Abstract
Potential roles of salicylic acid (SA) on seed germination have been explored in many plant species. However, it is still controversial how SA regulates seed germination, mainly because the results have been somewhat variable, depending on plant genotypes used and experimental conditions employed. We found that SA promotes seed germination under high salinity in Arabidopsis. Seed germination of the sid2 mutant, which has a defect in SA biosynthesis, is hypersensitive to high salinity, but the inhibitory effects are reduced in the presence of physiological concentrations of SA. Abiotic stresses, including high salinity, impose oxidative stress on plants. Endogenous contents of H2O2 are higher in the sid2 mutant seeds. However, exogenous application of SA reduces endogenous level of reactive oxygen species (ROS), indicating that SA is involved in plant responses to ROS-mediated damage under abiotic stress conditions. Gibberellic acid (GA), a plant hormone closely associated with seed germination, also reverses the inhibitory effects of high salinity on seed germination and seedling establishment. Under high salinity, GA stimulates SA biosynthesis by inducing the SID2 gene. Notably, SA also induces genes encoding GA biosynthetic enzymes. These observations indicate that SA promotes seed germination under high salinity by modulating antioxidant activity through signaling crosstalks with GA.
Key words: arabidopsis, gibberellic acid, reactive oxygen species, salicylic acid, salt stress, seed germination
Pathogen infection and abiotic stresses, such as heat, drought and high soil salinity, significantly influence plant growth and crop productivity. Growth hormones, including abscisic acid (ABA) and gibberellic acid (GA), play an important role in plant responses to biotic and abiotic stresses. Salicylic acid (SA) is also closely related with abiotic stress responses as well as plant responses to pathogen infection. It has been recently reported that SA induces stress tolerance and improves plant growth under osmotic stress.1
Germination is a critical developmental stage for plant establishment and growth. When environmental conditions, including soil water content, oxygen and temperature, are favorable, seeds are released from dormancy and produce roots into the ground. Seed germination is quite sensitive to environmental conditions, because defense mechanisms are not fully established in the germinating seeds. Several growth hormones play a role in seed germination under stressful conditions. In particular, it has been reported that SA regulates seed germination under certain growth conditions.2–4 However, it is largely unknown how SA influences seed germination. Both promotive and repressive effects of SA have been reported in different studies, depending on plant genotypes and assay conditions employed.
Two major SA-deficient plants, sid2 mutant and NahG transgenic line, are frequently used in seed germination assays. Both have a defect in SA accumulation. However, they respond differentially to high salinity. While germination of the sid2 seeds is greatly delayed under high salinity, that of the NahG transgenic seeds is slightly accelerated under the same conditions. The NahG transgenic lines express a salicylate hydroxylase gene and SA is metabolized to catechol, causing SA deficiency.5 Catechol is known to possess an antioxidant property. It is therefore possible that catechol may affect plant responses to reactive oxygen species (ROS) produced under high salinity, explaining at least in part the differential germination responses of the sid2 mutant and NahG transgenic seeds to high salinity.
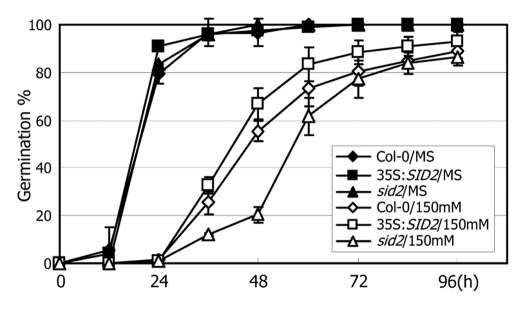
Under normal growth conditions, germination phenotypes of the sid2 mutant and 35S:SID2 transgenic seeds are indistinguishable from those of wild-type seeds. In contrast, germination of the sid2 seeds is significantly delayed under high salinity. Exogenous application of physiological concentrations of SA recovers the delayed seed germination, indicating that SA promotes seed germination under high salinity.6 Interestingly, germination of the 35S:SID2 transgenic seeds were delayed to a lesser degree in the presence of 150 mM NaCl (Fig. 1), suggesting that catechol may have a role in germination responses to high salinity.
Figure 1.
Effects of high salinity on seed germination. Seeds air-dried for two weeks at room temperature were routinely used for germination assays. They were imbibed at 4°C on MS-agar plates for three days in the dark and allowed to germinate at 22°C under long day conditions. Emergence of visible radicles was used as a morphological marker for germination. Three independent measurements, each consisting of 40 seeds, were averaged. Bars denote standard error of the mean. h, hours after cold-imbibition. To examine the effects of high salinity on germination, the seeds of the Col-0, 35S:SID2 and sid2 plants were cold-imbibed and germinated on MS-agar plates supplemented with 150 mM NaCl.
Oxidative damages caused by high salinity have been studied extensively in plants. Although ROS is generally perceived as toxic molecules, it has been shown that ROS also acts as signaling molecules when present at low concentrations.7 Previous studies have demonstrated that SA is closely related with ROS. A representative example is hypersensitive response (HR).8 When plants are infected by pathogens, SA stimulates ROS biosynthesis to induce cell death on the infected region. However, the functional relationship between SA and ROS is poorly understood in plant responses to abiotic stresses. In Arabidopsis, it has been suggested that SA is linked with ROS-mediated damages under abiotic stress conditions.
It has been shown that SA increases ROS-mediated oxidative damage and induce H2O2 production.9 However, SA induces resistance to diverse abiotic stresses, suggesting that SA reduces ROS-mediated oxidative damages.1,10 We also found that high salinity generates hydrogen peroxide in germinating seeds.6 Endogenous contents of H2O2 in the sid2 seeds were higher both under normal and high salt conditions. However, SA of 1 µM reduced the levels of H2O2, strongly supporting that SA promotes seed germination under osmotic stress by reducing ROS. Consistent with the notion that SA reduces ROS, while peroxidase activities were elevated in the sid2 seeds compared to those in wild-type seeds under normal growth conditions, they were lower in the sid2 seeds exposed to high salinity.
Although expression of the NADPH oxidase genes, such as RESPIRATORY BURST OXIDASE HOMOLOG (Atrboh),11 were altered in the sid2 mutant under normal growth conditions, it was uninfluenced in the sid2 seeds as well as in the wild-type seeds under high salinity. The expression patterns of genes encoding antioxidant-metabolizing enzymes, such as VITAMIN C DEFFECTIVE 1 (VTC1), VTC2, CADMIUM SENSITIVE 2 (CAD2) and NONPHOTOCHEMICAL QUENCHING 1 (NPQ1),12 also exhibited no significant differences in the germinating seeds of the sid2 mutant and wild-type plants under high salinity, suggesting that the reduction of ROS by SA in the germinating seeds under high salinity is regulated by complicated antioxidant mechanism.
GA promotes seed germination under abiotic stress conditions. Seed germination and seedling establishment of the FsGASA4 transgenic plants overexpressing a GA-responsive gene are resistant to high salinity and oxidative and heat stresses.2 Applications of exogenous GA promotes seed germination under abiotic stress conditions. Interestingly, the SID2 gene is induced and endogenous content of SA is elevated in the transgenic plants. In contrast, the sid2 mutant is insensitive to GA, indicating that GA reduces the inhibitory effects of adverse environmental conditions during seed germination and seedling growth by inducing SA biosynthesis.
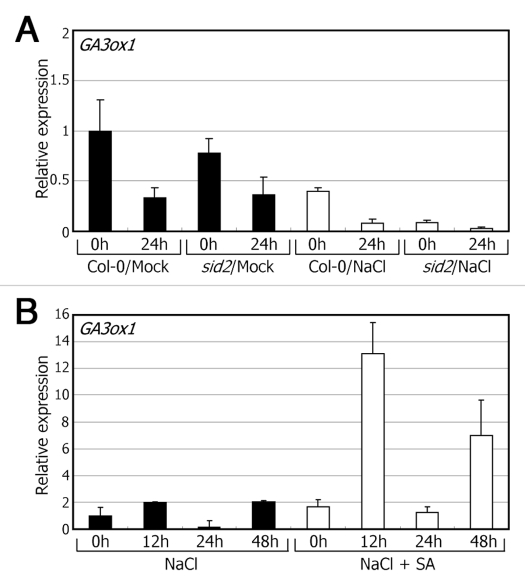
Meanwhile, our data suggest that endogenous levels of SA also influence GA biosynthesis. The transcript levels of the GA3 oxidase 1 (GA3ox1) gene, which is involved in GA biosynthesis,13 were lower in the sid2 seeds than in wild-type seeds under high salinity. In contrast, the expression patterns of the GA3ox1 gene were essentially identical in the wild-type and sid2 mutant seeds under normal growth conditions. Furthermore, the transcript levels of the GA3ox1 gene were greatly elevated in germinating wild-type seeds in the presence of 150 mM NaCl and 1 µM SA (Fig. 2). Together, these observations suggest that seed germination is promoted under high salinity through a positive feedback regulation of GA and SA signals.
Figure 2.
Analysis of transcript levels of the GA3ox1 gene. Seeds were allowed to germinate in the presence of 150 mM NaCl and 1 µM SA for the indicated time periods and used for extraction of total RNAs. Transcript levels were determined by quantitative real-time RT-PCR. Biological triplicates were averaged. Bars denote standard error of the mean. (A) Transcript levels of the GA3ox1 gene under high salinity. (B) Effects of SA on the transcription of the GA3ox1 gene under high salinity.
A small group of DELLA proteins, which act as negative regulators of GA signaling, have been extensively studied in GA-mediated plant growth and developmental processes. In germinating seeds, GA biosynthesis is induced and the RGL2 protein is degraded by the ubquitin/26S proteasome-dependent pathway.14 High salinity represses GA biosynthesis, causing delayed seed germination. However, the underlying molecular mechanisms have not been elucidated yet.
Taken together, it is evident that seed germination under high salinity is promoted by modulation of antioxidant activity by SA through signaling cross-talks with GA (Fig. 3). It will be interesting to examine how antioxidant activity is regulated and how SA is linked with GA signaling. Extensive measurements of various ROS species and examination of seed germination phenotypes of diverse mutants having defects in growth hormone signaling and biosynthesis under different growth diverse conditions will provide clues as to the molecular mechanisms underlying GA-SA signaling crosstalks during seed germination under stressful conditions.
Figure 3.
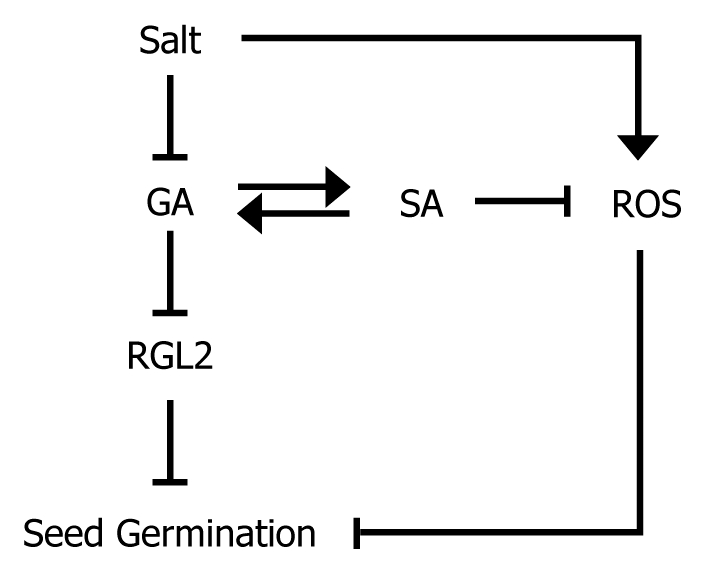
Schematic working model of SA in seed germination under high salinity. SA reduces ROS through signaling cross-talks with GA in seed germination under high salinity. It seems that the SA regulation of ROS may not be directly linked with known peroxidase pathways.
Acknowledgements
This work was supported by the Brain Korea 21 and Biogreen 21 (20080401034001) programs and by grants from the Plant Signaling Network Research Center (2010-0001453), the National Research Foundation of Korea (2009-0087317 and 2007-03415) and from the Agricultural R & D Promotion Center (309017-5), Korea Ministry for Food, Agriculture, Forestry and Fisheries. Sangmin Lee is grateful for the Seoul Science Fellowship Award.
Abbreviations
- ABA
abscisic acid
- GA
gibberellic acid
- GA3ox1
GA3 oxidase 1
- ROS
reactive oxygen species
- SA
salicylic acid
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13159
References
- 1.Singh B, Usha K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul. 2003;39:137–141. [Google Scholar]
- 2.Alonso-Ramírez A, Rodríguez D, Reyes D, Jiménez JA, Nicolás G, López-Climent M, et al. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 2009;150:1335–1344. doi: 10.1104/pp.109.139352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borsani O, Valpuesta V, Botella MA. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 2001;126:1024–1030. doi: 10.1104/pp.126.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajjou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C, et al. Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol. 2006;141:910–923. doi: 10.1104/pp.106.082057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, et al. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Kim SG, Park CM. Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol. 2010;188(2):626–637. doi: 10.1111/j.1469-8137.2010.03378.x. [DOI] [PubMed] [Google Scholar]
- 7.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trands Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 9.Harfouche AL, Rugini E, Mencarelli F, Botondi R, Muleo R. Salicylic acid induces H2O2 production and endochitinase gene expression but not ethylene biosynthesis in Castanea sativa in vitro model system. J Plant Physiol. 2008;165:734–744. doi: 10.1016/j.jplph.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Vlot AC, Dempsey DA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 11.Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006;140:1222–1232. doi: 10.1104/pp.105.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;149:1797–1809. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi S, Kamiya Y, Sun T. Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J. 2001;28:443–453. doi: 10.1046/j.1365-313x.2001.01168.x. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell. 2003;15:1591–1604. doi: 10.1105/tpc.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]