Abstract
MicroRNAs (miRNAs) have been recognized as important regulators in plant response to nutrient deficiencies. Of particular interest is the discovery that miR399 functions systemically in the maintenance of phosphate (Pi) homeostasis in response to external Pi fluctuation. Recent studies have further implicated both miR399 and sugars (mainly sucrose) as potential signal molecules in the shoot-to-root communication of phosphorus (P) status. Given that both miR399 and sucrose are transported via the phloem, their potential interaction (or cross-talk) along the signaling pathway is especially appealing for further exploration. In this mini-review, we highlight recent progress in unraveling crucial roles of both sucrose and miR399 in P-deficiency signaling. In particular, we further discuss recent findings that photosynthetic carbon (C) assimilation and subsequent partitioning, by overriding signaling of low external Pi, act as checkpoints upstream of miR399 for the onset of a systemic P-deficiency status.
Key words: sucrose, microRNA399, systemic signaling, P deficiency
Phosphorus (P) is an essential macronutrient for plant growth and development. Phosphate (Pi) availability is a limiting factor for crop productivity in many parts of the world's arable land.1 Because P fertilizer is a non-renewable resource and its mining is becoming ever more expensive, P has been recently highlighted as “the disappearing nutrient” of strategic importance in a recent NEWS FEATURE in the Nature.2
Plant acclimation to P deficiency is a highly coordinated process with an extensive re-programming of biochemical and metabolic pathways. Altered carbon allocation between shoots and roots is a hallmark of most P-deficient plants resulting in a higher root-to-shoot ratio. In this process, sucrose, the main form of carbon (C) source from shoots to roots, has also been implicated to act as a secondary messenger for shoot-to-root signaling of P status to regulate gene expression and Pi uptake in roots.3 Sucrose has been found to be either required for or to enhance P deficiency-regulated gene expression in several plant species.4–6 In recent years, microRNAs (miRNAs) have been recognized as crucial regulators in plant response to P deficiency. The mode of miRNA action is strictly based on the degree of sequence complementarity with target gene(s). It has been demonstrated that miR399 serves as a systemic signaling molecule in regulating systemic Pi homeostasis.7–9 Both sucrose and miR399 are phloemmobile.10–14 Several excellent reviews have been published recently to elucidate the roles of sucrose, miR399 and other aspects of P signaling.3,14–18 However, a paradox arises between the seemingly ubiquitous role of sucrose in signaling various nutrient deficiencies, including those of nitrogen (N) and P, and the stringent specificity of plant responses to a particular nutrient deficiency. Here, we summarize recent advances in understanding the roles of both sucrose and miR399, as modulated by light regime and phloem transport, and discuss how plants may adopt C as a “common currency”, primarily in the form of sucrose, to initiate specific responses to P deficiency by regulating miRNA399 expression.
Implications of Sucrose as a Signaling Molecule between Shoots and Roots
Photosynthetic C assimilation provides the primary C source for higher plants and is also the basis for the existence of most living beings on Earth. Plants have evolved a mechanism of coordinated responses to P deficiency by tightly coupling C assimilation and source-to-sink C allocation. The dual functions of sucrose as both a carrier of assimilated C and a messenger molecule have been elucidated previously in several articles.10,19,20 Phloem-transported sucrose provides the main form of C allocated from shoots (source) to roots (sink) to optimize root growth and development leading to a higher root-to-shoot ratio under P-limiting growth conditions.3,21–23 Sucrose levels in both shoots and roots are enhanced in P-deficient common bean and soybean plants.24–26 In other plants, such as Arabidopsis and white lupin, increased sucrose flux from shoots to roots via the phloem coincides with changes in root morphology and architecture, including a decrease in primary root length and an increase in lateral root growth.27–30 It has been proposed that sucrose increases root responsiveness to auxin to promote lateral root and root hair formation during P deficiency.31 Exogenous sucrose appears to function synergistically with P deficiency for increasing expression of P-responsive genes in Arabidopsis.5,32 Several high-affinity phosphate transporter genes, PHT2, PHT1;4 and PHT3;1, are induced by sucrose.33,34 In transgenic Arabidopsis lines expressing a Pht1;4 promoter-luciferase reporter gene, exogenous sucrose partly complements the adverse effect of dark treatment on P deficiency-induced luciferase activity.6 Genome-wide analysis of the Arabidopsis leaf transcriptome sheds more light on the scope of co-regulation of a large number of genes by sucrose availability and P deficiency.5 Interestingly, P deficiency-responsive genes of various functional categories (e.g., LaPT1, LaSAP1) are also induced by exogenous sucrose under P-sufficient conditions in young white lupin (Lupinus albus L.) seedlings grown in continuous dark.4 Furthermore, cluster root formation, a hallmark feature of P deficiency-induced root architecture in white lupin,1 appears to be modulated by sucrose. Exogenous sucrose induces cluster (proteoid) root formation in white lupin even under P-sufficient conditions.30
In addition to the application of exogenous sucrose, manipulating steady-state levels and/or phloem transport of endogenous sucrose have provided further evidence for a crucial role of sucrose in signaling P deficiency. In Arabidopsis, impaired phloem loading of sucrose leads to attenuated P-deficiency responses as demonstrated by characterization of the pho3 mutant.35 Because the gene (SUC2) encoding sucrose transporter for phloem loading is inactivated in the pho3 mutant, these data strongly support an essential role of phloem-transported sucrose in signaling P-deficiency responses.36 Alternatively, stem-girdling is an effective and straightforward technique to block phloem transport from shoots to roots. Liu et al.4 reported that more than 95% of phloem transport of sucrose was blocked, as demonstrated with radioactive 14C-sucrose in white lupin grown under the standard 16 h/8 h photoperiod. In the cluster roots of stem-girdled P-deficient plants, expression of several P deficiency-responsive genes (e.g., LaSAP1, LaMATE1, LaPT1) was markedly reduced to very low or non-detectable levels, indicating that phloem transport of shoot-derived sucrose is required for regulating these genes in response to low external Pi.
Shoot sucrose concentrations can be significantly reduced by growing plants in either extended or continuous dark.37,38 An abrupt decrease in the steady-state levels of sucrose in shoots could potentially impair the signaling cascades from low external Pi to the onset of a P deficiency status. Liu et al.4 demonstrated the impact of dark treatments on the expression of three P deficiency-responsive genes, LaSAP1, LaMATE1 and LaPT1, in the cluster roots of white lupin. When plants grown under 16 h/8 h photoperiod were transferred to continuous dark for 24 h, the transcript levels of all three genes were dramatically reduced. Moreover, the effect of 24 h dark-treatment on gene expression was shown to be reversible. When P-deficient dark-treated plants were returned to continuous light for 48 h, P deficiency-responsive gene expression was fully restored.4,17 Based on these data and those with stem-girdled plants, Liu et al.4 proposed that both C assimilation in shoots and subsequent shoot-to-root partitioning via the phloem are essential for P deficiency-regulated gene expression in cluster roots.
Low external Pi to roots appears to be insufficient for sustaining systemic signaling of P deficiency from shoots to roots under a C-limiting status. The notion that Pi per se is not a systemic signal for P-deficiency status has been tested previously from ‘split-root’ experiments with M. truncatula. Signaling of shoot P status for regulating gene expression in roots was not affected by Pi supply (i.e., +Pi and −Pi) to the two halves of roots.39 In contrast to systemic signaling, however, local Pi sensing in roots appears to be mediated by a different pathway in which Pi acts directly as a signal for regulating lateral root formation and root hair development.40–43 In common bean (Phaseolus vulgaris L), rapid downregulation of a phosphatase gene, PvHAD1, in P-deficient roots within an hour of Pi supply is caused by local sensing of Pi, not influenced by C-limiting status of the shoot or blocking of phloem transport by stem-girdling.44 The mechanism for local sensing of Pi is not within the scope of this mini-review.
Importance of Phloem-mobile miR399 and Onset of Systemic P-deficiency Status
miRNAs are a large family of small endogenous non-coding RNAs ubiquitous to both animals and plants. Mature miRNAs, typically of 20∼22 nucleotides (nt) in length, are the products from a multi-step processing of primary transcripts. Steady-state levels of miRNAs are controlled by a fine-tuning system involving both biogenesis and degradation. miRNAs are assembled into active RNA-induced silencing complexes to regulate gene expression by targeting messenger RNA for degradation or via translational repression.45–48
Although numerous miRNAs appear to be expressed constitutively in plants, the expression levels of several miRNAs are influenced by abiotic stresses and various nutrient deficiencies, such as -P, -Sulfur (S) and -Copper (Cu).8,9,48–52 Cloning from miRNA libraries and recent efforts in deep sequencing of miRNAs led to the identification of P deficiency-induced miRNAs: miR399, miR2111, miR778 and miR827 in Arabidopsis.12,13,53 miR399 is the most thoroughly studied, and its regulatory function in the maintenance of Pi homeostasis is crucial for plants to adapt to low P growth conditions. miR399 exerts its function by targeting the messenger RNA of PHO2 for degradation to repress PHO2 expression. PHO2 is a ubiquitin E2-conjugating enzyme that negatively regulates the uptake of Pi into roots.7–9,54 Therefore, increased miR399 expression eventually leads to enhanced Pi uptake in the roots of P-deficient plants. Conversely, miR399 expression is downregulated in plants grown under high external Pi to avoid Pi toxicity. miR399 overexpressing lines of Arabidopsis display symptoms typical of Pi toxicity, such as necrosis in the margins of leaves due to over-accumulation of Pi in shoots.9
miR399 gene appears to be partly regulated by the transcription factor, PHR1, in that miR399 promoter contains putative binding sites for PHR1, and the primary transcripts of miR399 are markedly reduced in the phr1 mutant.7 Given that mature miRNA accumulation is the result of both a multi-step biogenesis and degradation process, it is unclear whether mature miR399 is significantly reduced in the phr1 mutant.
Despite the accumulation of mature miR399 to a high steady-state level in P-deficient plants, paradoxically, not all mature miR399 molecules are “free to function”. Franco-Zorrilla et al.55 revealed that the function of miR399 is modulated by a group of non-coding RNAs of At4/Mt4/PSI1 family, which sequester miR399 via “target mimicry”. There is a high homology between miR399 and a conserved region of 22 nt in the At4/Mt4/PSI family RNAs. Due to a mismatch at the position crucial for cleavage and subsequent degradation, imperfect duplex At4/miR399, unlike the double-stranded structure formed between miR399 and target PHO2 mRNA, is recalcitrant to degradation. It is intriguing that miR399 abundance is not affected by elevated levels of At4 RNA in At4 overexpression lines. In animals, some miRNA/target RNA interactions, which involve various extents of sequence complementarity between miRNA and competing target RNAs, also affect stability of the miRNA itself.56–58
In recent years, accumulating evidence supports the role of phloem-mobile miR399 as a systemic signal molecule to transmit P-deficiency status from shoots to roots in several plant species. In particular, the biological significance of shoot-derived miR399 resides in that miR399 from shoots (as “source”) is responsible for the degradation of PHO2 mRNA in roots (as “sink”) prior to miR399 expression in roots. Two groups independently demonstrated that miR399 is transported via the phloem from shoots to roots in both Arabidopsis and tobacco in micro-grafting experiments.12,13 When shoots of a miR399 overexpressing line were grafted onto the wild-type rootstocks lacking miR399, high-level accumulation of mature miR399, instead of the corresponding primary transcript, was detected in roots. As a result, the grafted plants accumulated five-fold more Pi in shoots compared to the wild-type plants. Mature miR399 has also been identified in the phloem sap of several other plant species, such as oil rape seed and pumpkin,11 indicating that the regulatory mechanism in the maintenance of Pi homeostasis involves shoot-derived miR399 as a systemic signal molecule, and such a mechanism is highly conserved in higher plants.
C Assimilation/Partitioning and miR399 Expression: Correlation or a Causal Relationship?
The concept of miR399 as a phloem-mobile signal has become more widely accepted. However, it remains unknown how plants transmit the systemic signaling of low external Pi to initiate de novo miR399 biogenesis. In pursuit of the mechanism in the cross-talk between sugars and P deficiency, recent data from Liu et al.44 shed more light on how plants coordinate the systemic signaling of P deficiency with photosynthetic C assimilation and subsequent phloem transport of photosynthate in common bean. In control experiments, when P-sufficient plants were transferred to P-deficient growth conditions, miR399 was strongly induced in both shoots and roots within 48 h under standard 16 h light/8 h dark photoperiod. Surprisingly, miR399 was not induced in shoots and roots in plants subjected to continuous dark for 48 h. In addition, miR399 was not induced in roots when phloem transport was blocked by stem-girdling. The absence of miR399 induction in dark-treated or stem-girdled plants serves as direct evidence that low external Pi is not sufficient to trigger miR399-mediated shoot-to-root systemic signaling of P deficiency. Furthermore, these data suggest that plants have evolved a complex network by integrating a specific component (e.g., miR399) downstream of photosynthate (most likely sucrose) to optimize the essential plant function, i.e., photosynthetic C assimilation/partitioning under P deficiency.
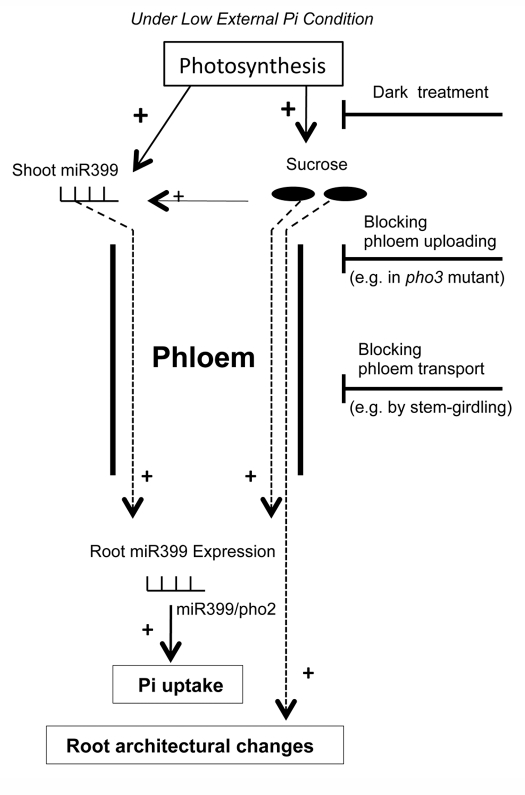
To summarize the crucial roles of sucrose and miR399 discussed in previous sections, we present a concise model to illustrate the integral link between sucrose and phloem-mobile miR399 in systemic signaling of low external Pi (Fig. 1). Under low external Pi, the generation of a systemic P-deficiency status in shoots requires both the induced expression of miR399 and the maintenance of sucrose level/flux, as occurs under standard 16 h/8 h photoperiod. miR399 and sucrose, as systemic signaling molecules from shoots to roots, are uploaded into and transported via the phloem to regulate various responses in roots, such as enhanced Pi uptake and altered root architecture. This signaling pathway could be disrupted by extended dark treatments, impaired phloem uploading, and/or blocking phloem transport by stem-girdling. Therefore, photosynthetic C assimilation and partitioning act as checkpoints, upstream of miR399, in the signaling cascades from low external Pi to the onset of a P-deficiency status.
Figure 1.
Systemic signaling of low external Pi for the onset of P-deficiency status. This model describes a causal relationship between photosynthetic C assimilation/partitioning and the onset of a systemic P-deficiency status under low external Pi. In this model, two types of phloem-mobile molecules (miR399 and sucrose) are both required for shoot-to-root communication to initiate P deficiency responses in roots. Under low external Pi, both the biogenesis of miR399 and the accumulation of sucrose require photosynthesis in shoots. After being transported to roots, shoot-derived miR399 and sucrose act as systemic signals to initiate a series of changes, such as a higher level of root miR399 and the subsequently enhanced Pi uptake by repressing target gene PHO2. Furthermore, sucrose also stimulates lateral root development, a hallmark feature of root architecture in P-deficient plants. This signaling pathway could be disrupted by any one, or a combination, of the three limiting steps: drastically reduced photosynthesis (e.g., by extended dark treatment), impaired phloem uploading of sucrose (e.g., in the pho3 mutant), and/or blocking of phloem transport (e.g., by stem-girdling). Therefore, low external Pi to roots is not sufficient for plants to initiate systemic P-deficiency responses in that low external Pi is superseded by C limitation.
Future Perspectives and Challenges
Natural resources for P fertilizer production are non-renewable, and mining of a dwindling resource is usually linked to ever-increasing and prohibitive costs. Unraveling the molecular mechanism of plant response to P deficiency provides a key foundation to improve phosphorus use efficiency (PUE). Although remarkable progress has been achieved in recent years, many puzzles remain unsolved: Is miR399 biogenesis or degradation modulated by light per se in shoots? If so, how? How is miR399 uploaded into phloem? Is the uploading process regulated? If so, how? Photosynthetic activity is significantly influenced by carbon dioxide concentration and light intensity in nature. Given the obvious causal relationship between photosynthetic C assimilation/partitioning and plant responses to low external Pi, improving PUE in the context of limited P fertilizer resources and climate changes will remain to be a long-term challenge.
Acknowledgements
The authors gratefully acknowledge the continuous funding from United States Department of Agriculture, National Research Initiative, CSREES Grant Number 2005-35100-16002; US Department of Agriculture, Agricultural Research Service CRIS Number 3640-21000-024-00D; and University of Minnesota, Specific Cooperative Agreement Number 58-3640-9-750. The authors also wish to thank Ginger Walker for proofreading of the manuscript.
Abbreviations
- miRNAs
microRNAs
- Pi
phosphate
- P
phosphorus
- N
nitrogen
- C
carbon
- nt
nucleotide
- S
sulfur
- Cu
copper
- h
hour
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13293
References
- 1.Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a non-renewable resource. New Phytol. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert N. The disappearing nutrient. Nature. 2009;461:716–718. doi: 10.1038/461716a. [DOI] [PubMed] [Google Scholar]
- 3.Hammond JP, White PJ. Sucrose transport in the phloem: integrating root response to phosphorus starvation. J Exp Bot. 2008;59:93–109. doi: 10.1093/jxb/erm221. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Samac DA, Bucciarelli B, Allan DL, Vance CP. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant J. 2005;41:257–268. doi: 10.1111/j.1365-313X.2004.02289.x. [DOI] [PubMed] [Google Scholar]
- 5.Müller R, Morant M, Jarmer H, Nilsson L, Nielsen TH. Genome-wide analysis of Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol. 2007;143:156–171. doi: 10.1104/pp.106.090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG. Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta. 2007;225:907–918. doi: 10.1007/s00425-006-0408-8. [DOI] [PubMed] [Google Scholar]
- 7.Bari R, Pant BD, Stitt M, Scheible WR. PHO2, microRNA399 and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL. Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell. 2006;18:412–421. doi: 10.1105/tpc.105.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation responses in Arabidopsis. Curr Biol. 2005;15:2038–2043. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhtz A, Springer F, Chappell L, Baulcombe DC, Kehr J. Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J. 2008;53:739–749. doi: 10.1111/j.1365-313X.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 12.Pant BD, Buhtz A, Kehr J, Scheible WR. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J. 2008;53:731–738. doi: 10.1111/j.1365-313X.2007.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin SI, Chiang SF, Lin WY, Chen JW, Tseng CY, Wu PC, et al. Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol. 2008;147:732–746. doi: 10.1104/pp.108.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu TY, Chang CY, Chiou TJ. The long-distance signaling of mineral macronutrients. Curr Opin Plant Biol. 2009;12:312–319. doi: 10.1016/j.pbi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Chiou TJ. The role of microRNAs in sensing nutrient stress. Plant Cell Environ. 2007;30:323–332. doi: 10.1111/j.1365-3040.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 16.Doerner P. Phosphate starvation signaling: a threesome controls systemic Pi homeostasis. Curr Opin Plant Biol. 2008;11:536–540. doi: 10.1016/j.pbi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Tesfaye M, Liu J, Allan DL, Vance CP. Genomic and genetic control of phosphate stress in legumes. Plant Physiol. 2007;144:594–603. doi: 10.1104/pp.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vance CP. QTLs, epigenetics, sugars and miRNAs: Quatern In phosphate acquisition and use. Plant Physiol. 2010;I154(2):582–588. doi: 10.1104/pp.110.161067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford BG. The role of long-distance signaling in plant responses to nitrate and other nutrients. J Exp Bot. 2002;53:39–43. [PubMed] [Google Scholar]
- 20.Smeekens S. Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Cakmak I, Hengeler C, Marschner H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium-deficiency in bean plants. J Exp Bot. 1994;45:1251–1257. [Google Scholar]
- 22.Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006;11:610–617. doi: 10.1016/j.tplants.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Hernández G, Ramirez M, Valdés-Lopez O, Tesfaye M, Graham MA, Czechowski T, et al. Phosphorus stress in common bean: root transcript and metabolic responses. Plant Physiol. 2007;144:752–767. doi: 10.1104/pp.107.096958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredeen AL, Rao IM, Terry N. Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989;89:225–230. doi: 10.1104/pp.89.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciereszko I, Gniazdowska A, Mikulska M, Rychter AM. Assimilate translocation in bean plants (Phaseolus vulgaris L.) during phosphate deficiency. J Plant Physiol. 1996;149:343–348. [Google Scholar]
- 26.Ciereszko I, Barbachowska A. Sucrose metabolism in leaves and roots of bean (Phaseolus vulgaris L.) during phosphate deficiency. J Plant Physiol. 2000;156:640–644. [Google Scholar]
- 27.Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, et al. Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ. 2003;26:1053–1066. [Google Scholar]
- 28.Ciereszko I, Johabsson H, Kleczkowski LA. Interactive effects of phosphate deficiency, sucrose and light/dark conditions on gene expression of UDP-glucose pyrophosphorylase in Arabidopsis. J Plant Physiol. 2005;162:343–353. doi: 10.1016/j.jplph.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Nacry P, Canivene G, Muller B, Azmi A, Van Onckelen H, Rossignol M, et al. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol. 2005;138:2061–2074. doi: 10.1104/pp.105.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou K, Yamagishi M, Osaki M, Masuda K. Sugar signaling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin. J Exp Bot. 2008;59:2749–2756. doi: 10.1093/jxb/ern130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, et al. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol. 2007;144:232–247. doi: 10.1104/pp.106.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco-Zorrilla JM, Martín AC, Leyva A, Paz-Ares J. Interaction between phosphate-starvation, sugar and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol. 2005;138:847–857. doi: 10.1104/pp.105.060517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lejay L, Gansel X, Cerezo M, Tilland P, Muller C, Krap A, et al. Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase. Plant Cell. 2003;15:2218–2232. doi: 10.1105/tpc.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lejay L, Wirth J, Pervent M, Cross JMF, Tillard P, Gojon A. Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol. 2008;146:2036–2053. doi: 10.1104/pp.107.114710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zakhleniuk OV, Raines CA, Lloyd JC. pho3: a phosphorus-deficient mutant of Arabidopsis thaliana (L.) Heynh. Planta. 2001;212:529–534. doi: 10.1007/s004250000450. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd JC, Zakhleniuk OV. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot. 2004;55:1221–1230. doi: 10.1093/jxb/erh143. [DOI] [PubMed] [Google Scholar]
- 37.Klein D, Morcuende R, Stitt M, Krapp A. Regulation of nitrate reductase expression in leaves by nitrate and nitrogen metabolism is completely overridden when sugars fall below a critical level. Plant Cell Environ. 2000;23:863–871. [Google Scholar]
- 38.Stitt M, Müller C, Matt P, Gibon Y, Carillo P, Morcuende R, et al. Steps towards an integrated view of nitrogen metabolism. J Exp Bot. 2002;53:959–970. doi: 10.1093/jexbot/53.370.959. [DOI] [PubMed] [Google Scholar]
- 39.Burleigh SH, Harrison M. The downregulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots. Plant Physiol. 1999;119:241–248. doi: 10.1104/pp.119.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Bucio J, Cruiz-Ramirez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 41.Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, et al. Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet. 2007;39:792–796. doi: 10.1038/ng2041. [DOI] [PubMed] [Google Scholar]
- 42.Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J. 2004;37:801–814. doi: 10.1111/j.1365-313x.2004.02005.x. [DOI] [PubMed] [Google Scholar]
- 43.Ticconi C, Lucero R, Sakhonwasee S, Adamson A, Creff A, Nussaume L, et al. ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proc Natl Acad Sci USA. 2009;106:14174–14179. doi: 10.1073/pnas.0901778106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Allan DL, Vance CP. Systemic signaling and local sensing of phosphate in common bean: cross-talk between photosynthate and microRNA399. Mol Plant. 2010;3:428–437. doi: 10.1093/mp/ssq008. [DOI] [PubMed] [Google Scholar]
- 45.Chen X. Small RNAs-secrets and surprises of the genome. Plant J. 2010;61:941–958. doi: 10.1111/j.1365-313X.2009.04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- 47.Mallory AC, Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat Genet. 2006;38:31–36. doi: 10.1038/ng1791. [DOI] [PubMed] [Google Scholar]
- 48.Sunkar R, Chinnusamy V, Zhu JK. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci. 2007;12:301–309. doi: 10.1016/j.tplants.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Kawashima CG, Yoshimoto N, Maruyama-Nakashita A, Tsuchiya YN, Saito K, Takahashi H, et al. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J. 2009;57:313–321. doi: 10.1111/j.1365-313X.2008.03690.x. [DOI] [PubMed] [Google Scholar]
- 50.Sunkar R, Zhu JK. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16:2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamasaki H, Abdel-Ghany SE, Cohu CM, Kobayashi Y, Shikanai T, Pilon M. Regulation of copper homeostasis by micro-RNA in Arabidopsis. J Biol Chem. 2007;282:16369–16378. doi: 10.1074/jbc.M700138200. [DOI] [PubMed] [Google Scholar]
- 52.Valdés-Lopez O, Yang SS, Aparicio-Fabre R, Graham P, Reyes JL, Vance CP, et al. MicroRNA expression profile in common bean (Phaseolus vulgaris) under nutrient deficiency stresses and manganese toxicity. New Phytol. 2010;187:805–818. doi: 10.1111/j.1469-8137.2010.03320.x. [DOI] [PubMed] [Google Scholar]
- 53.Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, et al. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol. 2009;151:2120–2132. doi: 10.1104/pp.109.147280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 2006;141:1000–1011. doi: 10.1104/pp.106.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- 56.Pasquinelli AE. Paring miRNAs through pairing. Science. 2010;328:1494–1495. doi: 10.1126/science.1191531. [DOI] [PubMed] [Google Scholar]
- 57.Ameres SL, Horwich MD, Hung JH, Xu J, Ghildiyal M, Weng Z, et al. Target RNA-directed trimming and tailing of small silencing RNAs. Science. 2010;328:1534–1539. doi: 10.1126/science.1187058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cazalla D, Yario T, Steitz J. Downregulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science. 2010;328:1563–1566. doi: 10.1126/science.1187197. [DOI] [PMC free article] [PubMed] [Google Scholar]