Abstract
Alternative respiratory pathway (AP) plays an important role in plant thermogenesis, fruit ripening and responses to environmental stresses. AP may participate in the adaptation to salt stress since salt stress increased the activity of the AP. Recently, new evidence revealed that ethylene and hydrogen peroxide (H2O2) are involved in the salt-induced increase of the AP, which plays an important role in salt tolerance in Arabidopsis callus, and ethylene may be acting downstream of H2O2. Recent observations also indicated both ethylene and nitric oxide (NO) act as signaling molecules in responses to salt stress, and ethylene may be a part of the downstream signal molecular in NO action. In this addendum, a hypothetical model for NO function in regulation of H2O2- and ethylene-mediated induction of AP under salt stress is presented.
Key words: alternative respiratory pathway, ethylene, hydrogen peroxide, nitric oxide, salt stress, signaling molecule
Respiration plays a pivotal role in the metabolism of plants by providing adequate energy and carbon sources to drive the cellular metabolism and transport processes. In addition to the cytochrome pathway (CP), plant mitochondria have a cyanide-resistant respiration electron transport pathway, the alternative respiratory pathway (AP). Alternative oxidase (AOX) is used as the terminal oxidase in the AP and located in the inner membrane of mitochondria. It is well known that the AP plays an important role in plant thermogenesis, fruit ripening and responses to environmental stresses.1–3 It is thought that the AP may play a role in preventing the formation of toxic reactive oxygen species (ROS) when the main CP is inhibited or restricted.4 Salt stress can lead to an accumulation of high levels of ROS, such as superoxide (O2−·), hydrogen peroxide (H2O2) and hydroxyl radicals (OH·).5,6 These may disturb cellular redox homeostasis, and then lead to oxidative damage. It has been shown that the AP may participate in the adaptation to salt stress since salt stress increased the activity of the AP.7,8 However, whether respiration could be involved in the prevention of ROS formation under salt stress is not reported. Furthermore, the mechanism of AP regulation affected by salinity remains unknown.
Ethylene and H2O2 are both able to induce AP in plant cells.3,9 The essential role of ethylene for AP induction was reported by Simons et al. (1999) in Arabidopsis, and it was shown that AP operation is ethylene dependent.11 In several studies, H2O2 is considered as the second messenger to induce AOX activity by directly oxidizing transcription factors or by modulating phosphorylation processes.9,11 Although ethylene and H2O2 have been found to be possibly involved in AP induction, the interaction between them in the induction of the AP during environmental stresses remains unclear.
Our results indicated that both H2O2 and ethylene induced AP activity in wild-type (WT) Arabidopsis but not in ethylene-insensitive mutant under salt stress, suggesting ethylene signaling is required for AP induction. Subsequently, we set out to investigate the relationship between H2O2 and ethylene under salt stress. It was found that H2O2 stimulated ethylene emission, while ethylene reduced H2O2 production under salt stress. Further results indicated H2O2 and ethylene modulated salt-induced AOX gene (AOX1a) expression and the increase in pyruvate content. These results suggest ethylene and H2O2 are involved in the salt-induced increase of the AP, which plays an important role in salt tolerance in WT calluses, and ethylene may be acting downstream of H2O2. There are reports that nitric oxide (NO) greatly induces AOX1a expression in Arabidopsis cell cultures and in tobacco plants.11,12 Our previous work demonstrated that salt possibly induced NO accumulation resulting from stimulating nitric oxide synthase (NOS) in Arabidopsis callus, and NO stimulated ethylene emission by increasing 1-aminocyclopropane-1-carboxylic acid synthase (ACS) activity under salt stress,13 suggesting ethylene may be a part of the downstream signal molecular in NO action in response to salt stress. These observations imply that NO may also be involved in AP induction under salt stress. In addition, salt-induced NO production was involved in H2O2 generation by stimulating plasma membrane (PM) NADPH oxidase activity.14 Growing evidences suggest that PM NADPH oxidase is responsible for H2O2 accumulation under stresses.14,15 In addition to functioning as an endogenous oxidant, H2O2 has been suggested as a diffusible signal for selective induction of defense mechanisms in plant cells.16,17 Summing up these observations that allows us to speculate NO maybe regulate H2O2- and ethylene-dependent AP induction under salt stress.
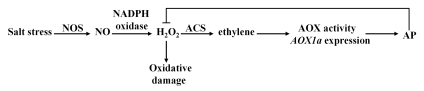
Based on the results obtained so far, a model for the function of NO, H2O2 and ethylene in AP induction under salt stress is proposed (Fig. 1). According to our model, the increased NO accumulation under salt stress is involved in ethylene-dependent AP induction. Under salt stress, NO generated from NOS acts as a signal molecule to activate PM NADPH oxidase activity to stimulate H2O2 generation. The accumulated H2O2 activates ACS activity to induce ethylene emission. The increased ethylene emission induces AOX1a expression and pyruvate content, thus resulting in enhanced AP activity. Eventually, the enhanced AP can dampen H2O2 generation in excess to avoid ROS damage in plant cells. The model we have proposed here should provide further insights into the mechanism of AP induction regulated by NO, H2O2 and ethylene signal molecules under salt stress.
Figure 1.
Hypothetical model for the potential function of NO, H2O2 and ethylene as signaling molecules in AP induction under salt stress in Arabidopsis. Salt stress activates a signal transduction cascade that leads to the increased activity of AOX, whose expression leads to enhanced AP activity. NO is generated by NOS, H2O2 is likely generated by PM NADPH oxidase attributed to NO, and ethylene emission is stimulated by ACS attributed to H2O2. The AP activity is regulated by ethylene directly under salt stress.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 90917019), Specialized Research Fund for the Doctoral Program of Higher Education of China (ratification number 20050730017), the Foundation of Science and Technology of Gansu Province (3ZS051-A25-018) and China Postdoctoral Science Foundation funded project (20100470884).
Abbreviations
- ACS
1-aminocyclopropane-1-carboxylic acid synthase
- AP
alternative respiratory pathway
- AOX
alternative oxidase
- CP
cytochrome pathway
- H2O2
hydrogen peroxide
- NO
nitric oxide
- NOS
nitric oxide synthase
- PM
plasma membrane
- ROS
reactive oxygen species
- WT
wild type
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13775
References
- 1.Vanlerberghe GC, McIntosh L. Lower temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiol. 1991;100:115–119. doi: 10.1104/pp.100.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chivasa S, Carr JP. Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell. 1998;10:1489–1498. doi: 10.1105/tpc.10.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, et al. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol. 2006;142:595–608. doi: 10.1104/pp.106.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 6.Liu YG, Wu RR, Wan Q, Xie GQ, Bi YR. Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol. 2007;48:511–522. doi: 10.1093/pcp/pcm020. [DOI] [PubMed] [Google Scholar]
- 7.Costa JH, Jolivet Y, Hasenfratz-Sauder MP, Orellano EG, da Guia Silva Lima M, Dizengremel P, et al. Alternative oxidase regulation in roots of Vigna unguiculata cultivars differing in drought/salt tolerance. J Plant Physiol. 2007;164:718–727. doi: 10.1016/j.jplph.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Smith CA, Melino VJ, Sweetman C, Soole KL. Manipulation of alternative oxidase can influence salt tolerance in Arabidopsis thaliana. Physiol Plant. 2009;137:459–472. doi: 10.1111/j.1399-3054.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagner AM. A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in Petunia hybrida cells. FEBS Lett. 1995;368:339–342. doi: 10.1016/0014-5793(95)00688-6. [DOI] [PubMed] [Google Scholar]
- 10.Simons BH, Millenaar FF, Mulder L, Van Loon LC, Lambers H. Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv. tomato. Plant Physiol. 1999;120:529–538. doi: 10.1104/pp.120.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neill SJ, Desikan R, Hancock J. Hydrogen peroxide signaling. Curr Opin Plant Biol. 2002;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, von Rad U, Durner J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta. 2002;215:914–923. doi: 10.1007/s00425-002-0828-z. [DOI] [PubMed] [Google Scholar]
- 13.Wang HH, Liang XL, Wan Q, Wang XM, Bi YR. Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta. 2009;230:293–307. doi: 10.1007/s00425-009-0946-y. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Wang YP, Yang YL, Wu H, Wang D, Liu JQ. Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ. 2007;30:775–785. doi: 10.1111/j.1365-3040.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Wang YP, Wang D. Role of nitric oxide and hydrogen peroxide during the salt resistance response. Plant Signal Behavior. 2007;2:473–474. doi: 10.4161/psb.2.6.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Silva H, Klessig RF. Active oxygen species in the induction of plant systemic acquired resistance by SA. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- 17.Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 1994;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]