Abstract
Objective
Although iliac crest autograft is the gold standard for lumbar fusion, the morbidity of donor site leads us to find an alternatives to replace autologous bone graft. Ceramic-based synthetic bone grafts such as hydroxyapatite (HA) and b-tricalcium phosphate (b-TCP) provide scaffolds similar to those of autologous bone, are plentiful and inexpensive, and are not associated with donor morbidity. The present report describes the use of Polybone® (Kyungwon Medical, Korea), a beta-tricalcium phosphate, for lumbar posterolateral fusion and assesses clinical and radiological efficacy as a graft material.
Methods
This study retrospectively analyzed data from 32 patients (11 men, 21 women) who underwent posterolateral fusion (PLF) using PolyBone® from January to August, 2008. Back and leg pain were assessed using a Numeric Rating Scale (NRS), and clinical outcome was assessed using the Oswestry Disability Index (ODI). Serial radiological X-ray follow up were done at 1, 3, 6 12 month. A computed tomography (CT) scan was done in 12 month. Radiological fusion was assessed using simple anterior-posterior (AP) X-rays and computed tomography (CT). The changes of radiodensity of fusion mass showed on the X-ray image were analyzed into 4 stages to assess PLF status.
Results
The mean NRS scores for leg pain and back pain decreased over 12 months postoperatively, from 8.0 to 1.0 and from 6.7 to 1.7, respectively. The mean ODI score also decreased from 60.5 to 17.7. X-rays and CT showed that 25 cases had stage IV fusion bridges at 12 months postoperatively (83.3% success). The radiodensity of fusion mass on X-ray AP image significantly changed at 1 and 6 months.
Conclusion
The present results indicate that the use of a mixture of local autologous bone and PolyBone® results in fusion rates comparable to those using autologous bone and has the advantage of reduced morbidity. In addition, the graft radiodensity ratio significantly changed at postoperative 1 and 6 months, possibly reflecting the inflammatory response and stabilization.
Keywords: Postero-Lateral fusion, PolyBone®, Radiodensity
INTRODUCTION
Although the iliac crest autograft is considered the best material for lumbar fusion, the iliac crest harvest is associated with a morbidity rate of 8.4-24%. As such, alternatives to autologous bone grafts have been explored, including allografts of cancellous chips and cortical spacers, such as demineralized bone matrix products. In addition, human recombinant bone morphogenetic proteins (BMPs) are showing promise in clinical trials2,4).
Ceramic-based synthetic bone grafts such as hydroxyapatite (HA) and b-tricalcium phosphate (b-TCP) provide scaffolds similar to those of autologous bone, are plentiful and less expensive than BMPs or demineralized bone matrix (DBM), and are not associated with donor morbidity2). Scaffolds made of HA and b-TCP mixtures provide osteoconduction for bone production as well as long-term stability and immediate ostogenic activity2,6,8).
PolyBone® (Kyungwon Medical, Korea) is made of beta-tricalcium phosphate. PolyBone® pore size ranges from 200-500 micrometers macroscopically, and to smaller than 10 micrometers microscopically, and pores constitute 75% of each granule.
This retrospective study analyzed the clinical outcome and radiological fusion rate following the use of mixture of local autologous bone and PolyBone® for instrumented posterolateral fusion (PLF) in degenerative lumbar spine surgery. In addition the authors report the serial radiological change of PLF mass and quantitative radiodensity ratio of fusion mass calculated with the function of picture archiving and communication system (PACS) (PetaVision 2.1, Seoul, Korea). We also discuss the relation between the radiological change of fusion mass and bone resorption, which is routinely observed radiologically and histologically3,12).
MATERIALS AND METHODS
We retrospectively assessed outcomes in 32 patients (11 men, 21 women) who underwent lumbar spinal surgery including PLF using mixture of local autologus bone and PolyBone® from January to August, 2008. All patients had degenerative lumbar spinal disease. The mean patient age was 58 years (range, 23-77 years), and the mean follow-up period was 24 months (range, 21-28 months). Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
Chronic alcoholic : Patient who consumes >40 g alcohol/day. BMI : Body Mass Index [Weight (kg)/Height^2 (m)]. T-score of BMD (Bone Mineral Density) was measured using Dual Energy X-ray absorptiometry at L2-4.
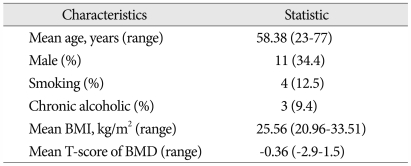
All patients first underwent wide decompression. In the majority of operated levels, interbody fusion using polyetheretherketone (PEEK) cages filled with local autologous bone was done. PLF of all the operated levels was attempted by connecting each transverse process with remained local autologous bone after interbody fusion and PolyBone®. Before paving with those fusion materials, the transverse process was decorticated with a drill. We paved approximately one layer of local autologous bone (2.5 cc per one level), remained after use for interbody fusion, on the transverse processes and intertransverse membrane. Then 2.5 cc of PolyBone® per one level were then added to each side as PLF expander (Fig. 1).
Fig. 1.
A : The yellow arrows indicate that local autologous bones (2.5 cc per one level) are prepared. B : The yellow arrows indicate that 2.5 cc PolyBone® per one level are added on each side as a posterolateral fusion expander.
Clinical and radiological follow-up was done at postoperative 1, 3, 6 and 12 months. We used a Numeric Rating Scale (NRS) to measure back and leg pain, and the Oswestry Disability Index (ODI) to assess clinical outcome.
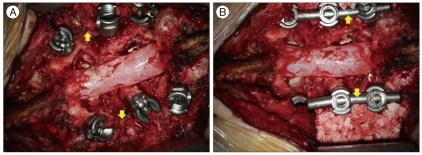
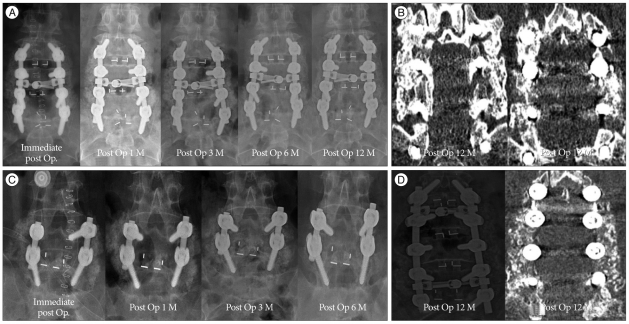
Serial radiological X-rays were done at 1, 3, 6 and 12 months postoperatively. The radiographs were digitized at 200 um spatial resolution and 16-bit gray-scale resolution. In addition, computed tomography (CT) at 12 months postoperatively was checked to assess a precise status of PLF mass. Radiological assessment was done by a neurosurgeon and a radiologist. We classified fusion mass on X-ray AP image into 4 stages according to its serial change : stage I, the fusion mass density is slightly denser compared with that of the adjacent vertebrae; stage II, the fusion mass becomes denser than stage I and the fusion mass particles can be seen more prominently; stage III, the particles begin to disappear, the density decreases, and the gap among bone chips decreases; stage IV, the fusion mass appears as a connected bone bridge with a density similar to that of the adjacent vertebra (Fig. 2).
Fig. 2.
A : Stage I. The fusion mass density is similar to that of the adjacent vertebra and small particles are seen. B : Stage II. The density of the fusion mass seems to be denser and particles are more prominent. C : Stage III. The particles begin to disappear, and the density of the fusion mass seems to lower than in stage II. The gaps, which is seen low density, among the bone chips begin to disappear. D : Stage IV. The fusion mass appears to be a connected bone bridge with a density similar to the adjacent vertebra.
Quantitative radiodensity of fusion mass on the AP X-ray was calculated. We used the radiodensity of the titanium rod as a reference for correcting any variations in film exposure or processing. The frame of the PLF bridge and the titanium rod was drawn twice by a neurosurgeon and a radiologist in simple AP X-ray image. If the frame was determined, the mean radiodensity on the X-ray image could be calculated with our PACS system. We used the mean of two values calculated this way. Interclass correlation coefficient (ICC) value was 0.878 (0.744-0.944). A ICC value >0.8 was considered to indicate valid inter/intra variability. The ratio of mean density (PLF mass/Titanium rod) could be determined using a simple AP X-ray image (Fig. 3). The serial change of radiodensity ratio was analyzed.
Fig. 3.
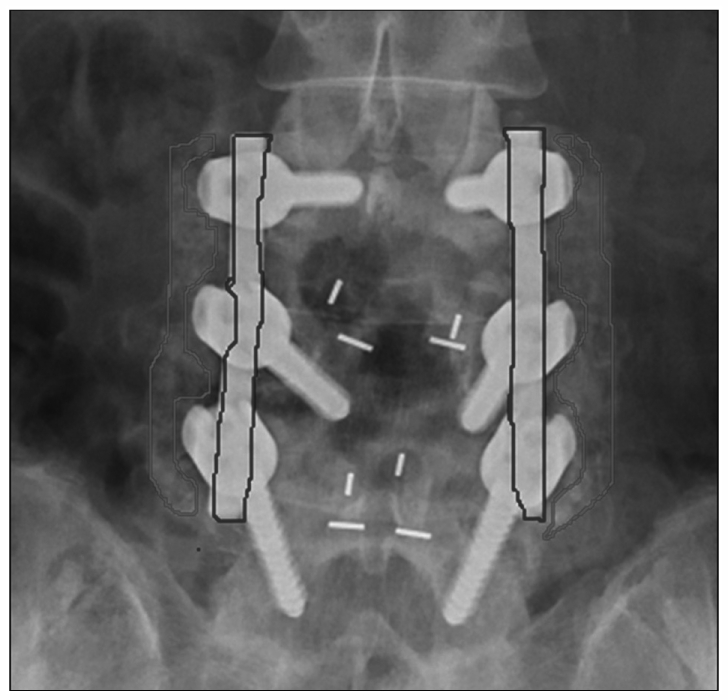
We used the mean radiodensity from the red frame (fusion mass) and blue frame (titanium rod) drawn two times by different assessors. (i.e., the mean radiodensity from the red frame/the mean radiodensity from the blue frame).
SAS 9.1 and R 2.9.0 software were used for statistical analysis. Data were analyzed using the Linear Mixed Model, Wilcoxon Rank sums test and Fisher's exact test.
RESULTS
We performed surgical correction on 32 patients with degenerative lumbar disease using decompression, interbody fusion and posterolateral fusion with PolyBone®. The diseases comprised 17 cases of spondylolisthesis (10 degenerative and 7 lytic), 4 cases of stenosis and 11 cases of spondylolisthesis (7 degenerative and 4 lytic) with stenosis. A total of 57 levels were operated upon, from L1-2 to L5-S1. There were 17 cases involving one level, 7 cases involving two levels, 6 cases involving three levels, and 2 case involving four levels with disease (Table 2).
Table 2.
The Changes of Posterolateral Graft with Time
Alcohol : Patient consumed>40 g alcohol/day (i.e., alcoholic), T-score of BMD (Bone Mineral Density) : Measured using Dual Energy X-ray absorptiometry in L2-L4, BMI : Body Mass Index [Weight (kg)/Height^2 (m)], SPL : spondylolisthesis, ST : stenosis, 3D : fusion stage on postoperative 3 day image, 1M : fusion stage on postoperative 1 month image, 3M : fusion stage on postoperative 3 month image, 6M : fusion stage on postoperative 6 month image, 12M : fusion stage on postoperative 12 month image. F : fusion failure (absence of fusion bridge), NI : no image was obtained, F/L : follow-up loss
Clinical outcome assessment at 12 months showed that the mean NRS score for leg and back pain decreased from 8.0 to 1.0 and 6.7 to 1.7, respectively. The mean ODI score decreased from 60.5 (range, 31.0-85.0) to 17.7 (range, 0-45.0) (p<0.05).
Radiological assessment
The PLF fusion mass on the AP X-ray image of all patients showed stage I state in the immediate postoperative period. The 1-month postoperative X-rays showed all but 6 cases were at stage II. Of those 6 cases, 4 moved rapidly to stage III, one case remained at stage I, and the other case was lost to follow-up. Postoperative 3-month X-rays were not performed in 5 cases. For the remaining cases, 11 were at stage II, and 16 were at stage III. Postoperative 6-month X-rays showed loss of the fusion mass in two cases, and five cases were not X-rayed. Of the remaining cases, one was at stage II, 14 were at stage III, and 10 were at stage IV. At 12 months, 2 cases were lost to follow-up, 3 cases showed loss of fusion mass, 2 cases were at stage III and 25 cases were at stage IV, and those stage IV fusion mass were also identified as a complete fusion bridge in postoperative 12 month CT images. In addition, CT image also showed loss of fusion mass in 3 patients who showed identical finding in X-ray image. CT of 2 cases who were at stage III showed incomplete fusion bridge, because fusion mass in the CT seem to contain unabsorbed particle (Fig. 4).
Fig. 4.
A : X-ray images of a 65-year-old female patient show serial change from stage I to IV. B : Post operative 12 month CT of same patient also shows complete fusion bridge. C : Although the X-ray images of 51-year-old female patient show also gradual change until postoperative 3 month, fusion mass suddenly disappear on the post operative 6 month X-ray image. These serial images show that fusion failure occurs during the follow up. D : Post operative 12 month X-ray and CT of a 66-year-old female patient seems that hyperdense bone chips still remain. We believe fusion mass is still on the stage III.
We defined fusion at postoperative 12 month, if PLF mass showed stage IV in X-ray image and CT coronal image also showed complete fusion bridge. The failure was defined if PLF mass disappeared in X-ray or CT. We didn't consider interbody fusion result in our study. Therefore, the rate of PLF success at postoperative 12 months was 83.3% (25/30). Failure rate of PLF was 10% (3/30) and other 2 cases were remained undetermined (Table 2).
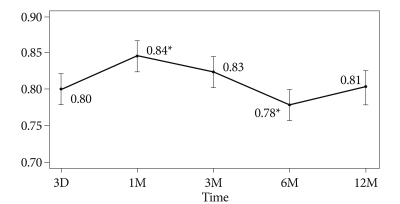
The mean radiodensity ratios in all cases were serially analyzed. The mean density ratio value increased up to 1 month postoperatively (p<0.01), remained stable up to 3 months, and then decreased up to 6 months (p<0.01). The mean ratio at 12 months decreased to a level similar to that of the immediate postoperative state (Fig. 5).
Fig. 5.
A Linear Mixed Model was used for statistical analysis. The radiodensity ratio changed over time. The graph shows the mean radiodensity ratio and the 95% confidence intervals. Asterisks indicate a significant difference of change compared mean value of radiodensity at previous state (p<0.05).
We could not find any correlation between PLF success and smoking, alcohol abuse, body mass index (BMI) or T-score of bone mineral density (BMD).
DISCUSSION
Autogenous iliac bone is the 'gold standard' material for spinal fusion. However, such grafts are associated with increased surgical time, significant blood loss, postoperative pain and iliac fracture5,7,10). Therefore, ceramics such as hydroxyapatite and b-tricalcium phosphate have been used as bone graft materials, and good results have been reported2,4,5,9,11).
We used PolyBone® which is made of b-tricalcium phosphate, with local autologous bone which was harvested during laminectomy, for lumbar posterolateral fusion. We believe this approach reduced operative times and morbidity related to iliac harvest. Like other ceramics, PolyBone® is an osteoconductive material2,4). We also used local autologous bone which was left over after interbody fusion, and has osteogenic capacity for posterolateral fusion. We always used 5 cc PolyBone® with 5 cc local autologous bone for bilateral one-level fusion to make optimum use of the osteoinductivity of autologous bone.
Clinical outcomes were assessed using NRS and ODI scores. Both scores decreased over 12 months postoperatively, indicating improvement. Those improvements were likely due to decompression and stabilization.
Radiological fusion assessment was done using plain AP X-rays and coronal CT reconstruction by a radiologist and a neurosurgeon. We didn't consider the result of interbody fusion in our study because we wanted to find the efficacy of PolyBone® as a bone graft expander. We achieved an 83% radiological fusion rate at 12 months postoperatively. We believe that fusion was continuing to occur in the two cases at stage III at 12 months. The lumbar posterolateral fusion rate when using autologous iliac bone is reported to be 40-98%1,7). The present results indicate the use of a mixture of local autologous bone and PolyBone® results in comparable fusion rates, and has the advantage of reduced morbidity associated with iliac harvest.
Bone graft status cannot be quantified simply using radiodensity alone. However, using the ratio of the graft and titanium rod radiodensities is likely to reduce variability associated with obesity, clothing, and viewing angle for radiation exposure. In addition, we used the mean value measured within frame the graft and rod drawn by the two assessors to make much accuracy. The mean density ratio serially changed over time. The mean density ratio value increased up to 1 month postoperatively, then decreased up to 6 months, and increased up to 12 months. This trend was probably due to an inflammatory reaction in response to graft resorption until 6 month and subsequent graft stabilization after 6 month9). Although more studies are required, the present results suggest that the radiodensity ratio may be one of predictive indicator of graft status, such as resorption or stabilization.
Even though we did not find that other factors, such as smoking, obesity, alcohol and osteoporosis were linked to failure, we believe more cases may show such factors become statistically significant.
CONCLUSION
We retrospectively analyzed outcomes following the use of PolyBone® as a fusion expander for posterolateral fusion in degenerative lumbar spine surgery. We found that pain and ODI improved postoperatively, and that the radiological posterolateral fusion success rate was 83.3%. The graft radiodensity ratio significantly changed at postoperative 1 and 6 months, possibly reflecting the inflammatory response and stabilization. The present results indicate that the use of a mixture of local autologous bone and PolyBone® results in posterolateral fusion rates comparable to those achieved using autologous bone, and has the advantage of reduced morbidity. Although more data are needed, the change of radiodensity ratio are promising predictive value of the status of graft material.
Footnotes
The corresponding author is an individual stockholder of Kyungwon Medical, Korea. However, this research was not financially supported.
References
- 1.Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years : influence of technique on fusion rate and clinical outcome. Spine (Phila Pa 1976) 2004;29:455–463. doi: 10.1097/01.brs.0000090825.94611.28. discussion Z5. [DOI] [PubMed] [Google Scholar]
- 2.Brandoff JF, Silber JS, Vaccaro AR. Contemporary alternatives to synthetic bone grafts for spine surgery. Am J Orthop (Belle Mead NJ) 2008;37:410–414. [PubMed] [Google Scholar]
- 3.Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983:28–42. [PubMed] [Google Scholar]
- 4.Burger EL, Patel V. Calcium phosphates as bone graft extenders. Orthopedics. 2007;30:939–942. doi: 10.3928/01477447-20071101-06. [DOI] [PubMed] [Google Scholar]
- 5.Chang CH, Lin MZ, Chen YJ, Hsu HC, Chen HT. Local autogenous bone mixed with bone expander : an optimal option of bone graft in single-segment posterolateral lumbar fusion. Surg Neurol. 2008;70(Suppl 1):S1:47–S1:49. doi: 10.1016/j.surneu.2008.05.022. discussion : 49. [DOI] [PubMed] [Google Scholar]
- 6.Delécrin J, Takahashi S, Gouin F, Passuti N. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis : a prospective, randomized study. Spine (Phila Pa 1976) 2000;25:563–569. doi: 10.1097/00007632-200003010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Dimar JR, 2nd, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY. Two-year fusion and clinical outcomes in 224 patients treated with a single-level instrumented posterolateral fusion with iliac crest bone graft. Spine J. 2009;9:880–885. doi: 10.1016/j.spinee.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autogenous bone graft : Efficacy and indications. J Am Acad Orthop Surg. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hing KA, Wilson LF, Buckland T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007;7:475–490. doi: 10.1016/j.spinee.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine (Phila Pa 1976) 1989;14:1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Moro-Barrero L, Acebal-Cortina G, Suárez-Suárez M, Pérez-Redondo J, Murcia-Mazón A, López-Muñiz A. Radiographic analysis of fusion mass using fresh autologous bone marrow with ceramic composites as an alternative to autologous bone graft. J Spinal Disord Tech. 2007;20:409–415. doi: 10.1097/bsd.0b013e318030ca1e. [DOI] [PubMed] [Google Scholar]
- 12.Togawa D, Bauer TW, Lieberman IH, Sakai H. Lumbar intervertebral body fusion cages: histological evaluation of clinically failed cages retrieved from humans. J Bone Joint Surg Am. 2004;86-A:70–79. [PubMed] [Google Scholar]