Abstract
We have recently found that microbial species ranging from Gram-negative and Gram-positive bacteria to different fungi emit volatiles that strongly promote starch accumulation in leaves of both mono- and di-cotyledonous plants. Transcriptome and enzyme activity analyses of potato leaves exposed to volatiles emitted by Alternaria alternata revealed that starch over-accumulation was accompanied by enhanced 3-phosphoglycerate to Pi ratio, and changes in functions involved in both central carbohydrate and amino acid metabolism. Exposure to microbial volatiles also promoted changes in the expression of genes that code for enzymes involved in endocytic uptake and traffic of solutes. With the overall data we propose a metabolic model wherein important determinants of accumulation of exceptionally high levels of starch include (a) upregulation of ADPglucose-producing SuSy, starch synthase III and IV, proteins involved in the endocytic uptake and traffic of sucrose, (b) downregulation of acid invertase, starch breakdown enzymes and proteins involved in internal amino acid provision and (c) 3-phosphoglycerate-mediated allosteric activation of ADPglucose pyrophosphorylase.
Key words: starch, microbial volatiles, sucrose synthase, ADPglucose, carbohydrate metabolism
Plants produce starch as predominant storage carbohydrate to cope with temporary starvation imposed by the environment. This branched homopolysaccharide is synthesized by starch synthase using ADPglucose as the sugar donor molecule. Starch biosynthesis in leaves has generally been considered to take place exclusively in the chloroplast, and segregated from the sucrose biosynthetic process that takes place in the cytosol.1,2 According to this view, starch is considered the end-product of a unidirectional pathway wherein the allosterically regulated ADPglucose pyrophosphorylase exclusively catalyses the synthesis of ADPglucose, and functions as the major regulatory step in the starch biosynthetic process.1,2 However, mounting evidences have indicated the occurrence of additional/alternative pathway(s) of starch biosynthesis wherein ADPglucose is extraplastidially produced by enzymes such as sucrose synthase,3–6 and then is transported to the plastid by the action of a still unidentified ADPglucose translocator.7,8 According to this view both plastidic phosphoglucomutase and ADPglucose pyrophosphorylase play an important role in the scavenging of glucose units derived from hydrolytic starch breakdown.9
Plants perceive biotic stimuli by recognizing a multitude of different signaling compounds originating from the interacting organisms. Microbes synthesize and emit many volatile compounds with molecular masses less than 300 Da, low polarity and a high vapor pressure.10–12 We have recently explored the effect on starch metabolism of volatiles released from different microbial species ranging from Gram-negative and Gram-positive bacteria to different fungi. We found that all microbial species tested (including plant pathogens and microbes that normally do not interact with plants) emitted volatiles that strongly promoted starch accumulation in leaves of both mono- and di-cotyledonous plants when microbes were cultured in minimal media.13 Transcriptome, metabolite content and enzyme activity analyses of potato leaves exposed to volatiles emitted by Alternaria alternata revealed that starch over-accumulation was accompanied by increase of 3-phosphoglycerate to Pi ratio (positive and negative effectors of ADPglucose pyrophosphorylase, respectively) and upregulation of sucrose synthase, acid invertase inhibitors, starch synthase class III and IV, starch branching enzyme, glucose-6-phosphate (G6P) transporter and enzymes involved in glycolytic, respiratory and fermentative pathways. This phenomenon, designated as MIVOISAP (Microbial Volatiles Induced Starch Accumulation Process), was also accompanied by downregulation of acid invertase, plastidial thioredoxins, plastidial β-amylase and starch phosphorylase, proteins involved in the conversion of plastidial triose-phosphates into cytosolic G6P, proteins involved in internal amino acid provision such as proteases and cysteine synthase and less well defined mechanisms involving bacteria-type stringent response.13
Phosphatidylinositol (PI) is synthesized in the cytosol from G6P in a three-step process, involving inositol-phosphate synthase, inositol monophosphatase and phosphatidylinositol synthase.14 PI is then converted into phosphatidylinositol-3-phosphate (PI3P) and phosphatidylinositol-4-phosphate (PI4P) by means of PI3P kinase (PI3K) and PI4P kinase (PI4K), respectively. PI3P and PI4P have been implicated in diverse physiological functions, including increased plasma membrane endocytosis, vesicle traffic and vacuole biogenesis and organization.15,16 Recent studies have provided strong evidence that an important pool of sucrose incorporated into cells is taken up by PI3K- and/or PI4K-mediated endocytosis and vesicle traffic processes prior to its conversion into starch.17,18 In line with this observations, Im et al.19 have shown that enhanced phosphoinositide metabolism results in increased utilization of sugars from the medium. Noteworthy, our transcriptome analyses revealed that genes coding for PI4K, PI3K, inositol-phosphate synthase and inositol monophosphatase are upregulated by volatiles emitted by A. alternata (3.55-, 3.12-, 4.62- and 3.78-fold increase, respectively).
Plant actin cytoskeleton is a dynamic scaffolding that plays a crucial role in organelle movement, vesicle trafficking, cytoplasmic streaming, plant defenses against pathogens, etc., in response to internal and external signals.20,21 Recently, evidence has been provided indicating that actin cytoskeleton is also involved in the endocytic uptake and traffic of sucrose linked to starch biosynthesis in cultured cells of sycamore.18 Sucrose synthase associates with the actin cytoskeleton,22 which further indicates that actin cytoskeleton can act as a determinant of starch metabolism. Actin-depolymerizing factors are modulators of the dynamic organization of the actin cytoskeleton, modulating the rate of actin filament turnover and networking cellular signals into cytoskeletal-dependent processes.23,24 Consistent with the view that actin cytoskeleton-mediated endocytosis and/or vesicle traffic may be involved in MIVOISAP, array analyses revealed that actin-depolymerizing factor expression is upregulated by treatment with volatiles emitted by A. alternata (3.26 fold increase).
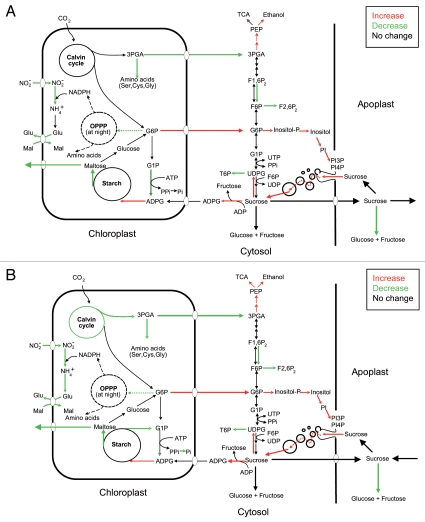
Our currently ongoing research is consistent with the idea that potato MIVOISAP is regulated, at least in part, at the transcriptional level. Based on our transcriptome, metabolite content and enzyme activity analyses we would like to propose a metabolic model of potato MIVOISAP wherein important determinants of accumulation of exceptionally high levels of starch include (a) upregulation of ADPglucose-producing SuSy, acid invertase inhibitors, starch synthase III and IV, proteins involved in the endocytic uptake and traffic of sucrose, (b) downregulation of acid invertase, starch breakdown enzymes and proteins involved in internal amino acid provision and (c) 3-phosphoglycerate-mediated allosteric activation of ADPglucose pyrophosphorylase (Fig. 1). During MIVOISAP the triose-P/Pi translocator is downregulated, whereas the G6P translocator is upregulated when plants are cultured in both autotrophic and heterotrophic conditions. Therefore, according to this model, the G6P translocator would play a role in transporting photosynthetically produced G6P from the plastid to the cytosol to be subsequently used for the synthesis of sucrose and PI necessary for the endocytic uptake of sucrose. During MIVOISAP there occurs a downregulation of the synthesis of plastidial proteins and of proteins involved in internal amino acid provision (especially cysteine) such as proteases and enzymes involved in synthesis de novo of amino acids.25 It is thus likely that, under conditions of limited protein breakdown occurring during exposure to microbial volatiles, excess ATP and carbon will be diverted from protein metabolism towards starch biosynthesis.
Figure 1.
Suggested metabolic model of MIVOISAP in leaves of potato plants cultured in the absence and presence of sucrose (A and B, respectively). According to this model, major determinants of accumulation of exceptionally high levels of starch in leaves of plants exposed to microbial volatiles include (A) upregulation of ADPglucose-producing SuSy, acid invertase inhibitors, starch synthase III and IV, proteins involved in the endocytic uptake and traffic of sucrose, and G6P export from the stroma to the cytosol and (B) downregulation of acid invertase, starch breakdown enzymes, synthesis of plastidial proteins and proteins involved in internal amino acid provision (especially cysteine) such as proteases, enzymes involved in synthesis de novo of amino acids, and less well defined mechanisms involving bacteria-type stringent response.25
Acknowledgements
This research was partially supported by grant BIO2007-63915 from the Comisión Interministerial de Ciencia y Tecnología and Fondo Europeo de Desarrollo Regional (Spain), and by Iden Biotechnology S.L. M.O. was partly supported by grant No. APVV-0432-06 from the Grant Academy APVV and by grant No. 2/0200/10 from the Grant Academy VEGA. I.E. acknowledges a pre-doctoral fellowship from the Spanish Ministry of Education and Science.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13808
References
- 1.Neuhaus HE, Häusler RE, Sonnewald U. No need to shift the paradigm on the metabolic pathway to transitory starch in leaves. Trends Plant Sci. 2005;10:154–156. doi: 10.1016/j.tplants.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Streb S, Egli B, Eicke S, Zeeman SC. The debate on the pathway of starch synthesis: a closer look at low-starch mutants lacking plastidial phosphoglucomutase supports the chloroplast-localised pathway. Plant Physiol. 2009;151:1769–1772. doi: 10.1104/pp.109.144931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baroja-Fernández E, Muñoz FJ, Saikusa T, Rodríguez-López M, Akazawa T, Pozueta-Romero J. Sucrose synthase catalyzes the de novo production of ADPglucose linked to starch biosynthesis in heterotrophic tissues of plants. Plant Cell Physiol. 2003;44:500–509. doi: 10.1093/pcp/pcg062. [DOI] [PubMed] [Google Scholar]
- 4.Baroja-Fernández E, Muñoz FJ, Zandueta-Criado A, Morán-Zorzano MT, Viale AM, Alonso-Casajús N, et al. Most of ADP-glucose linked to starch biosynthesis occurs outside the chloroplast in source leaves. Proc Natl Acad Sci USA. 2004;101:13080–13085. doi: 10.1073/pnas.0402883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muñoz FJ, Baroja-Fernández E, Morán-Zorzano MT, Viale AM, Etxeberria E, Alonso-Casajús N, et al. Sucrose synthase controls the intracellular levels of ADPglucose linked to transitory starch biosynthesis in source leaves. Plant Cell Physiol. 2005;46:1366–1376. doi: 10.1093/pcp/pci148. [DOI] [PubMed] [Google Scholar]
- 6.Baroja-Fernández E, Muñoz FJ, Montero M, Etxeberria E, Sesma MT, Ovecka M, et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009;50:1651–1662. doi: 10.1093/pcp/pcp108. [DOI] [PubMed] [Google Scholar]
- 7.Pozueta-Romero J, Ardila F, Akazawa T. ADPglucose transport by adenylate translocator in chloroplasts is linked to starch biosynthesis. Plant Physiol. 1991;97:1565–1572. doi: 10.1104/pp.97.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozueta-Romero J, Frehner M, Viale AM, Akazawa T. Direct transport of ADPglucose by adenylate translocator is linked to starch biosynthesis in amyloplasts. Proc Natl Acad Sci USA. 1991;88:5769–5773. doi: 10.1073/pnas.88.13.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz FJ, Morán-Zorzano MT, Alonso-Casajús N, Baroja-Fernández E, Etxeberria E, Pozueta-Romero J. New enzymes, new pathways and an alternative view on starch biosynthesis in both photosynthetic and heterotrophic tissues of plants. Biocatal Biotransform. 2006;24:63–76. [Google Scholar]
- 10.Schöller CEG, Gürtler H, Pedersen R, Molin S, Wilkins K. Volatile metabolites from actinomycetes. J Agric Food Chem. 2002;50:2615–2621. doi: 10.1021/jf0116754. [DOI] [PubMed] [Google Scholar]
- 11.Schulz S, Dickschat JS. Bacterial volatiles: the smell of small organisms. Nat Prod Rep. 2007;24:814–842. doi: 10.1039/b507392h. [DOI] [PubMed] [Google Scholar]
- 12.Splivallo R, Fischer U, Göbel C, Feussner I, Karlovsky P. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 2009;150:2018–2029. doi: 10.1104/pp.109.141325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezquer I, Li J, Ovecka M, Baroja-Fernández E, Muñoz FJ, Montero M, et al. Microbial volatile emissions promote accumulation of exceptionally high levels of starch in leaves in mono- and di-cotyledonous plants. Plant Cell Physiol. 2010;51:1674–1693. doi: 10.1093/pcp/pcq126. [DOI] [PubMed] [Google Scholar]
- 14.Gardocki ME, Jani N, Lopes JM. Phosphatidylinositol biosynthesis: biochemistry and regulation. Biochim Biophys Acta. 2005;1735:89–100. doi: 10.1016/j.bbalip.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Leshem Y, Seri L, Levine A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007:51185–51197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- 16.Peleg-Grossman S, Volpin H, Levine A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J Exp Bot. 2007;58:1637–1649. doi: 10.1093/jxb/erm013. [DOI] [PubMed] [Google Scholar]
- 17.Etxeberria E, Baroja-Fernández E, Muñoz FJ, Pozueta-Romero J. Sucrose inducible endocytosis as a primary mechanism of nutrient uptake in heterotrophic plant cells. Plant Cell Physiol. 2005;46:474–481. doi: 10.1093/pcp/pci044. [DOI] [PubMed] [Google Scholar]
- 18.Baroja-Fernández E, Etxeberria E, Muñoz FJ, Morán-Zorzano MT, Alonso-Casajús N, González P, et al. An important pool of sucrose linked to starch biosynthesis is taken up by endocytosis in heterotrophic cells. Plant Cell Physiol. 2006;47:447–456. doi: 10.1093/pcp/pcj011. [DOI] [PubMed] [Google Scholar]
- 19.Im YJ, Perera IY, Brglez I, Davis AJ, Stevenson-Paulik J, Phillippy BQ, et al. Increasing plasma membrane phosphatidylinositol-(4,5)-bisphosphate biosynthesis increases phosphoinositide metabolism in Nicotiana tabacum. Plant Cell. 2007;19:1603–1616. doi: 10.1105/tpc.107.051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkmann D, Baluska F. Actin cytoskeleton in plants: from transport networks to signaling networks. Microsc Res Tech. 1999;47:135–154. doi: 10.1002/(SICI)1097-0029(19991015)47:2<135::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Hussey PJ, Ketelaar T, Deeks MJ. Control of the actin cytoskeleton in plant cell growth. Annu Rev Plant Biol. 2006;57:109–125. doi: 10.1146/annurev.arplant.57.032905.105206. [DOI] [PubMed] [Google Scholar]
- 22.Duncan KA, Huber SC. Sucrose synthase oligomerization and F-actin association are regulated by sucrose concentration and phosphorylation. Plant Cell Physiol. 2007;48:1612–1623. doi: 10.1093/pcp/pcm133. [DOI] [PubMed] [Google Scholar]
- 23.Clément M, Ketelaar T, Rodiuc N, Banora MY, Smertenko A, Engler G, et al. Actin-depolymerizing factor2-mediated actin dynamics are essential for root-knot nematode infection of Arabidopsis. Plant Cell. 2009;21:2963–2979. doi: 10.1105/tpc.109.069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian M, Chaudhry F, Ruzicka DR, Meagher RB, Staiger CJ, Day B. Arabidopsis actin-depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol. 2009;150:815–824. doi: 10.1104/pp.109.137604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson WA, Roach PJ, Montero M, Baroja-Fernández E, Muñoz FJ, Eydallin G, et al. Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol Rev. 2010;34:952–985. doi: 10.1111/j.1574-6976.2010.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]