Abstract
Virulence in plant pathogenic fungi is controlled through a variety of cellular pathways in response to the host environment. Nitrogen limitation has been proposed to act as a key signal to trigger the in planta expression of virulence genes. Moreover, a conserved Pathogenicity mitogen activated protein kinase (MAPK) cascade is strictly required for plant infection in a wide range of pathogens. We investigated the relationship between nitrogen signaling and the Pathogenicity MAPK cascade in controlling infectious growth of the vascular wilt fungus Fusarium oxysporum. Several MAPK-activated virulence functions such as invasive growth, vegetative hyphal fusion and host adhesion were strongly repressed in the presence of the preferred nitrogen source ammonium. Repression of these functions by ammonium was abolished by L-Methionine sulfoximine (MSX) or rapamycin, two specific inhibitors of Gln synthetase and the protein kinase TOR (Target Of Rapamycin), respectively, and was dependent on the bZIP protein MeaB. Supplying tomato plants with ammonium rather than nitrate resulted in a significant delay of vascular wilt symptoms caused by the F. oxysporum wild type strain, but not by the ΔmeaB mutant. Ammonium also repressed invasive growth in two other pathogens, the rice blast fungus Magnaporthe oryzae and the wheat head blight pathogen Fusarium graminearum. Our results suggest the presence of a conserved nitrogen-responsive pathway that operates via TOR and MeaB to control infectious growth in plant pathogenic fungi.
Key words: nitrogen, virulence, MAPK, TOR, MeaB, rapamycin
Fungal pathogens use an arsenal of virulence mechanisms to invade their hosts, overcome their defences and colonize their tissues. Accumulating evidence suggests that these infection-related processes are tightly controlled by a complex network of cellular pathways that respond to a combination of signals from the host environment. For example, it was suggested that nitrogen limitation triggers the expression of virulence genes in plant pathogens.2,13 Moreover, fungal infection on plants requires a conserved Pathogenicity MAPK cascade homologous to the filamentous growth cascade of Saccharomyces cerevisiae.17 In the vascular wilt fungus F. oxysporum, a soil-borne pathogen that attacks a wide range of economically important crops, the orthologous MAPK Fmk1 controls multiple virulence-related processes such as root adhesion and penetration, as well as invasive growth on living plant tissue.5
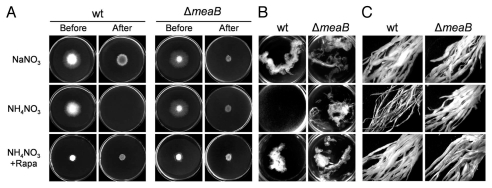
Previous work established that the capacity of F. oxysporum to penetrate cellophane membranes defines a major virulence function that requires the Fmk1 MAPK and its downstream component, the homeodomain transcription factor Ste12.11,12 We noted that cellophane penetration was dramatically affected by nitrogen source: F. oxysporum invades cellophane membranes in the presence of sodium nitrate, but not on ammonium nitrate, ammonium sulfate or ammonium tartrate (Fig. 1A). The repressing effect of the nitrogen source ammonium was conserved in two other plant pathogen species, the rice blast fungus M. oryzae and the wheat head blight pathogen F. graminearum, indicating that nitrogen regulation of invasive growth may be functional in many fungal plant pathogens.8
Figure 1.
Nitrogen source regulates invasive growth, hyphal aggregation and root adhesion via TOR and MeaB. (A) Effect of nitrogen source, MeaB and TOR on cellophane penetration by Fusarium oxysporum. Fungal colonies were grown 4 days at 28°C on plates covered with a cellophane membrane (before). The cellophane with the fungal colony was removed and plates were incubated for an additional day to determine the presence of mycelial growth on the plate (after). (B) Hyphal aggregates forming after 36 hours conidial germination. Cultures were vortexed to dissociate weakly adhered hyphae and observed in a binocular microscope. (C) Root adhesion assay. Roots of tomato seedlings were immersed for 36 hours in microconidial suspensions, then washed by vigorous shaking in water and observed in a binocular microscope. Adhering fungal mycelium is visible as a white mass covering the roots.
Besides ammonium, Gln was the only one among different amino acids tested to at least partially repress cellophane penetration. In agreement with this finding, MSX, an irreversible inhibitor of Gln synthetase4 fully restored invasive growth of F. oxysporum in the presence of ammonium. The requirement of Gln synthetase activity for nitrogen repression suggests that Gln rather than ammonium may act as the repressing signal.8 In S. cerevisiae, Gln levels regulate the activity of TOR, a conserved serine/threonine kinase that orchestrates cell growth in response to nutrients.3,4 We found that rapamycin, a highly specific inhibitor of TOR, also restored cellophane penetration in the presence of repressing concentrations of ammonium (Fig. 1A). This result directly implicates TOR in the transmission of the nitrogen signal.8 Besides cellophane invasion, ammonium also repressed additional virulence-related functions that require the Fmk1 MAPK cascade, such as vegetative hyphal fusion and fungal adhesion to tomato roots, in a TOR-dependent manner (Fig. 1B and C). Thus, nitrogen source affects the regulation of a broad range of virulence functions.8
Nitrogen utilization in fungi is a tightly regulated process. TOR is a central player of nitrogen catabolite repression in S. cerevisiae.1,3 We found that several genes involved in nitrogen catabolism of F. oxysporum were significantly upregulated by rapamycin, supporting a role of TOR in nitrogen catabolite repression.8 We also investigated the role of MeaB, a bZIP protein that mediates nitrogen catabolite repression in the saprophytic ascomycete Aspergillus nidulans through inhibition of the wide domain regulator AreA.16 Nitrogen utilization phenotypes of ΔmeaB and ΔareA mutants, as well as cross-species complementation and transcriptional analysis of nitrogen catabolic genes, showed that MeaB and AreA have highly conserved roles in regulation of nitrogen catabolism between A. nidulans and F. oxysporum.8
Strikingly, the ΔmeaB strain was able to perform cellophane invasion, vegetative hyphal fusion and root adhesion in the presence of repressing concentrations of ammonium (Fig. 1). Interestingly, invasive growth of the ΔmeaB mutant was further enhanced by addition of rapamycin, indicating that MeaB and TOR both contribute to ammonium repression in an independent and additive manner.8 To test whether the role of MeaB involves inhibition of the wide domain regulator AreA, virulence-related functions were scored in a ΔareA mutant. Unexpectedly, the ΔareA strain was still able to perform these functions, even in the presence of the repressing source ammonium, suggesting that AreA acts as a repressor in nitrogen-regulation of virulence mechanisms, in contrast to its well-described role as an activator of nitrogen catabolite genes.8
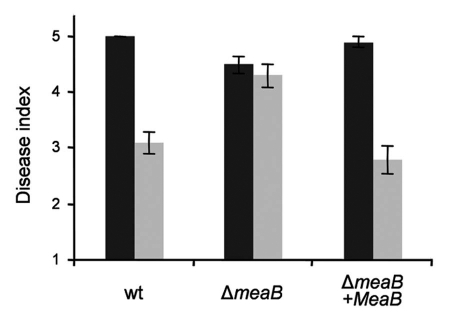
We next asked whether nitrogen source also affects virulence of F. oxysporum during infection of its natural host plant tomato. Seedlings whose roots were inoculated with conidia of the wild type strain and supplied with an ammonium solution, showed a significant delay in the development of wilt symptoms compared to plants supplied with nitrate. Importantly, no differential effect of nitrogen source on the severity of disease symptoms was detected in plants infected with the ΔmeaB strain (Fig. 2). Moreover, transcript levels of the virulence gene Six1,14 in the wild type strain, but not in the ΔmeaB mutant, were dramatically reduced during infection of tomato roots in the presence of ammonium compared with nitrate, and this repression was completely abolished by rapamycin.8
Figure 2.
Nitrogen source and MeaB regulate plant infection. Incidence of F. oxysporum wilt on tomato plants 20 days after inoculation with the indicated strains and supplied with solutions containing 25 mM of NaNO3 (black columns) or NH4NO3 (grey columns). Severity of disease symptoms was recorded using an index ranging from 1 (healthy plant) to 5 (dead plant). Bars represent standard errors calculated from 10 plants.
How do Nitrogen Response and Pathogenicity MAPK Pathways Interact to Regulate Virulence Functions?
Our results highlight a novel role of nitrogen source in the regulation of fungal virulence on plants, but also raise a number of questions that need to be addressed in future studies. Transmission of the nitrogen repression signal requires independent inputs from the protein kinase TOR and the bZIP protein MeaB, two components that have not been associated previously with fungal pathogenicity on plants. A common feature shared by the virulence functions subject to nitrogen control in F. oxysporum is that they all require the Fmk1 MAPK cascade.8 Intriguingly, the filamentation and invasive growth response in S. cerevisiae is also repressed by high concentrations of ammonium7 and requires the orthologous MAPK Kss1.9
A central question is how the conserved nitrogen response and MAPK pathways interact to control the shared virulence targets. Using a combination of pharmacological and genetic approaches, we showed that neither rapamycin treatment nor deletion of meaB could restore invasive growth in the Δfmk1 strain.8 These results place the nitrogen repression pathway either independently or upstream of the Fmk1 MAPK cascade. However, there is also evidence for a direct link between the two pathways. Previous studies showed a pivotal role of the homeodomain transcription factor Ste12 in regulating invasive growth and plant pathogenicity downstream of the Pathogenicity MAPK.10,12 Ste12 transcript levels were strongly downregulated on ammonium compared to nitrate, suggesting that Ste12 expression is controlled by nitrogen source. Transcriptional repression of Ste12 in F. oxysporum required both MeaB and AreA, consistent with the phenotypic effects of ΔmeaB and ΔareA mutations of reversing nitrogen repression of virulence functions.8
A second key issue concerns the common downstream targets of the nitrogen response and MAPK pathways, and their role during invasive growth, adhesion and plant infection. Some of these genes are likely to encode extracellular or surface proteins such as Six1. However, other cellular responses associated with nitrogen starvation such as autophagy or generation of reactive oxygen species have recently been linked to fungal virulence on plants.6,15 Future work is needed to expand our understanding of how nitrogen and MAPK signaling interact to control infectious growth in plant pathogenic fungi.
Acknowledgements
This work was supported by grant BIO2007-62661 from the Ministerio de Educación y Ciencia and by the Marie Curie Research Training Network MRTN-CT-2005-019277 (SIGNALPATH). M.S.L.B. and R.P.R. had Ph.D., fellowships from the Ministerio de Educación y Ciencia.
Abbreviations
- MAPK
mitogen activated protein kinase
- TOR
target of rapamycin
- MSX
L-methionine sulfoximine
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13729
References
- 1.Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- 2.Coleman M, Henricot B, Arnau J, Oliver RP. Starvation-induced genes of the tomato pathogen Cladosporium fulvum are also induced during growth in planta. Mol Plant Microbe Interact. 1997;10:1106–1109. doi: 10.1094/MPMI.1997.10.9.1106. [DOI] [PubMed] [Google Scholar]
- 3.Crespo JL, Hall MN. Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2002;66:579–591. doi: 10.1128/MMBR.66.4.579-591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crespo JL, Powers T, Fowler B, Hall MN. The TOR-controlled transcription activators GLN3, RTG1 and RTG3 are regulated in response to intracellular levels of glutamine. Proc Natl Acad Sci USA. 2002;99:6784–6789. doi: 10.1073/pnas.102687599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Pietro A, Garcia-Maceira FI, Meglecz E, Roncero MI. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol Microbiol. 2001;39:1140–1152. [PubMed] [Google Scholar]
- 6.Egan MJ, Wang ZY, Jones MA, Smirnoff N, Talbot NJ. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci USA. 2007;104:11772–11777. doi: 10.1073/pnas.0700574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Berges MS, Rispail N, Prados-Rosales RC, Di Pietro A. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. Plant Cell. 2010;22:2459–2475. doi: 10.1105/tpc.110.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhani HD, Styles CA, Fink GR. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 1997;91:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 10.Park G, Bruno KS, Staiger CJ, Talbot NJ, Xu JR. Independent genetic mechanisms mediate turgor generation and penetration peg formation during plant infection in the rice blast fungus. Mol Microbiol. 2004;53:1695–1707. doi: 10.1111/j.1365-2958.2004.04220.x. [DOI] [PubMed] [Google Scholar]
- 11.Prados Rosales RC, Di Pietro A. Vegetative hyphal fusion is not essential for plant infection by Fusarium oxysporum. Eukaryot Cell. 2008;7:162–171. doi: 10.1128/EC.00258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rispail N, Di Pietro A. Fusarium oxysporum Ste12 controls invasive growth and virulence downstream of the Fmk1 MAPK cascade. Mol Plant Microbe Interact. 2009;22:830–839. doi: 10.1094/MPMI-22-7-0830. [DOI] [PubMed] [Google Scholar]
- 13.Snoeijers SS, Perez-Garcia A, Joosten MH, De Wit PJ. The effect of nitrogen on disease development and gene expression in bacterial and fungal plant pathogens. Eur J Plant Pathol. 2000;106:493–506. [Google Scholar]
- 14.van der Does HC, Duyvesteijn RG, Goltstein PM, van Schie CC, Manders EM, Cornelissen BJ, et al. Expression of effector gene SIX1 of Fusarium oxysporum requires living plant cells. Fungal Genet Biol. 2008;45:1257–1264. doi: 10.1016/j.fgb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Veneault-Fourrey C, Barooah M, Egan M, Wakley G, Talbot NJ. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science. 2006;312:580–583. doi: 10.1126/science.1124550. [DOI] [PubMed] [Google Scholar]
- 16.Wong KH, Hynes MJ, Davis MA. Recent advances in nitrogen regulation: a comparison between Saccharomyces cerevisiae and filamentous fungi. Eukaryot Cell. 2008;7:917–925. doi: 10.1128/EC.00076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X, Mehrabi R, Xu JR. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot Cell. 2007;6:1701–1714. doi: 10.1128/EC.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]