Sage E., Leng M. Chemically modified nucleic acids as immunodetectable probes in hybridization experiments. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3466–3470. doi: 10.1073/pnas.81.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdlov E. D., Monastyrskaya G. S., Guskova L. I., Levitan T. L., Sheichenko V. I., Budowsky E. I. Modification of cytidine residues with a bisulfite-O-methylhydroxylamine mixture. Biochim Biophys Acta. 1974 Mar 8;340(2):153–165. [PubMed] [Google Scholar]
- Virtanen M., Palva A., Laaksonen M., Halonen P., Söderlund H., Ranki M. Novel test for rapid viral diagnosis: detection of adenovirus in nasopharyngeal mucus aspirates by means of nucleic-acid sandwich hybridisation. Lancet. 1983 Feb 19;1(8321):381–383. doi: 10.1016/s0140-6736(83)91500-3. [DOI] [PubMed] [Google Scholar]
- Virtanen M., Syvänen A. C., Oram J., Söderlund H., Ranki M. Cytomegalovirus in urine: detection of viral DNA by sandwich hybridization. J Clin Microbiol. 1984 Dec;20(6):1083–1088. doi: 10.1128/jcm.20.6.1083-1088.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Woolley P., Wills P. R. Excluded-volume effect of inert macromolecules on the melting of nucleic acids. Biophys Chem. 1985 Jun;22(1-2):89–94. doi: 10.1016/0301-4622(85)80029-6. [DOI] [PubMed] [Google Scholar]


