Abstract
Recently we showed that ABA is at least partly responsible for the induction of the polyamine exodus pathway in Vitis vinifera plants. Both sensitive and tolerant plants employ this pathway to orchestrate stress responses, differing between stress adaptation and programmed cell death. Herein we show that ABA is an upstream signal for the induction of the polyamine catabolic pathway in Vitis vinifera. Thus, amine oxidases are producing H2O2 which signals stomata closure. Moreover, the previously proposed model for the polyamine catabolic pathway is updated and discussed.
Key words: plant growth, abscissic acid, polyamines, amine oxidases, signaling, oxidative stress, programmed cell death
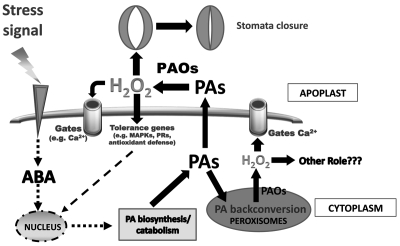
We have shown that tobacco salinity induces an exodus of the polyamine (PA) spermidine (Spd) into the apoplast where it is oxidized by polyamine oxidase (PAO) generating hydrogen peroxide (H2O2). Depending on the size of H2O2, it signals either tolerance-effector genes or the programmed cell death syndrome1 (PCD). PAs are ubiquitous and biologically active molecules. In the recent years remarkable progress has been accomplished regarding the regulation of PAs biosynthesis and catalysis, not only under normal physiological but also under stress conditions.1 The most studied PAs are the diamine Putrescine (Put) and its derivatives the triamine Spd and the tetramine spermine (Spm). They are present in the cells in soluble form (S), or conjugated either to low molecular weight compounds (soluble hydrolyzed form, SH) or to “macro” molecules or cell walls (pellet hydrolyzed form, PH). In higher plants, Put is synthesized either directly from ornithine via ornithine decarboxylase (ODC; EC 4.1.1.17) or indirectly from arginine via arginine decarboxylase (ADC; EC 4.1.1.19). Spd and Spm are synthesized via Spd synthase (EC 2.5.1.16, SPDS) and Spm synthase (EC 2.5.1.22, SPMS), respectively, by sequential addition of aminopropyl groups to Put, catalyzed by S-adenosyl-L-methionine decarboxylase (SAMDC; EC 4.1.1.50).2,3 In plants, PAs are present in the cytoplasm, as well as in cellular organelles.4 Recently it was shown that during stress, they are secreted into the apoplast where they are oxidized by amine oxidases (AOs), such as diamine oxidase for Put (DAO, E.C. 1.4.3.6) and polyamine oxidase (PAO, E.C. 1.4.3.4) for Spd and Spm.1,5,6 Oxidation of PAs generates, amongst other products, H2O21,7,8 which is involved in cell signaling processes coordinated by abscissic acid (ABA),9 but also acts as efficient oxidant and, at high concentration, orchestrates the PCD syndrome.6,10 Two types of PA catabolism by PAO are known in plants: the terminal and the back-conversion pathways. The terminal one takes place in the apoplast, produces except H2O2, 1,3-diaminopropane and an aldehyde depending on the species. On the other hand, the back-conversion pathway is intracellular (cytoplasm and peroxisomes) resulting to the production of H2O2 and the sequential production of Put by Spm via Spd.1,7 Now we have shown that PA exodus also occurs in Vitis vinifera and this phenomenon is at least partially induced by abscissic acid (ABA).11 Thus, exogenous application of ABA results to PA exodus into the apoplast of grapevine. PA is oxidized by an AO resulting to production of H2O2. When the titer of H2O2 is below a threshold, expression of tolerance-effector genes is induced, while when it exceeds this threshold the programmed cell death (PCD) syndrome is induced.
Stress Conditions Facilitate the Induction of the Polyamine Exodus Pathway
Under stress conditions, the stress signal is perceived and PA homeostasis is re-adjusted in order to further orchestrate stress responses. The perceived signal involves the ABA signaling pathway.11 With respect to the PA catabolic pathway, Moschou et al.1 and Toumi et al.11 showed that stress induces exodus of PAs into the apoplast, were they are catabolized by PAO. Grape cultivars showing contrasting tolerance to drought stress exhibit differential PA biosynthesis rate and titers. In both, the tolerant and the sensitive genotypes, stress activates the exodus route of PAs, but only in the tolerant one the higher PA biosynthetic rate in the cellular compartment eliminates potential detrimental effects exerted by PAO-derived H2O2 in the apoplast. On the contrary, in the sensitive genotype intracellular homeostasis of PAs is not restored, and their levels are insufficient to ameliorate the intervening effects of H2O2; thus, the PCD syndrome is signaled and executed.11
Polyamines under stress could affect vital cellular functions varying from development and growth to PCD. For instance under salinity, excessive amounts of ions are absorbed by cells resulting to osmotic and toxic phenomena which are sensed by cells and are translated as cascades of molecular signals, mainly under the control of ABA.12 The response of PAs to ABA was shown in Arabidopsis13 and results show that, in contrast to wild type, neither the ABA mutant, aba2-3, nor the ABA insensitive, abi1-1, plants were able to regulate the PAs biosynthetic genes; exogenous application of ABA to these lines resulted to higher ADC2 and SAMDC levels, suggesting that PAs upregulation is ABA-dependent under stress.13
Correlation of Polyamine Exodus Pathway with Biotic Stress Responses and Morphogenesis
Recently, the PA exodus pathway was also correlated with biotic stress tolerance. Engineering the PA catabolic pathway in tobacco to increase the generation of H2O2, by overexpression of the PAO gene, resulted in tolerance to certain pathogens, such as Pseudomonas syringae and Phytopthtora infestans.14 Increased expression of PAO gene and PAO protein led to the induction of an array of defensive proteins; the two major proteins induced were the salicylic-inducible protein kinase (SIPK) and wound inducible protein kinase (WIPK). These two MAPKs activate a series of proteins involved in defense organization. Moreover, PA catabolism by PAO was correlated with the elongation of pollen tube in Arabidopsis and Pyrus pyrifolia, since loss-of-function mutant alleles of the AtPAO3 gene showed reduced seed set due to defects in pollen tube growth.15 In this study, PAO derived-H2O2 through Spd oxidation was shown to induce a Ca2+ permeable channel in the pollen tube tip which is crucial for proper pollen tube elongation. Thus, Spd derived-H2O2 through PAO may play a crucial role in the induction of the stress-dependent Ca2+ permeable channels.
In the grapevine (Vitis vinifera L.) exogenous Spd resulted to H2O2 accumulation as documented using the cell-permeable 2′,7′-dichlorfluorescein-diacetate (DCFH-DA) H2O2-specific dye in cell suspension cultures (Fig. 1A). Application of aminoguanidine and guazatine, DAO and PAO potent inhibitors respectively, abrogated accumulation of H2O2, and the same effect was observed when supplementing the cell suspension culture with ascorbate, a H2O2 scavenger (Fig. 1A). PAO activity resulted to H2O2 accumulation in planta as shown in leaves by DAB staining (Fig. 1B). More importantly, DAB deposits forming in the presence of H2O2 were localized primarily in the vascular tissue (Fig. 1B). Transmission electron microscopy (TEM) specific for H2O2 (coupled with CeCl3 staining) revealed that the apoplast is the main location of PAs oxidation in grapevine (Fig. 1C). Furthermore, results by scanning electron microscopy (SEM) showed that in Spdtreated leaf epidermis, Spd induced stomata closure (Fig. 1D), which was restricted by simultaneous application of aminoguanidine and guazatine (Fig. 1D). Additionally, post-treatment with Spd, H2O2 was shown to be localized particularly in guard cells (Fig. 1E). Previously, An et al.17 showed that DAO is involved in the ABA-induced stomata closure in Vicia faba. Paschalidis et al.18,19 showed that in Nicotiana tabacum plants PAO activity correlates with vascular tissues as observed here for Vitis vinifera. These results reinforce the view of direct involvement of PAOs-derived H2O2 in stomata closure signaling cascade. Moreover, it seems likely that PAOs are a downstream target of the ABA signaling network.
Figure 1.
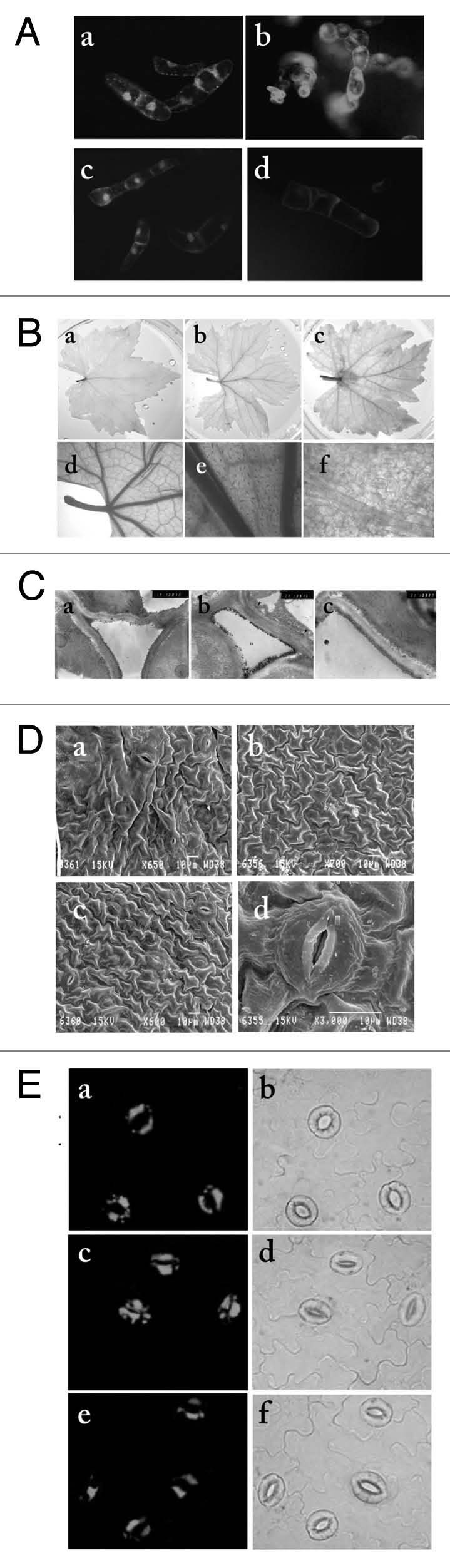
Polyamine oxidase-induced H2O2 accumulation by Spermidine oxidation in Vitis vinifera. (A) In situ H2O2 epifluorescne in control grapevine cells (a), in cells treated with 10 mM Spd for 1 h (b), in cells treated with 10 mM Spd, 0.5 mM aminoguanidine and 0.5 mM guazatine for 1 h (c) and in cells treated with 10 mM Spd and 1 mM ascorbate for 1 h (d). (B) In situ DAB detection of H2O2 in fully developed leaves (15th) of grapevine. Control leaf (a); leaf infiltrated with 10 mM Spd for 10 min prior to DAB infiltration (b); leaf infiltrated with 10 mM Spd for 1 h prior to DAB infiltration (c); close-up pictures of (b) showing H2O2 accumulation in secondary veins (d and e) and in closed stomata (f). (C) Hydrogen peroxide detection by transmission Electron microscopy (TEM) in control leaf (a), leaf treated with 10 mM Spd for 1 h (b) and with 10 mM Spd, 0.5 mM aminoguanidine and 0.5 mM guazatine for 1 h (c). Hydrogen peroxide was observed as cerium perhydroxide deposits (CPD) in vascular parenchyma cells. In (b), staining was heavy in cell corner middle lamella and significantly increased in cell walls and in plasma membrane (arrow heads). Staining was sparse in (a) and even lower in (c). CML, cell corner middle lamella; CW, cell wall; IS, intercellular space. (D) Scanning electron microscopy (SEM) of control leaves (a), leaves treated with 10 mM Spd for 1 h (b) and leaves treated with Spd supplemented with 0.5 mM aminoguanidine and 0.5 mM guazatine (c and d). (E) In situ Spd-induced H2O2 accumulation in guard cells and stomatal closure. DCFH-DA fluorescence in control strips (a), in leaf strips treated with 10 mM Spd (c) and in strips treated with 0.5 mM aminoguanidine and 0.5 mM guazatine (e). (b, d and f) bright field images of (a, c and e), respectively. Methods were described previously in Moschou et al.1
An Emerging New Picture for Polyamine Catabolism
PA catabolism seems that it is not simply a biochemical process in plants. The products of this reaction and especially the reactive oxygen species H2O2 seems to actively participate in the signaling network with numerous targets. In our previous work and herein, we show that PA cellular homeostasis is tightly controlled via PAs sequestration, by regulation of the biosynthetic and catabolic PA genes to facilitate H2O2 generation.1 Hydrogen peroxide, depending on its ‘size’, signals different cellular processes ranging from induction of tolerance-effector and defensive genes to PCD. In addition, new developmental roles for PA catabolism are revealed. A paradigm is the involvement of Spd catabolism on pollen tube elongation. Downstream targets of Spd-derived H2O2 are the Ca2+ channels.15 Furthermore, another downstream target of ABA is the exodus of PAs into the apoplast where they are oxidized by PAOs to generate H2O2. Moschou et al.16 reported that ABA is inducing AtPAOs in Arabidopsis, enhancing the back-conversion pathway of PAs in peroxisomes. Although the peroxisomal pathway is functionally divergent from the apoplastic PA catabolic pathway described by Moschou et al.1 and Toumi et al.11 both pathways are induced by ABA (Fig. 2).
Figure 2.
Proposed model for PAO-derived H2O2 participation in stress and development. That PAO correlates with development was previously highlighted by Paschalidis and Roubelakis-Angelakis18,19 and Paschalidis et al.20 During development, intrinsic clues induce PA catabolism and generation of H2O2. This molecule seems to act as signal to activate Ca2+ channels,15 and stomata closure (herein). Higher Spd titers or increased PAO activity results to high levels of H2O2 that activate Ca2+ channels, but the Ca2+ influx is either not restricted or the influx is exceedingly high leading to deleterious effects.15 Under stress, PA exodus into the apoplast is signaled where it is oxidized by PAO. The generated H2O2 induces either expression of tolerance-effector genes or the PCD syndrome.1 The decision depends both, on the size of the H2O2 and on the restoration of intracellular PA homeostasis, which is brought about by the simultaneous induction of the PA biosynthetic genes.1 All the previous reinforce the view that only optimal oxidation of Spd by PAO can lead to normal molecular responses. Moreover, in the case of the control of cation channels the back-conversion pathway seems to possess significant role,15 but yet a role for this pathway in other responses, like effector-genes induction remains to be established.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12679
References
- 1.Moschou PN, Paschalidis KA, Delis ID, Andrioupoulou AH, Lagiotis GD, Yakoumakis DI, et al. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that depict tolerance. Plant Cell. 2008;20:1708–1724. doi: 10.1105/tpc.108.059733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-Tanguy J. Metabolism and function of polyamines in plants: recent development (new approaches) Plant Growth Reg. 2001;34:135–148. [Google Scholar]
- 3.Wang P, Song CP. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 2007;178:703–718. doi: 10.1111/j.1469-8137.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 4.Kusano T, Yamaguchi K, Berberich T, Takahashi Y. Advances in polyamine research. J Plant Res. 2007;120:345–350. doi: 10.1007/s10265-007-0074-3. [DOI] [PubMed] [Google Scholar]
- 5.Sebela M, Radova A, Angelini R, Tavladoraki P, Frebort I, Pec P. FAD-containing polyamine oxidases: a timely challenge for researchers in biochemistry and physiology of plants. Plant Sci. 2001;160:197–207. doi: 10.1016/s0168-9452(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 6.Yoda H, Yoshinobu H, Hiroshi S. Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 2006;142:193–206. doi: 10.1104/pp.106.080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moschou PN, Sammartin M, Andriopoulou AH, Rojo E, Sanchez-Serrano JJ, Roubelakis-Angelakis KA. Bridging the gap between plant and mammalian polyamine catabolism: A novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in Arabidopsis. Plant Physiol. 2008;147:1845–1857. doi: 10.1104/pp.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asthir B, Duffus CM, Smith RC, Spoor W. Diamine oxidase is involved in H2O2 production in the chalazal cells during barley grain filling. J Exp Bot. 2002;53:677–682. doi: 10.1093/jexbot/53.369.677. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–1448. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan LJ, Zhang B, Shi WW, Li HY. Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol. 2008;50:2–18. doi: 10.1111/j.1744-7909.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 11.Toumi I, Moschou PN, Paschalidis KA, Bouamama B, Ben Salem-Fnayou A, Ghorbel AW, et al. Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway. J Plant Physiol. 2010;167:519–525. doi: 10.1016/j.jplph.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Davies WJ, Kudoyarova G, Hartng W. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant's response to drought. J Plant Growth Regul. 2005;24:285–295. [Google Scholar]
- 13.Alcazar R, Cuevas JC, Patron M, Altabella T, Tiburcio AF. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol Plant. 2006;128:448–455. [Google Scholar]
- 14.Moschou PN, Sarris PF, Scandalis N, Andriopoulou AH, Paschalidis KA, Panopoulos NJ, Roubelakis-Angelakis KA. Engineered polyamine catabolism pre-induces SA-independent immunity and enhances tolerance to bacteria and fungi in tobacco. Plant Physiol. 2009;149:1970–1981. doi: 10.1104/pp.108.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Qu H, Shang Z, Jiang X, Moschou PN, Roubelakis-Angelakis KA, et al. Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant J. 2010 doi: 10.1111/j.1365-313X.2010.04301.x. In press. [DOI] [PubMed] [Google Scholar]
- 16.Moschou PN, Delis ID, PAschalidis KA, Roubelakis-Angelakis KA. Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol Plant. 2008;133:140–156. doi: 10.1111/j.1399-3054.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 17.An Z, Jing W, Liu Y, Zhang W. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J Exp Bot. 2008;59:815–825. doi: 10.1093/jxb/erm370. [DOI] [PubMed] [Google Scholar]
- 18.Paschalidis KA, Roubelakis-Angelakis KA. Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age, cell division/expansion and differentiation. Plant Physiol. 2005;138:142–152. doi: 10.1104/pp.104.055483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paschalidis KA, Roubelakis-Angelakis KA. Sites and regulation of polyamine catabolism in the tobacco plant. Correlations with cell division/expansion, cell cycle progression and vascular development. Plant Physiol. 2005;138:2174–2184. doi: 10.1104/pp.105.063941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paschalidis KA, Moschou PN, Toumi I, Roubelakis-Angelakis KA. Polyamine anabolic/catabolic regulation along the woody grapevine plant axis. J Plant Physiol. 2009;166:1508–1519. doi: 10.1016/j.jplph.2009.03.013. [DOI] [PubMed] [Google Scholar]