Abstract
We recently demonstrated that non-pathogenic and non-symbiotic microbes E. coli and yeast are taken up by roots and used as a source of nutrients by the plant. Although this process appears to be beneficial for the plant, the nutritional gain of microbe incorporation has to exceed the energy expense of microbe uptake and digestion, and the question remains whether the presence of microbes triggers pathogen- and other stress-induced responses. Here, we present evidence that digesting microbes is accompanied by strong downregulation of genes linked to stress response in Arabidopsis. Genome-wide transcription analysis shows that uptake of E. coli by Arabidopsis roots is accompanied by a pronounced downregulation of heat shock proteins. Plants upregulate heat shock proteins in response to environmental stresses including temperature, salt, light and disease agents including microbial pathogens. The pronounced downregulation of heat shock proteins in the presence of E. coli indicates that uptake and subsequent digestion of microbes does not induce stress. Additionally it suggests that resources devoted to stress resistance in control plants may be re-allocated to the process of microbe uptake and digestion. This observation adds evidences to the notion that uptake of microbes is an active, purposeful and intentional behavior of the plant.
Key words: heat shock proteins, Arabidopsis, plant-microbe interactions, stress response
Interactions between microbes and plants include beneficial and detrimental relationships for the plant. Beneficial relationships include symbioses1 such as diazotrophic endophytes that supply nitrogen2,3 and other endophytic associations that promote plant growth.4 Detrimental relationships involve fungal, bacterial and virus pathogens.1 Beside these well established interactions, there is evidence for a new type of interaction that is beneficial for the plant only. Plants take up and digest non-pathogenic and non-symbiotic microbes such as E. coli and yeast and use them as a nutrient source.5 The incorporation of microbes into roots occurs by mechanisms that appear to be controlled by the plant and include the generation of an extracellular cell wall-like structure for enclosing microbes at the root surface and induction of genes encoding cell wall synthesizing, loosening and degrading enzymes, to facilitate incorporation of microbes into root cells.5
We therefore concluded that in the absence of pathogenic or symbiotic relationships, plants coordinate the entry of E. coli and yeast into root cells with an apparent expenditure of energy.5 However, for this process to be of evolutionary significance, benefits of accessing microbes as a nutrient source have to outweigh the energy expense. Such notion is in agreement with plant behavior being active and purposeful and relying on cost-benefit assessment.6 Therefore, to fully demonstrate the benefits of this process for the plant, an analysis of nutritional gain versus energy expenditure is required. We commenced this analysis by scrutinizing the processes involved in the incorporation and digestion of microbes via the plant's metabolic changes.
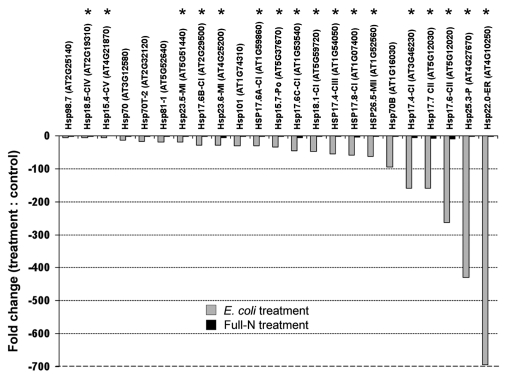
Arabidopsis plants cultivated in axenic hydroponic culture for three weeks with full-N supplied (10 mM NH4NO3) were grown without N for 3 days and incubated for a further 24 h in the presence of E. coli Bl21 (final OD600 = 2, E. coli treatment) or 10 mM NH4NO3 (full-N treatment), or without any addition of nutrients (control). RNA was extracted from roots to probe an Agilent microarray (Agilent Technologies, USA). Expression values corresponded to treatments versus control. Comparative analysis of gene expression between treatments revealed that the expression of heat shock proteins (HSPs) was dramatically downregulated in plants treated with E. coli Bl21, compared to full-N treatment (Fig. 1). Heat shock proteins contribute to stress tolerance and their expression is induced in response to heat stress and multiple environmental stresses arising from biotic and abiotic stimuli.7–9 HSPs act as molecular chaperones that regulate the folding, localization, accumulation and degradation of proteins.10 HSPs are classified into a number of families based on their molecular mass (HSP100, HSP90, HSP70, HSP60 and small HSPs (sHSPs)).10 Our results show that 17 sHSPs out of the 19 members of the sHSP superfamily that constitute the majority of HSPs, are downregulated in E. coli treatment (Fig. 1). All sHSP genes in plants are induced in response to environmental stresses,7 with the exception of two genes (Hsp21.7-CVI and Hsp14.7-CVII) that are involved in specific housekeeping functions of plant cells and are constitutively expressed.9 These two sHSPs were not downregulated in E. coli-treated plants confirming that the downregulation of HSPs observed in our experiments is caused by a suppressed stress response.
Figure 1.
Differential expression of heat shock protein genes in Arabidopsis roots incubated with E. coli Bl21 (gray bar) or ammonium nitrate (black bar). Expression values correspond to treatments versus control (plants grown without N). Small heat shock proteins (sHSPs) are indicated by asterisk. Gene expressions greater than 2-fold change compared to control are shown for E. coli treatment. For microarray experiments and analysis, refer to reference 5.
Thus, we argue that the absence of an induction of HSPs upon E. coli uptake is an indication that E. coli incorporation into roots does not cause stress, corroborating the notion that this process is directed by the plant and not microbes. The downregulation of numerous HSPs may be an indication that the plant undergoes resources re-allocation. Plants trade-off resources as required to remain competitive.6,11 Resistance response to abiotic and biotic stress requires substantial resources,12,13 providing potential for resource re-allocation. It is worth mentioning that full-nitrogen supplied plants did not significantly downregulate HSPs compared to E. coli treated plants (Fig. 1), which excludes the possibility that a replete N status is the cause of HSPs downregulation. That resources generally devoted to stress resistance may be used for the metabolic systems involved in uptake and digestion of microbes and could be crucial for optimizing the cost/benefit balance of microbial digestion.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13760
References
- 1.Baron C, Zambryski PC. Notes from the underground: Highlights from plant-microbe interactions. Trends Biotechnol. 1995;13:7–9. [Google Scholar]
- 2.Reinhold-Hurek B, Hurek T. Life in grasses: diazotrophic endophytes. Trends Microbiol. 1998;6:139–144. doi: 10.1016/s0966-842x(98)01229-3. [DOI] [PubMed] [Google Scholar]
- 3.Triplett EW. Diazotrophic endophytes: Progress and prospects for nitrogen fixation in monocots. Plant Soil. 1996;186:29–38. [Google Scholar]
- 4.Lugtenberg B, Kamilova F. Plant-Growth-Promoting Rhizobacteria. Annu Rev Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- 5.Paungfoo-Lonhienne C, Rentsch D, Robatzek S, Webb RI, Sagulenko E, Nasholm T, et al. Turning the table: plants consume microbes as a source of nutrients. PLoS One. 2010;5:11915. doi: 10.1371/journal.pone.0011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trewavas A. What is plant behaviour? Plant Cell Environ. 2009;32:606–616. doi: 10.1111/j.1365-3040.2009.01929.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun W, Van Montagu M, Verbruggen N. Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta. 2002;1577:1–9. doi: 10.1016/s0167-4781(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 8.Swindell WR, Huebner M, Weber AP. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics. 2007;8:125. doi: 10.1186/1471-2164-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddique M, Gernhard S, von Koskull-Doring P, Vierling E, Scharf KD. The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones. 2008;13:183–197. doi: 10.1007/s12192-008-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. doi: 10.1007/BF00039383. [DOI] [PubMed] [Google Scholar]
- 11.Matyssek R, Agerer R, Ernst D, Munch JC, Osswald W, Pretzsch H, et al. The plant's capacity in regulating resource demand. Plant Biol (Stuttg) 2005;7:560–580. doi: 10.1055/s-2005-872981. [DOI] [PubMed] [Google Scholar]
- 12.Gale J, Zeroni M. The cost to plants of different strategies of adaptation to stress and the alleviation of stress by increasing assimilation. Plant Soil. 1985;89:57–67. [Google Scholar]
- 13.Heckathorn SA, Poeller GJ, Coleman JS, Hallberg RL. Nitrogen availability alters patterns of accumulation of heat stress-induced proteins in plants. Oecologia. 1996;105:413–418. doi: 10.1007/BF00328745. [DOI] [PubMed] [Google Scholar]