Abstract
Background:
Changes in the arterial partial pressure of CO2 (PaCO2) has a direct though transient effect on the cerebral vasculature and cerebral circulation. Decreased PaCO2 levels lead to vasoconstriction and can result in dangerously low levels of cerebral perfusion that resolve in 4–6 h. It is currently believed that perfusion abnormalities contribute to intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL) in the neonate. PaCO2-induced vasoconstriction may contribute to the pathology of IVH and PVL.
Methods:
Near-infrared spectroscopy [NIRS; (INVOS cerebral/somatic oximeter; Somanetics Corporation, Troy, MI, USA)] was utilized to determine changes in regional oxygenation (rSO2) of the brain in response to changes in ventilation in isoflurane anesthetized newborn piglets.
Results:
Changes in cerebral rSO2 correlated significantly with end-tidal CO2 levels and to blood flow in the common carotid artery. This correlation was significant during baseline conditions, after periods of CO2 loading and during periods of hypothermia.
Conclusions:
The results of the study demonstrate the utility of NIRS to accurately reflect changes in cerebral oxygenation and flow to the brain in response to changes in CO2 levels in anesthetized, ventilated neonatal piglets. The use of NIRS may provide an early alert of low levels of cerebral blood flow and brain oxygenation, potentially helping in preventing the progression of IVH or PVL in the neonate.
Keywords: Common carotid artery blood flow, INVOS, neonatal piglets, PaCO2, regional oxygen saturation (rSO2)
INTRODUCTION
PaCO2 is an important regulator of the cerebral circulation independent of autoregulation[17,25] and has been shown to result in changes in the peak blood flow velocity in the middle cerebral artery.[15] Relative change in cerebral blood flow during alterations of PaCO2 depends on several factors, including baseline blood flow, cerebral perfusion pressure, and use of anesthetic drugs. The vascular response to CO2 is thought to be due to changes in intracellular pH that is attenuated as intracellular buffers return the pH to normal after several hours.[12,22,23] The return to eucapnic levels following hypocapnia has been associated with a significant overshoot of cerebral blood flow (CBF).[22] The manipulation of PaCO2 is commonly used in the operating room and the intensive care unit to manage CBF. Hypocapnia is used to treat increased intracranial pressure, inducing vasoconstriction and reducing CBF, while both hypocapnia and hypercapnia have been used to treat cerebral ischemia.[7]
High-frequency ventilation (HFV) and continuous positive airway pressure (CPAP) are used in premature neonates to keep immature lungs open and promote normal gas exchange. HFV is often used as a therapy in cardiopulmonary care of the neonates as well, especially for persistent pulmonary hypertension. The use of HFV and CPAP has improved the survival rate of premature infants but can lead to rapid loss of CO2 and hypocapnia[18] if not closely monitored. While assisted ventilation promotes an adequate O2 uptake, it has the potential to negatively impact cerebral and peripheral O2 delivery by altering PaCO2 and cardiac output. The use of pulse oximetry and blood gas analysis provides information on the effectiveness of ventilation but not of the downstream physiologic responses to changes in PaCO2 and cardiac output.
The impact of HFV and CPAP on blood flow to the brain in neonates is not currently established in premature infants but both can cause rapid and extreme changes in CO2 levels resulting in changes in CBF that can go undetected. Acute hypocapnia induces the constriction of cerebral blood vessels reducing cerebral blood flow and it has been suggested that prolonged deep hypocapnia is one of the risk factors for periventricular leukomalacia (PVL) in neonates.[19,3] In addition, Erickson et al. have demonstrated an association between hypocapnia and the risk of severe intraventricular hemorrhage (IVH) and PVL.[10]
To gain a better understanding of the specific CO2 -induced changes in the brain, the present study utilized NIRS to determine oxygen delivery to the brain during periods of the changing ventilation rate. NIRS has been used extensively over the past decade during cardiac and vascular surgeries to monitor oxygen delivery to the brain and spine in adults, children, and infants.[5,8,9,11,20,26] The use of NIR light (700–1000 nm wavelength) for in vivo analysis of hemoglobin saturation is based on the fact that very few substances in tissue absorb NIR wavelengths. NIRS is the basis of pulse oximetry for the estimation of arterial hemoglobin saturation and in cerebral oximetry to measure regional hemoglobin saturation in the capillary beds, reflecting venous saturation. The use of NIRS for monitoring brain O2 delivery is based on the observation that internal jugular vein hemoglobin saturation (SijvO2) reflects global cerebral perfusion. A high correlation of SijvO2 with rSO2 has been reported in studies in adults, children, and infants[1,16,21] and also in animal studies.[4]
The current study utilized the INVOS system (Somanetics Corporation, Troy, MI, USA) to determine the effects of ventilatory changes on regional saturation of the brain. This report discusses the response of common carotid (CC) flow and regional oxygen saturation of the cerebral tissue in response to changes in end-tidal CO2 (etCO2) caused by the ventilatory rate change in a neonatal piglet model. The effects of CO2 loading and hypothermia on the animal's ability to respond to etCO2 changes were also investigated.
MATERIALS AND METHODS
Guidelines for animal research
The procedures used in this study are in agreement with the guidelines of the Providence Hospital Institutional Animal Care and Use Committee (IACUC). Surgical assistance and veterinary care were provided by a licensed veterinary technician. The Animal Care and Use Program at Providence Hospital, Detroit, MI, conforms to the standards described in the National Institutes of Health Guide of the Care and Use of Laboratory Animals [DHHS publication no. (HIH) 86-23]. The study protocols were approved by the IACUC at Providence Hospital.
Surgical preparation
Neonatal Yorkshire Duroc piglets of 10 ± 3 days (range = 6–14) of age weighing 2.4 ± 0.6 kg (range = 1.9–2.9 kg) were preanesthetized with an intramuscular injection of ketamine (33 mg/kg) and atropine (0.05 mg/kg). After intubation, the piglets received 1.5–3.0% isofluorane anesthesia while being mechanically ventilated (Draeger Medical Apollo Anesthesia Machine; Draeger, Germany). Arterial O2 saturation (SpO2) and partial pressure of carbon dioxide (PaCO2) were maintained by appropriate ventilatory adjustments in the 95–99% and 35–45 mmHg range, respectively. A warming mat and hot-air fan were used to maintain the core body temperature at 38 ± 0.5°C. Animals received intravenous (IV) heparin (200 units/kg/h), and an IV maintenance solution of physiologic saline (10 mL/kg/h) throughout the experiment. Serum glucose, lactate, base excess, and electrolyte levels were monitored closely and maintained in the normal range using 10 g/dL of dextrose–water (D10W), sodium bicarbonate, potassium chloride, and lactated Ringer's solution as needed. The left femoral vein and artery were cannulated for IV fluid administration, arterial blood pressure measurements, and blood sampling, respectively. Catheters were flushed with heparinized saline and secured with ligatures.
Arterial blood pressure, heart rate, SpO2, and body temperature were continuously monitored by the Datex-Ohmeda monitor (GE Healthcare, Milwaukee, WI, USA). etCO2, fraction of inspired oxygen (fiO2), and pulmonary compliance were also continuously monitored (Draeger Medical Apollo Anesthesia Machine; Draeger). The common carotid artery (CCA) was also isolated and instrumented for continuous blood flow measurements using a T400 series ultrasonic flow-meter (Transonic Systems Inc., Ithaca, NY, USA). Regional tissue O2 saturation of the brain (CrSO2), kidney (KrSO2), gut (GrSO2), and muscle (MrSO2) were monitored continuously by the INVOS regional tissue oxygenation monitor (INVOS 5100C; Somanetics) throughout the experiments. This monitor generates two wavelengths of light (730 and 810 nm) from light-emitting diodes, which are alternately illuminated, and utilizes a disposable sensor, incorporating two light-emitting diodes and two detectors, spaced 3 and 4 cm from the light-emitting diode. The 3-cm detector is assumed to measure primarily the light passing through the shallow structures while the 4-cm detector is measures the light passing through both the shallow structures and a deeper tissue. An empirically derived subtraction algorithm developed from data obtained in normal adult volunteers[13] is used to correct for the signal from the shallow tissues, and rSO2 is displayed as follows: ratio of oxyhemoglobin to total hemoglobin × 100, expressed as percent rSO2. Data are collected continuously and averaged at time intervals of 5 s.
Regions of the skin where the INVOS sensors were placed were cleaned with warm soap and water, and hair was removed by shaving and the application of a depilatory. The sensors were placed on the head, the dorsal region of the abdomen over the right kidney, on the abdomen lateral to the umbilicus, and on the right leg over the gluteus muscle to continuously measure regional CrSO2, KrSO2, GrSO2, and MrSO2, respectively. All the data acquired by the Datex-Ohmeda monitor, Apollo Anesthesia Machine, the INVOS, and CCA blood flow were collected in real time at a sampling rate of 12, 46, 9–10, and 240 samples/min, respectively, and stored on a personal computer for offline analysis (the RugLoop program; Dmed, Temse, Belgium).
Serum electrolytes and glucose levels and hemoglobin concentration were checked at the start of the experiment and every 30 min while serum lactate levels, and arterial blood gasses (ABG) were measured 15 min after each ventilation rate change by iSTAT (Abbott Point of Care Inc. Princeton, NJ, USA). The hemoglobin concentration was also determined by co-oximetry (Instrumentation Laboratory, Lexington, MA, USA).
Experimental protocols
Hypocapnia during normothermic conditions
The changes in BP, HR, CCA blood flow, and cerebral, renal, intestinal, and muscle rSO2 in response to changes in the ventilation rate were followed in normotensive anesthetized neonatal piglets[Figure 1]. In addition, serum electrolytes, glucose and lactate levels, and hemoglobin concentration were measured every 30 min. Blood gasses were obtained at the end of every ventilatory period. Following the stabilization period, the baseline measurements were recorded followed by the initiation of changes in the ventilation rate.
Figure 1.
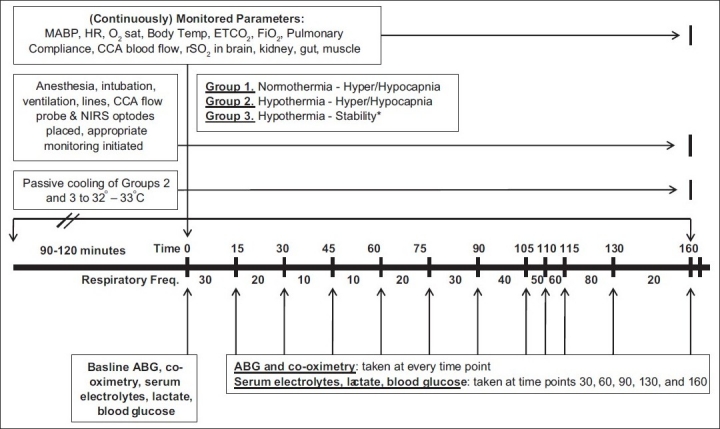
Experimental protocol
Animals were allowed to stabilize with a ventilator frequency of 20. The ventilation settings were subsequently increased to cause a decrease in the etCO2. The initial ventilation settings were as follows: Pinsp = 20, Freq = 30, Tinsp = 0.4, PEEP = 3. Once stable hemodynamic values were achieved, the ventilation frequency was increased by increments of 10 until a value of 80 was achieved with 15 min between each change with the exception of rates from 50 to 80 where there were 5 min between each change.
Throughout the experiment, the SpO2 and tidal volume were maintained by modifying the fiO2 and ventilatory pressure, respectively.
Hypercapnia and hypocapnia during normothermic conditions
Animals were allowed to stabilize with ventilation settings to produce an etCO2 of 25–30 mmHg. The ventilation settings were varied sequentially to increase or reduce the etCO2. The initial ventilation settings were as follows: Pinsp = 20, Freq = 30, Tinsp = 0.4, PEEP = 3. Once stable hemodynamic and rSO2 values were achieved, the ventilation frequency was decreased by increments of 10 until a value of 10 was reached with 15 min between each change. Following the period of hypercapnia, hypocapnia was induced by increasing the frequency of ventilation. The frequency was increased in increments of 10 to a maximum of 80 with 15-min periods between each change with the exception of rates from 50 to 80 where there were 5 min between each change.
Throughout the experiment, the SpO2 and tidal volume were maintained by modifying the fiO2 and ventilatory pressure, respectively.
Capnia changes during hypothermia
The animals’ body temperature was allowed to decrease passively to 32–33°C. Briefly, the heating pad was turned off and the hot-air fan was turned to air only. All blankets were removed from the animal and the body temperature was allowed to decrease. After the animal's core body temperature reached 32–33°C, it was allowed to stabilize with ventilation settings to produce an etCO2 of 25–30 mmHg. The ventilation settings required to achieve the above-said etCO2 were as follows: Pinsp = 20, Freq = 30, Tinsp = 0.4, PEEP = 3. Once stable values were achieved, the frequency was decreased by increments of 10 until a value of 10 was reached with 15 min between each change. Following the period of hypercapnia, hypocapnia was induced by increasing the frequency of ventilation. The frequency was increased in increments of 10 to a maximum of 80 with 15-min periods between each change with the exception of rates from 50 to 80 where there were 5 min between each change. Throughout the experiment, the SpO2 and tidal volume were maintained by modifying the fiO2 and ventilatory pressure, respectively.
Serum electrolytes, glucose and lactate levels, and hemoglobin concentration were measured every 30 min. Blood gasses were obtained at the end of every ventilatory period.
Hypothermic stability studies
Four piglets were anesthetized, ventilated, and instrumented as previously described. The animals were allowed to cool to reach a body temperature of 33°C. To achieve cooling, after surgical preparation and instrumentation, the heating pad was turned off and the hot-air fan was turned to air only. All blankets were removed from the animal and the body temperature was allowed to decrease. After reaching a cooled temperature, the animals were monitored for 4 h to document the cardiovascular stability of the preparation.
Equipment and materials
The INVOS 5100C and pediatric soma-sensors were provided by Somanetics Corporation. Electrolyte and arterial blood gas cartridges for the iSTAT were purchased from Abaxis (Union City, CA, USA). All drugs and solutions used were obtained through the veterinary facility at Providence Hospital.
Statistical analysis
Data from the RugLoop program were saved in Excel workbooks. To analyze the effects of etCO2 changes, etCO2, and cerebral rSO2, the mean values of all parameters monitored were calculated for every 1-min block of data collection and the last 5 min of each ventilatory block. We used the mean values of the last 5 min of each ventilatory block as we assumed that this period represented the equilibrium of the effects at each ventilation rate most accurately. The Spearman correlation coefficient was used to prove correlations between CC flow, etCO2, and CrSO2. ANOVAs were done using SPSS 19 to determine significant differences in CrSO2, etCO2, and CC flow due to changes in the respiratory rate within a group. Additionally, Student's t-tests were done using SPSS 19 to determine significant differences between experimental and control groups.
RESULTS
CrSO2 and common carotid flow responses to changes in ventilation
Figure 2 shows the effects of changes in the ventilation on etCO2, cerebral regional oxygen saturation, and CC blood flow. As seen in panels A and B of Figure 2, CrSO2 and CC flow tracked etCO2 throughout the experiment.
Figure 2.
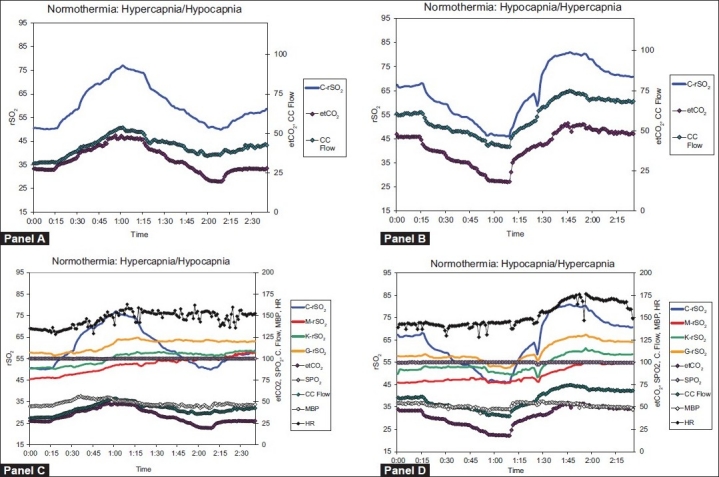
Average graphs for normothermic response to hypercapnia (Panels A and C; n = 6) and hypocapnia (Panels B and D; n = 6) in newborn piglets. Panels A and B show traces of cerebral rSO2, mean arterial blood pressure, and common carotid artery flow. Panels C and D depict all measurements recorded during the experiment
Hypercapnia during normothermic conditions
To determine the effects of hypercapnia on CrSO2 and CCA flow, the ventilation rate was decreased to increase the etCO2. Cerebral regional oxygen saturation, etCO2, and CC flow significantly increased with the decreasing ventilatory rate (P < 0.001; Figure 3A–C). The correlations between the cerebral rSO2, the CC flow, and etCO2 were determined. As seen in Figure 3D–F, there was a significant correlation between the change in CrSO2 and etCO2(r2 = 0.996; P < 0.001), the change in CC flow and etCO2 (r2 = 0.9652; P<0.001), and CrSO2 and CC flow (r2 = 0.971; P < 0.001).
Figure 3.
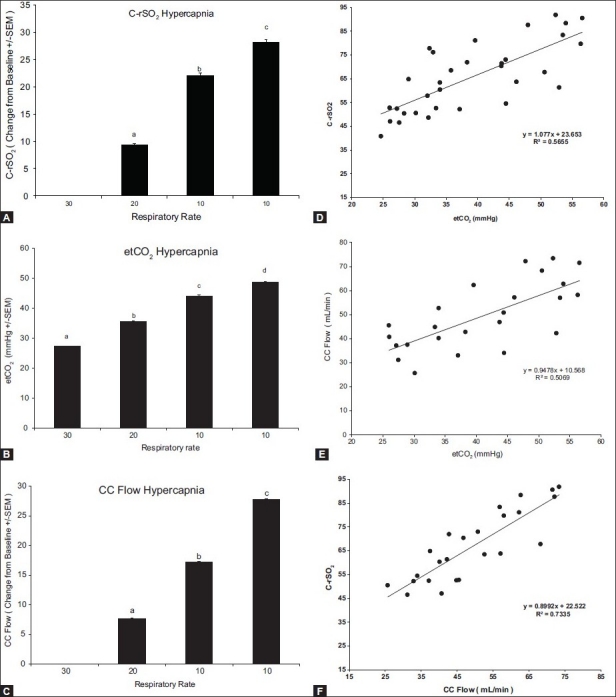
Response to hypercapnia. Change from baseline values for C-rSO2 (A), etCO2 (B), and CC flow (C). Bars represent the average of six animals and each letter represents a significant difference between the bars. Panels D–F depict the correlation between C-rSO2 and etCO2, CC flow and etCO2, and C-rSO2 and CC flow, respectively. All parameters correlate significantly
Hypocapnia during normothermic conditions
To determine the effects of hypocapnia on the cerebral vascular response, the ventilation rate was increased to decrease etCO2. There was a significant decrease in cerebral regional oxygen saturation, CC flow, and etCO2 with increasing ventilation rates (P < 0.001; Figure 4A–C). Figure 4D–F depicts the correlation between CrSO2 and etCO2, CC flow and etCO2, and also CrSO2 and CC flow, respectively. etCO2 correlated with CrSO2 (Figure 4, Panel D; r = 0.75, r2 = 0.57, P < 0.0001) and CC flow (Figure 4, Panel E; r = 0.71, r2 = 0.51, P < 0.0001). No changes in regional saturation of the kidney, gut, or muscle were observed during the manipulation of CO2 levels (data not shown). CrSO2 also correlated significantly with the CC flow (Figure 4, Panel F; r = 0.85, r2 = 0.73, P < 0.0001).
Figure 4.
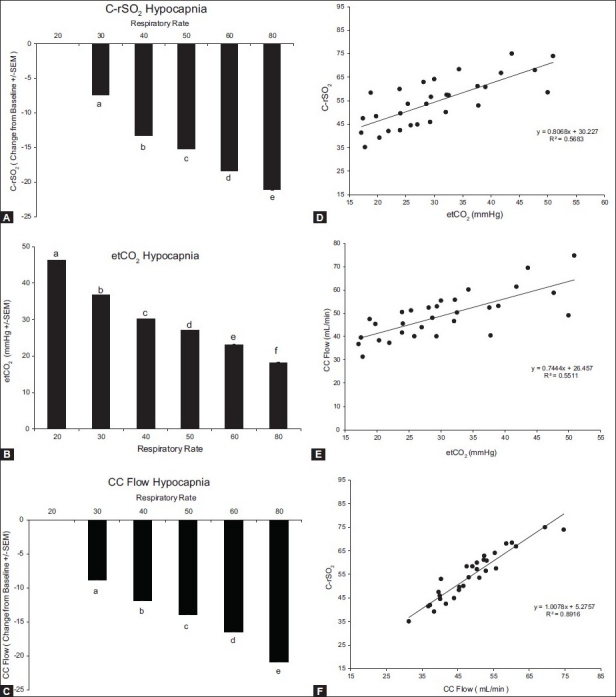
Response to hypocapnia. Change from baseline values for C-rSO2 (A), etCO2 (B), and CC flow (C). Bars represent the average of six animals and each letter represents a significant difference between the bars. Panels D-F depict the correlation between C-rSO2 and etCO2, CC flow and etCO2, and C-rSO2 and CC flow, respectively. All parameters correlate significantly
Effects of CO2 loading on hypocapnic response
Naïve animals responded to hyperventilation with a significant decrease in CrSO2 and CC flow that tracked the decrease in etCO2. A separate group of animals was run to determine the effects of hyperventilation in the presence of high PaCO2 levels. As seen before, there was a significant decrease in cerebral regional oxygen saturation, CC flow, and etCO2 with increasing ventilation rates (P < 0.001; Figure 5A–C). The magnitude of the response was muted in the animals that had high PaCO2 (P < 0.001). The decrease in etCO2 correlated with both the decrease in CrSO2(Figure 5, Panel D; r = 0.84, r2 = 0.70, P < 0.0001) and decrease in the CC flow (Figure 5, Panel E; r = 0.53, r2 = 0.28, P < 0.001). Cerebral oxygen saturation correlated significantly with the CC flow (Figure 5, Panel F; r = 0.74, r2 = 0.55, P < 0.0001).
Figure 5.
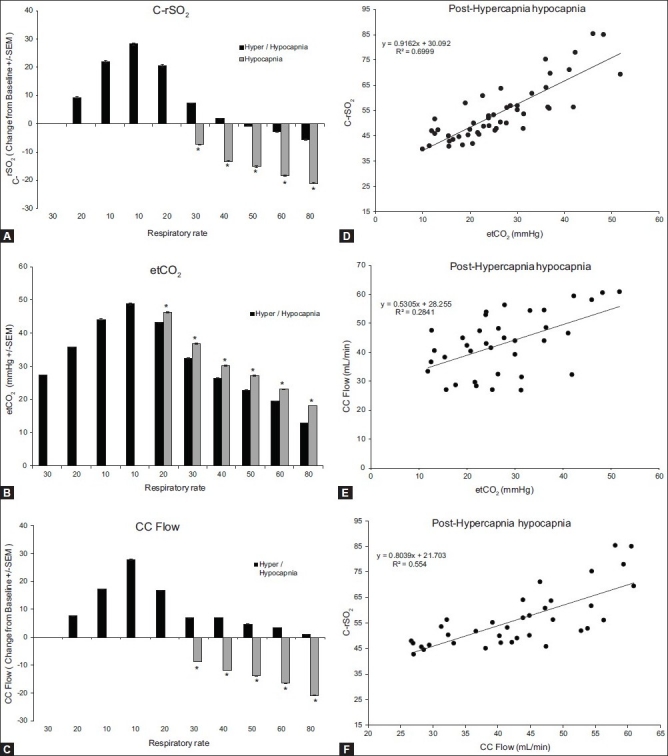
Response to hypocapnia after CO2 preloading. Change from baseline values for C-rSO2 (A), etCO2 (B), and CC flow (C). Bars represent the average of six animals and each letter represents a significant difference between the bars within the group. Asterisks represent a significant difference between the CO2-preloaded group and the naïve group. Panels D–F depict the correlation between C-rSO2 and etCO2, CC flow and etCO2, and C-rSO2 and CC flow, respectively. All parameters correlate significantly
Hypercapnia during hypothermic conditions
To determine the effects of hypothermia on hypercapnia, the piglet was cooled to 33°C and the hypercapnia experiment was repeated. Under cooled conditions, the pattern of changes in the response to etCO2 were similar to those in the normothermic animals; however, the magnitude of the change was significantly different in the hypothermic group (P < 0.001) Figures 6A–C. etCO2 increased with decreasing ventilation frequencies. CC flow and cerebral regional oxygen saturation (rSO2) mirrored the changes in etCO2. The changes in rSO2 during etCO2 changes were specific to the brain as seen by the fact that changes in rSO2 of the muscle, kidney, or gut were observed during the experiment (data not shown).
Figure 6.
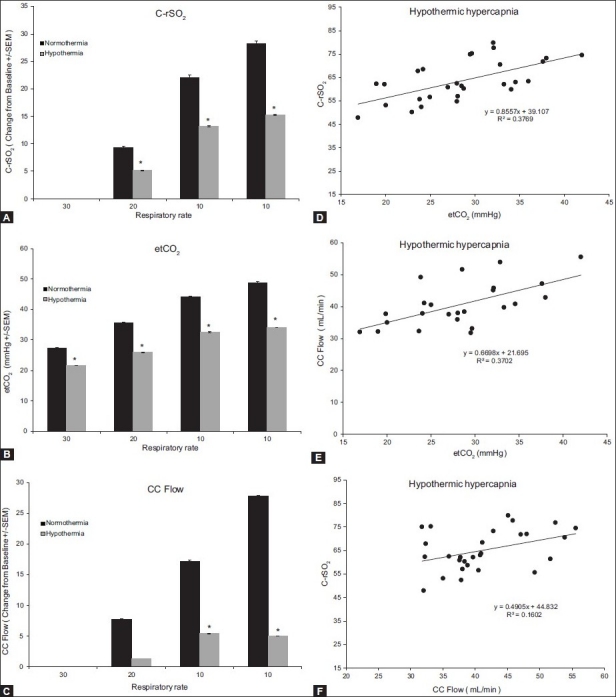
Response to hypercapnia during hypothermia. Change from baseline values for C-rSO2 (A), etCO2 (B), and CC flow (C). Bars represent the average of six animals and each letter represents a significant difference between the bars within the group. Asterisks represent a significant difference between the normothermic and hypothermic groups. Panels D–F depict the correlation between C-rSO2 and etCO2, CC flow and etCO2, and C-rSO2 and CC flow, respectively. All parameters correlate significantly
The increase in etCO2 correlated with both the increase in CrSO2 relative to baseline (Figure 6, Panel D; r = 0.61, r2= 0.38, P < 0.001) and increase in the CC flow relative to baseline values (Figure 6, Panel E; r = 0.61, r2 = 0.37, P < 0.01). Cerebral oxygen saturation correlated significantly with the CC flow (Figure 6, Panel F; r = 0.40, r2 = 0.16, P < 0.05).
Hypothermic stability studies
Four animals were allowed to cool to a body temperature of 33°C and were monitored for 4 h. As expected, animals with a low body temperature had a lower heart rate than normothermic animals. All other parameters were similar to animals with normal core body temperatures. All parameters (CrSO2, MrSO2, KrSO2, GrSO2, HR, MBP, and CC flow) were stable throughout the 4-h time period (data not shown).
DISCUSSION
Perfusion problems are common challenges in neonatal intensive care. PVL and IVH have both been associated with changes in cerebral perfusion.[10,14,24] PVL is characterized by the destruction of the white matter surrounding the ventricles which is thought to be caused by a lack of oxygen or blood flow to the periventricular area resulting in cell death and loss of function. Infants with PVL are at risk for motor disorders, delayed mental development, coordination problems, and vision and hearing problems. PVL is also associated with an increased risk for hemorrhage in the periventricular intraventricular area which can lead to cerebral palsy.[2,6] IVH involves hemorrhage of vessels in the germinal matrix that are fragile and rupture easily. Infants with respiratory problems are more likely to develop IVH and as with PVL, there is no real treatment for IVH. The first symptoms of either PVL or IVH occur after the damaging event has happened, forcing physicians to treat the symptoms rather than prevent the problem. We believe that the INVOS system can be an early indication of perfusion problems in the brain and may allow physicians to correct low perfusion before the tissue has become damaged.
Infants born at less than 30 weeks of gestational age have underdeveloped lungs and may require treatment surfactant therapy with conventional mechanical ventilation or high-frequency ventilation. Changes in the ventilatory rate or lung compliance have the possibility of rapidly altering the level of arterial CO2. The autoregulation of flow is not consistent in the neonatal brain and CO2 can cause the dilation or constriction of cerebral vessels by acting on the vessels directly, thereby avoiding the protective effects of autoregulation.[17] Currently, blood gasses are the best way to determine how ventilation is affecting the levels of CO2. However, blood gas determination is infrequent and may contribute to anemia in the preterm infant. The present study determined the ability to use the INVOS cerebral-somatic oximeter (Somanetics Corporation) to track the level of cerebral oxygenation during changes in ventilatory management in an anesthetized newborn piglet model.
INVOS noninvasively measures the regional hemoglobin oxygen saturation in tissue beneath the probe and is venous weighted. rSO2 is responsive to all the physiologic factors that influence oxygen availability in the brain such as vascular anatomical variability, hemoglobin dissociation, cardiac output, dyshemoglobinemias, blood pH, vascular permeability, vasoactive drugs, and metabolic demand, resulting in an arteriovenous (AV) difference. Independent of the cause, however, inappropriate cerebral O2 levels can result in neuronal damage producing neurocognitive or sensory/motor deficits, or retinal detachment in neonates. The regional oxygen saturation of the brain, or any tissue, is dependent on several factors including blood flow to the tissue, blood pressure, CO2 levels, fiO2, and metabolic demand. This study demonstrates the utility of the INVOS system for tracking the effects of CO2 changes on blood flow and oxygen delivery to the brain.
This study found a statistically significant correlation between etCO2 and cerebral rSO2(CrSO2; Figure 4, Panel D; r = 0.75, r2 = 0.57, P < 0.0001). Clinically, etCO2 does not always correlate with the arterial tension of CO2(PaCO2) and cannot be used as an accurate representation of CO2 levels in the patient. In our study, we have demonstrated that etCO2 significantly correlates with our periodic blood gas readings of PaCO2(r = 0.72, r2 = 0.51, P < 0.0001). We further demonstrated a significant correlation between etCO2 and CC flow (Figure 4, Panel E; r = 0.71, r2 = 0.51, P < 0.0001). Furthermore, the CCA flow significantly correlated with CrSO2(Figure 4, Panel F; r = 0.85, r2 = 0.73, P < 0.0001). This correlation indicates that, in our study, when demand of the tissue, fiO2, and all other variables affecting CrSO2 are held constant, then the increase or decrease in rSO2 that we observe is a result of an increase or decrease in cerebral blood flow. Notably, we can visualize when the brain is reaching a dangerously low level of blood flow or oxygen saturation, thereby possibly preventing dangerous conditions as associated with IVH and PVL which are thought to be a result of ischemic or low-flow state in the brain.
We also investigated the cerebral response to changes in ventilation after CO loading, which may be seen with birth asphyxia, low respiratory rate, or apnea before the use of high-frequency ventilation. Figure 5, Panels A–C, shows the response to hypocapnia following a hypercapnic period. When the animal was pretreated with high CO2 levels, the response to hypocapnia was muted compared to hypocapnia alone. As seen before, our model demonstrated a strong correlation between etCO2 and PaCO2 following CO2 loading (r = 0.87, r2 = 0.76, P < 0.0001). The effects of increased CO2 levels prior to hypocapnia were analyzed by determining the change in CrSO2 and CC flow from baseline. Rather than absolute CrSO2 and CC flow values, this analysis was used because the increased CO2 levels had an effect on the baseline value. As before, there was a statistically significant correlation between etCO2 and change in cerebral rSO2(CrSO2; Figure 5, Panel D; r = 0.84, r2 = 0.70, P < 0.0001). We also demonstrated a significant correlation between etCO2 and CC flow (Figure 5, Panel E; r = 0.53, r2 = 0.28, P < 0.001) as well as between CC flow and CrSO2(Figure 5, Panel F; r = 0.74, r2 = 0.55, P < 0.0001). Even in the presence of high blood levels of CO2, the change in cerebral rSO2 values indicate a change in the cerebral blood flow.
The third set of experiments aimed to determine the effects of hypothermia on response to ventilation. Hypothermia is thought to protect tissue due to reduced demand at cool temperatures and is used frequently in the NICU after birth asphyxia. Hypothermia stunted the response to CO2 changes as seen in both cerebral rSO2 and CCA flow. Figure 6, Panels A and C, demonstrates a reduced change in CrSO2 and CC flow due to changes in rate of ventilation. The reduction in CrSO2 was most likely due to the reduction in the CC flow. The oxygen demand of the cerebral tissue should decrease with cooling thereby increasing CrSO2 levels, but the decrease in the flow may have been greater than the decrease in demand of the tissue. Although the response was diminished, the changes in CrSO2 and CC flow still correlated significantly. As before, there was a statistically significant correlation between etCO2 and cerebral rSO2(CrSO2; Figure 6, Panel D; r = 0.61, r2 = 0.38, P < 0.001). We also demonstrated a significant correlation between etCO2 and CC flow (Figure 6, Panel E; r = 0.61, r2 = 0.37, P < 0.01) as well as between CC flow and CrSO2 (Figure 6, Panel F; r = 0.40, r2 = 0.16, P < 0.05).
In conclusion, our study has demonstrated the utility of the INVOS system in the neonatal ICU during ventilatory manipulation. The use of INVOS allows physicians to visualize the amount of oxygenation in the brain during various manipulations in ventilation. Furthermore, it gives neonatologists the ability to ensure proper oxygenation of the brain and prevent periods of ischemia or severe hypoxia which may lead to conditions such as IVH and PVL.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/65/81722
Contributor Information
Erin A. Booth, Email: boothea@umich.edu.
Christopher Dukatz, Email: cedukatz1@gmail.com.
Beena G Sood, Email: bsood@med.wayne.edu.
Michael Wider, Email: mwider@wayne.edu.
REFERENCES
- 1.Abdul-Kaliq H, Troitzsch D, Berger F, Lange PE. Comparison of regional transcranial oximetry with NIRS and jugular venous bulb oxygen saturation. Biomed Tech. 2000;45:328–32. doi: 10.1515/bmte.2000.45.11.328. [DOI] [PubMed] [Google Scholar]
- 2.Adams-Chapman I. Insults to the developing brain and impact on neurodevelopmental outcome. J Commun Disord. 2009;42:256–62. doi: 10.1016/j.jcomdis.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Ambalvanan N, Carlo WA. Hypocapnia and hypercapnia in respiratory management of newborn infants. Clin Perinatol. 2001;28:517–31. doi: 10.1016/s0095-5108(05)70104-4. [DOI] [PubMed] [Google Scholar]
- 4.Booth EA, Dukatz C, Ausman J, Wider M. Cerebral and somatic venous oximetry in adults and infants. Surg Neurol Int. 2010;1:75–80. doi: 10.4103/2152-7806.73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Montanini S. Monitoring cerebral oxygen saturation in elderly patients undergoing general abdominal surgery; a prospective cohort study. Eur J Anesth. 2006;24:59–65. doi: 10.1017/S0265021506001025. [DOI] [PubMed] [Google Scholar]
- 6.Chow PP, Horgan JG, Taylor KJ. Neonatal periventricular leukomalacia: Real-Time sonographic diagnosis with CT correlation. Am J Radiogr. 1985;145:155–60. doi: 10.2214/ajr.145.1.155. [DOI] [PubMed] [Google Scholar]
- 7.Curley G, Kavanagh BP, Laffey JG. Hypocapnia and the injured brain: More harm than benefit. Crit Care Med. 2010;38:1348–59. doi: 10.1097/CCM.0b013e3181d8cf2b. [DOI] [PubMed] [Google Scholar]
- 8.Denault A, Deschamps A, Murkin J. A proposed algorithm for the intraoperative use of cerebral NIRS. Semin Cardiothorac Vasc Anesth. 2007;11:274–81. doi: 10.1177/1089253207311685. [DOI] [PubMed] [Google Scholar]
- 9.Edmonds HL, Ganzel BL, Austin EH. Cerebral oximetry for cardiac and vascular surgery. Semin Cardiothorac Vasc Anesth. 2004;8:147–66. doi: 10.1177/108925320400800208. [DOI] [PubMed] [Google Scholar]
- 10.Erickson SJ, Grauaug A, Gurrin L, Swaminathan M. Hypocarbia in the ventilated preterm infant and its effect on intraventricular haemorrhage and bronchopulmonary dysplasia. J Pediatr Child Health. 2002;38:560–2. doi: 10.1046/j.1440-1754.2002.00041.x. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb EA, Fraser CD, Andropoulos DB, Diaz LK. Bilateral monitoring of cerebral oxygen saturation results in recognition of aortic cannula malposition. Pediatr Anesth. 2006;16:787–9. doi: 10.1111/j.1460-9592.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 12.Hansen NB, Nowicki PT, Miller RR, Malone T, Bickers RG, Menke JA. Alterations in cerebral blood flow and oxygen consumption during prolonged hypocarbia. Pediatr Res. 1986;20:147–50. doi: 10.1203/00006450-198602000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hongo K, Kobayashi S, Okudera H, Hokama M, Nakagawa F. Noninvasive cerebral optical spectroscopy; depth resolved measurements of cerebral haemodynamics using indocyanine green. Neurol Res. 1995;17:89–93. doi: 10.1080/01616412.1995.11740293. [DOI] [PubMed] [Google Scholar]
- 14.Hunt RW, Evans N, Rieger I, Kluckow M. Low SVC flow and neurodevelopment at 3 years in very preterm infants. J Pediatr. 2004;145:588–92. doi: 10.1016/j.jpeds.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 15.Ide K, Eliasziw M, Poulin MJ. Relationship between middle cerwebral artery blood velocity and end tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol. 2003;95:129–37. doi: 10.1152/japplphysiol.01186.2002. [DOI] [PubMed] [Google Scholar]
- 16.Kim MB, Ward DS, Cartwright CR, Kolano J, Chelebowski S, Henson LC. Estimation of jutular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit. 2000;16:191–9. doi: 10.1023/a:1009940031063. [DOI] [PubMed] [Google Scholar]
- 17.Kontos HA, Wei EP, Raper AJ, Patterson JL., Jr Local mechanism of CO2 action on cat pial arterioles. Stroke. 1977;8:226–9. doi: 10.1161/01.str.8.2.226. [DOI] [PubMed] [Google Scholar]
- 18.Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- 19.Murase M, Ishida A. Early hypocarbia of preterm infants: Its relationship to periventricular leukomalacia and cerebral palsy, and its perinatal risk factors. Acta Paediatrica. 2005;94:85–91. doi: 10.1111/j.1651-2227.2005.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 20.Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring Brain Oxygen Saturation During coronary artery bypass surgery: A randomized, Prospective Study. Anesth Analg. 2007;104:51–8. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 21.Nagdyman N, Ewert P, Peters B, Miera O, Fleck T, Berger F. Comparison of different NIRS cerebral oxygenation indices with central venous and jugular oxygenation saturation in children. Pediatr Anesth. 2008;18:160–6. doi: 10.1111/j.1460-9592.2007.02365.x. [DOI] [PubMed] [Google Scholar]
- 22.Poulin MJ, Liang PJ, Robbins PA. Fast and slow components of cerebral blood flow response to step decreases in etCO2 in humans. J Appl Physiol. 1998;85:388–97. doi: 10.1152/jappl.1998.85.2.388. [DOI] [PubMed] [Google Scholar]
- 23.Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol. 1970;23:394–403. doi: 10.1001/archneur.1970.00480290014002. [DOI] [PubMed] [Google Scholar]
- 24.Seri I. Low SVC flow during the first postnatal day and neurodevelopment in preterm neonates. J Pediatr. 2004;145:573–5. doi: 10.1016/j.jpeds.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Pelligrino D, Koenig HM, Albrecht RF. The role of endothelium and nitric oxide in rat pial arteriolar dilatory response to CO2 in vivo. J Cereb Blood Flow Metab. 1994;14:944–51. doi: 10.1038/jcbfm.1994.126. [DOI] [PubMed] [Google Scholar]
- 26.Yao F, Tseng C, Ho C, Levin S, Illner P. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;18:552–8. doi: 10.1053/j.jvca.2004.07.007. [DOI] [PubMed] [Google Scholar]


