Abstract
Understanding how plants sense and respond to heat stress is central to improve crop tolerance and productivity. Recent findings in Physcomitrella patens demonstrated that the controlled passage of calcium ions across the plasma membrane regulates the heat shock response (HSR). To investigate the effect of membrane lipid composition on the plant HSR, we acclimated P. patens to a slightly elevated yet physiological growth temperature and analysed the signature of calcium influx under a mild heat shock. Compared to tissues grown at 22°C, tissues grown at 32°C had significantly higher overall membrane lipid saturation level and, when submitted to a short heat shock at 35°C, displayed a noticeably reduced calcium influx and a consequent reduced heat shock gene expression. These results show that temperature differences, rather than the absolute temperature, determine the extent of the plant HSR and indicate that membrane lipid composition regulates the calcium-dependent heat-signaling pathway.
Key words: acclimation, calcium signaling, fatty acids, moss, Physcomitrella patens, stress response
In the context of the current global warming, an increase in the global temperature and in the frequency and severity of heat waves is expected to affect crops productivity and distribution.1–4 Land plants constantly encounter wide daily and seasonal thermal variations and the agricultural yields are tightly correlated to their effective tolerance to thermal stress.5,6 To face this environmental challenge and develop new strategies, it is crucial to fully understand the mechanisms by which mild variations in ambient temperature are accurately perceived, leading to a timely activation of the heat shock proteins (HSPs) and the establishment of an optimal thermotolerance. Temperature sensing in plants and other organisms has been the subject of numerous studies.5–7 For many years, the activation of HSPs following a sharp temperature increase was thought to be regulated by denatured cytosolic proteins, that upon sequestering Hsp70 and Hsp90, would derepress heat shock transcription factors (HSFs), thereby inducing the upregulation of heat shock genes.8–10 Yet, the overexpression of HSPs, so called the heat shock response (HSR), can be activated under mild physiological conditions that are unlikely to cause any protein denaturation in the cell.11–13 Although multiple HSR triggering mechanisms may co-exist,6,14–16 recent evidences in bacteria, algae, plants and mammalian cells point at the plasma membrane as being the most sensitive, earliest upstream responder to a mild temperature elevation.17–20
Cellular membranes are extremely sensitive to diverse environmental stimuli.21 The existence of membrane-associated thermosensors was repeatedly suggested but the exact nature of sensor components is still unknown. We recently showed that specific calcium channels in the moss P. patens plasma membrane regulate the heat signaling pathway. These channels regulate the onset of the acquired thermotolerance in the plant by controlling the intensity of the HSR via a preceding Ca2+ influx.20 Here, we report the effect of modulation of membrane lipid composition on the moss plant heat-induced calcium signature and HSR.
Membrane Fatty Acid Composition Change in Mosses Pre-adapted to Different Temperatures
Acclimation to different growth temperatures was shown to affect the intensity of the HSR.20 When organisms are exposed to mildly elevated temperatures, specific rearrangements of the membrane fatty acid composition occur to protect vital membrane-related processes, such as lipid saturation, preventing membrane disruption during heat-induced hyperfluidization.22 To investigate the effect of such rearrangements in the membrane composition on the heat-sensing and signaling pathway, we first measured the level of lipid unsaturation in membranes of P. patens grown at 22°C or 32°C for ten days.
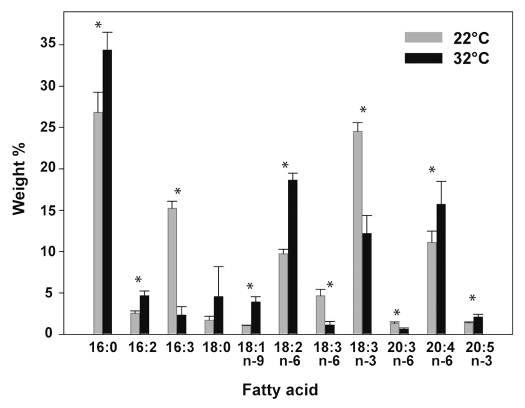
The total fatty acids (FA) were extracted and the abundance of the different species was estimated (Fig. 1). Membrane composition in tissues grown at 32°C showed a clear remodelling of membrane FAs in response to elevated growth temperature. In plants acclimated to higher growth temperature, we observed a general decrease in polyunsaturated FAs (PUFA), in particular within the C16 and C18 FAs. The most pronounced change occurred in 16:3 and α-Linolenic acid, 18:3 n-3, whose amounts were respectively decreased by 86% and by half at 32°C, as compared to plants grown at 22°C (Fig. 1). Conversely, the proportion of diunsaturated FAs, 18:2 and 16:2, and also the monounsaturated oleic acid (18:1) displayed an interesting opposite response and their levels almost doubled in plants grown at 32°C, as compared to plants grown at 22°C (Fig. 1). Similarly, the saturated palmitic acid, 16:0, displayed a 60% increase after ten days at 32°C. In the C20 FA series we were able to detect only polyunsaturated species. Although the trienoic acid, 20:3 n-6, decreased as growth temperature increased, like C16 and C18 analogs, the most abundant C20 FA, arachidonic acid (20:4 n-6, as well as 20:5 n-3), showed opposite changes (Fig. 1). The level of 20:4 significantly increased by 45% at 32°C compared to 22°C. These long-chain polyunsaturated FAs are abundant in P. patens and other algae, compared to higher plants.23 Their exact role during plant acclimation to higher temperatures is not known. The observation that C16, C18 and C20 FAs displayed distinct patterns, suggests that individual classes of FA have specific roles in maintaining optimal membrane functions. The overall membrane unsaturation level was expressed using the double bond index/saturated fatty acids ratio (DBI/sat).24 The DBI/sat significantly decreased as growth temperature increased (Fig. 3), indicating that at the higher temperatures, membranes are enriched with more saturated FAs. This effect is likely to reduce the non-bilayer propensity25 and increase the rigidity of the moss membranes thereby optimizing the dynamics of membrane associated processes during a heat-shock.26 Our findings are consistent with previous reports in Arabidopsis thaliana, where similar changes in individual FA species were observed when the growth temperature was shifted above 30°C.27 This suggests that the mechanisms leading to the remodelling of membrane lipids during temperature acclimation are conserved across land plants.
Figure 1.
Membrane lipid composition is modulated by the growth temperature. Percentage of saturated versus unsaturated fatty acids in P. patens membranes measured from tissues grown for ten days at 22°C or 32°C. Fatty acids were quantified by GC-MS and values are expressed as percentage of total weight and expressed as means ± S.D. (n = 4). Significant changes (p < 0.05) were marked by *.
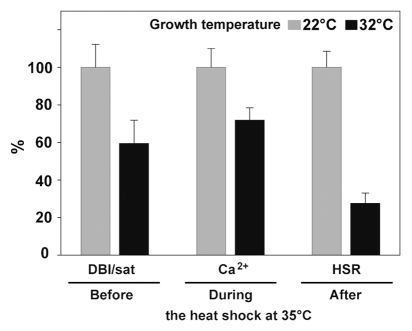
Figure 3.
Correlation of the unsaturation level of membrane FAs, maximal Ca2+ influx and the extent of HSR in tissues grown at 22 or 32°C. Sequential events occurring before, during and after 1 h heat shock at 35°C in tissues acclimated at indicated temperatures. Values of 22°C-grown tissues from Figures 1 and 2 were normalized to 100%. DBI/sat: Double Bond Index/saturated fatty acids ratio measured before the application of the HS. Ca2+: values of maximal cytosolic calcium concentration at the 15th minute of the HS. HSR: GUS specific activities from HSP-GUS line reflecting the extent of the HSR 8 h following the HS.
Heat-induced Ca2+ Influx is Influenced by the Growth Temperature
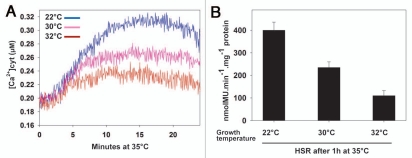
We have shown earlier that the extent of the HSR is tightly controlled by the intensity of a preceding heat-induced Ca2+ influx across the plasma membrane.20 To test whether plant membrane composition has an effect on the heat-induced Ca2+ entry, we monitored the fluctuations of Ca2+ concentrations during an abrupt temperature upshift in tissues grown at various temperatures (Fig. 2A). The UBI-AEQ moss line that constitutively expresses Aequoria victoria apoaequorin reporter,20 was grown either at 22, 30 or 32°C for ten days and then heat-treated at 35°C. Monitoring the relative cytoplasmic calcium concentration during the first minutes of temperature increase, showed that the amplitude of the heat-induced calcium influx in tissues pre-adapted at 30°C and 32°C was reduced compared to 22°C (Fig. 2A). In 32°C-grown tissues, the maximal Ca2+ amplitude, observed at the 15th minute of the temperature shift up to 35°C, was 30% lower than in tissues grown at 22°C challenged with the same heat-shock temperature (Figs. 2A and 3). This lower calcium transient correlated well with the consequent milder activation of the Hsp17.3B promoter observed in HSP-GUS line28 grown at the same temperatures and submitted to 1 h heat shock at 35°C (Fig. 2B). This indicates that exposure to a given elevated temperature (here 35°C) does not exclusively determine the intensity of Ca2+ entry, nor the subsequent activation of heat shock genes. Rather, the growth temperature plays an important role, likely by modulating the membrane composition and membrane physical state, which in turn influences the dynamics of the membrane-associated heat sensor. These findings are in line with earlier reports that membrane fluidizers, such as benzyl alcohol, cause a HSR at non-inducing temperatures in plants and bacteria.11,12,28 Thus, in Escherichia coli19 and Synechocystis29 preadaptation to different growth temperatures modulated the subsequent heat-induced expression levels of major classes of HSPs and affected thermotolerance.
Figure 2.
Effect of growth temperature on heat-induced calcium influx. (A) UBI-AEQ moss tissues were grown at 22, 30 or 32°C for ten days and the cytosolic calcium concentrations was measured during a heat shock at 35°C. Luminescence counts were integrated every 3 s. Displayed traces are representative of three replicates. (B) Hsp17.3B promoter activation reflected by specific GUS activity in HSP-GUS tissues grown at 22°C, 30°C or 32°C for ten days and heat shocked 1 h at 35°C. The values shown were measured after 8 h recovery at 22°C and correspond to means of three independent experiments. The standard deviations are shown.
Materials and Methods
Plant material and growth conditions.
Physcomitrella patens (Gransden wild type) was grown on moss solid minimal medium overlaid with a cellophane disk as in Saidi et al.28 and maintained in a temperature-controlled chamber at indicated temperatures and durations. HSP-GUS and UBI-AEQ lines were previously described in Saidi et al.28 and Saidi et al.20 respectively. To perform heat shocks, protonemal tissues were transferred to liquid minimal medium using Costar multiwell plates (Corning Incorporated, Corning, NY) as detailed in Saidi et al.20
Fatty acid analysis.
Moss tissues were grown under different temperatures using a solid minimal medium lacking ammonium tartrate. This will reduce the proportion of thylakoid membranes in the samples by reducing the density of chloroplast-enriched cells (chloronemata) and increasing the proportions of caulonemata, containing fewer chloroplasts. Total FAs were extracted as in Folch et al.30 with the following modifications: Grounded P. patens tissues were heated in 2 mL isopropanol for 5 min at 85°C. After temperature cooled down, 2 mL methanol (containing 0.001% butylated hydroxytoluene as antioxidant) and 8 mL chloroform were added. The mixture was left at room temperature for one hour with regular vortexing. Afterwards, 3 mL 0.2 M KCl was added to allow phase separation to occur [final composition of chloroform:alcohol:water (2:1:0.75, v/v)]. After vigorous vortexing, the sample was centrifuged at 600 g for 10 min. The lower chloroform phase was evaporated and the residue was resolubilized in chloroform:methanol (2:1, v/v). The extracted lipids were transmethylated in 1 ml 5% acetyl chloride in methanol, at 80°C for 2 h and the resulting fatty acid methyl esters were extracted with hexane. The total fatty acid composition was determined by a gas chromatography-mass spectrometry (GC-MS) system (GCMS-QP2010, Shimadzu) equipped with a BPX-70 capillary column (10 m × 0.1 mm ID, 0.2 µm film thickness) maintained at 150°C for 2 min, programmed at 6°C/min to 215°C, then at 20°C/min to 235°C and maintained isothermally for 2 min. Identification and calibration of peaks was made by comparison with authentic standards and confirmed with NIST (National Institute of Standards and Technology, Gaithersburg, MD) MS library spectra.
In vivo reconstitution of aequorin and Ca2+-dependent luminescence measurements.
In order to monitor calcium transients, reconstitution of aequorin was performed in vivo by incubating UBI-AEQ protonemal tissues, in darkness, for one hour in standard moss liquid medium supplemented with 3 µM coelenterazine followed by brief three times washing. The heat shock of UBI-AEQ tissues was performed in liquid minimal medium (containing 2 mM CaCl2) using SCREENMATES 96 well microtiter plates (Thermo Fisher Scientific, Hudson, NH), directly in the 1420 VICTOR light luminometer (PerkinElmer, Waltham, MA). The temperature in the medium was continuously recorded using YF-160 type-K thermocouple (Eppendorf, Hamburg, Germany). Luminescence counts (CPS) were integrated every 3 s and, at the end of each monitoring, a discharging solution (0.1 M CaCl2, 10% ethanol, 2% Nonidet P40) was added to measure remaining active aequorin in the sample. The concentration of cytosolic calcium was determined by calculating the pCa using the equation described in Plieth.31 Displayed traces are representative of three replicates.
GUS assays.
GUS specific activities were determined using 2 mM of the MUG substrate, as in Saidi et al.20 GUS activity was expressed in nmol of hydrolyzed 4-Methylumbelliferone (MU) produced per minute per milligram of total soluble proteins. GUS activities were measured eight hours after the heat shocks. The GUS activity values are means of three independent experiments and standard deviations are shown. Soluble protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA) according to the to manufacturer's instructions.
Conclusions
The analysis of the membrane lipid composition in the moss P. patens adapted to slightly elevated growth temperatures showed a significant decrease in the level of unsaturation of membrane FAs, an increase in the membrane rigidity that explains the lower heat-induced Ca2+-influx. This attenuated calcium signature in turn correlates with an attenuated outcome of the heat shock signaling pathway, as revealed by the reduced HSP-gene expression (Fig. 3). The sequential events described in here add to a growing body of evidence that sets the plasma membrane as one of the main cellular component of sensing thermal stress. In conclusion, our working hypothesis for future research is a scheme whereby a mild temperature upshift transiently increases the fluidity of the plant cell plasma membranes, that transiently opens specific Ca2+ channels, yet to be identified. Crossing Ca2+ ions bind to calmodulin-binding domains that activate a specific calmodulin- and kinase-dependent signaling pathway, that activates heat-shock factors, which upregulate HSP expression. This improves plant acquired thermotolerance32 (Sup. Fig. 1).
Acknowledgements
We are grateful to the technical help of Maude Muriset. This research was financed by Grant 3100A0-109290 from the Swiss National Science Foundation and by the Hungarian National Scientific Research Foundation (OTKA NK 68379 and OTKA NN 76716).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13163
Supplementary Material
References
- 1.Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;11:1–4. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Long SP, Ort DR. More than taking the heat: crops and global change. Curr Opin Plant Biol. 2010;13:240–247. doi: 10.1016/j.pbi.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Yi Z, Hong-Bo S. The responding relationship between plants and environment is the essential principle for agricultural sustainable development on the globe. Comptes Rendus Biologies. 2008;331:321–328. doi: 10.1016/j.crvi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Lobell DB, Asner GP. Climate and management contributions to recent trends in US agricultural yields. Science. 2003;299:1032. doi: 10.1126/science.1078475. [DOI] [PubMed] [Google Scholar]
- 5.Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, et al. Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci. 2004;29:471–487. doi: 10.1007/BF02712120. [DOI] [PubMed] [Google Scholar]
- 6.Hua J. From freezing to scorching, transcriptional responses to temperature variations in plants. Curr Opin Plant Biol. 2009;12:568–573. doi: 10.1016/j.pbi.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Fukao Y, Hayashi M, Fukazawa M, Suzuki I, Nishimura M. Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J Biol Chem. 2007;282:37794–37804. doi: 10.1074/jbc.M707168200. [DOI] [PubMed] [Google Scholar]
- 11.Saidi Y, Domini D, Choy F, Zryd JP, Schwitzguebel JP, Goloubinoff P. Activation of the heat shock response in plants by chlorophenols: transgenic Physcomitrella patens as a sensitive biosensor for organic pollutants. Plant Cell Environm. 2007;30:753–763. doi: 10.1111/j.1365-3040.2007.01664.x. [DOI] [PubMed] [Google Scholar]
- 12.de Marco A, Vígh L, Diamant S, Goloubinoff P. Native folding of aggregation-prone recombinant proteins in Escherichia coli by osmolytes, plasmid- or benzyl alcohol-overexpressed molecular chaperones. Cell Stress Chaperones. 2005;10:329–339. doi: 10.1379/CSC-139R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vígh L, Maresca B, Harwood JL. Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem Sci. 1998;23:369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- 14.Volkov RA, Panchuk II, Mullineaux PM, Schöffl F. Heat stress-induced H(2)O(2) is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol. 2006;61:733–746. doi: 10.1007/s11103-006-0045-4. [DOI] [PubMed] [Google Scholar]
- 15.Kumar SV, Wigge PA. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell. 2010;140:136–147. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Xuan Y, Zhou S, Wang L, Cheng Y, Zhao L. Nitric oxide functions as a signal and acts upstream of AtCaM3 in thermotolerance in Arabidopsis seedlings. Plant Physiol. 2010;153:1895–1906. doi: 10.1104/pp.110.160424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horváth I, Glatz A, Varvasovszki V, Török Z, Pali T, Balogh G, et al. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci USA. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shigapova N, Török Z, Balogh G, Goloubinoff P, Vígh L, Horváth I. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem Biophys Res Commun. 2005;328:1216–1223. doi: 10.1016/j.bbrc.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 19.Balogh G, Horváth I, Nagy E, Hoyk Z, Benko S, Bensaude O, et al. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 20.Saidi Y, Finka A, Muriset M, Bromberg Z, Weiss YG, Maathuis FJ, et al. The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell. 2009;21:2829–2843. doi: 10.1105/tpc.108.065318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glatz A, Vass I, Los DA, Vígh L. The Synechocystis model of stress: From molecular chaperones to membranes. Plant Physiol Biochem. 1999;37:1–12. [Google Scholar]
- 22.Quinn PJ, Joo F, Vígh L. The role of unsaturated lipids in membrane structure and stability. Prog Biophys Mol Biol. 1989;53:71–103. doi: 10.1016/0079-6107(89)90015-1. [DOI] [PubMed] [Google Scholar]
- 23.Kaewsuwan S, Cahoon EB, Perroud PF, Wiwat C, Panvisavas N, Quatrano RS, et al. Identification and functional characterization of the moss Physcomitrella patens delta5-desaturase gene involved in arachidonic and eicosapentaenoic acid biosynthesis. J Biol Chem. 2006;281:21988–21997. doi: 10.1074/jbc.M603022200. [DOI] [PubMed] [Google Scholar]
- 24.Sineriz F, Bloj B, Farias RN, Trucco RE. Regulation by membrane fluidity of the allosteric behavior of the (Ca2)-adenosine triphosphatase from Escherichia coli. J Bacteriol. 1973;115:723–726. doi: 10.1128/jb.115.3.723-726.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horváth I, Mansourian A, Vígh L, Thomas P, Joo F, Quinn PJ. Homogeneous catalytic hydrogenation of the polar lipids of pea chloroplasts in situ and the effects on lipid polymorphism. Chem Phys Lipids. 1986;39:251–264. [Google Scholar]
- 26.Vígh L, Escriba PV, Sonnleitner A, Sonnleitner M, Piotto S, Maresca B, et al. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res. 2005;44:303–344. doi: 10.1016/j.plipres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004;4:17. doi: 10.1186/1471-2229-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saidi Y, Finka A, Chakhporanian M, Zryd JP, Schaefer DG, Goloubinoff P. Controlled expression of recombinant proteins in Physcomitrella patens by a conditional heat-shock promoter: a tool for plant research and biotechnology. Plant Mol Biol. 2005;59:697–711. doi: 10.1007/s11103-005-0889-z. [DOI] [PubMed] [Google Scholar]
- 29.Lehel C, Gombos Z, Török Z, Vígh L. Growth temperature modulates thermotolerance and heat shock response of cyanobacterium Synechocystis PCC 6803. Plant Physiol Biochem. 1993;31:81–82. [Google Scholar]
- 30.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 31.Plieth C. Aequorin as a reporter gene. Methods in Molecular Biology Arabidopsis protocols. 2006;2:307–328. doi: 10.1385/1-59745-003-0:307. [DOI] [PubMed] [Google Scholar]
- 32.Ruelland E, Zachowski A. How plants sense temperature? Environ Exp Bot. 2010;69:225–232. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.