Abstract
The plant hormone ethylene (ET) plays a crucial role in the signalling network when plants have to respond to biotic stresses. We investigate the beneficial interaction between the model plant Arabidopsis thaliana and the endophytic fungus Piriformospora indica. Recently, we showed that ET signalling and ETHYLENE RESPONSE FACTOR (ERF)1 are important to balance beneficial and nonbeneficial traits in this symbiosis. 147 ERF genes in Arabidopsis encode transcriptional regulators with a variety of functions involved in development, physiological processes as well as plant/microbe interactions. In the beneficial symbiosis between Arabidopsis and P. indica, overexpression of ERF1 activates defence responses, strongly reduces root colonization and thus abolishes the benefits for the plants. Here we show that additional transcription factors of the ERF family, the ERF DOMAIN PROTEIN9 (ERF9) and the ETHYLENE-RESPONSIVE ELEMENT BINDING FACTOR14 (ERF14) are involved in the interaction between the two symbionts and are required for growth promotion of the host plant. Expression of these genes is upregulated in colonized wild-type roots. Insertional inactivation of ERF9 and ERF14 diminishes the P. indica-induced growth promotion and activates the expression of the PATHOGENESIS-RELATED (PR)-1 and PR-2 genes. We propose that ERF9 and ERF14 repress PR gene expression in colonized Arabidopsis roots and thus contribute to the establishment of a beneficial interaction.
Key words: Piriformospora indica, ethylene, ERF transcription factor, ERF9, ERF14, plant defense, plant/microbe interaction
Introduction
Recently we have shown that ethylene (ET) signalling and ET-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and the model plant Arabidospsis thaliana.1 P. indica belongs to the Sebacinales and colonizes roots of many plant species inter- and intracellularly including Arabidopsis. The fungus forms pear-shaped spores which accumulate in the roots as well as on the root surface, stimulates growth and seed production, confers resistance against abiotic (water and salt) stress and protects the plant against pathogen infections.2–4 Mutants impaired in ET perception, signal transduction or ET-targeted transcription factors were examined in Camehl et al.1 Growth of ETR1, EIN2 and EIN3/EIL1 deletion mutants was not promoted or even inhibited by P. indica. Overexpression of ERF1 promoted defence responses in the presence of the fungus and abolished the benefits for the plants. Besides ET, ERF1 is a target of jasmonic acid (JA) signalling in Arabidopsis.5 Inactivation of ERF1 is not feasible because of the redundant function of the ERF family members. Therefore we (like others) investigated seedlings overexpressing ERF1 under the control of the 35S promoter. P. indica-induced promotion of shoot growth was reduced and of root growth was completely abrogated compared to uncolonized 35S::ERF1 control seedlings. Expression of the defence genes PR-1, PR-2, PR-5 and PDF1.2, but not of PR-3, PR-4 and LOX1 was stimulated by the fungus in 35S::ERF1, but not in wild-type roots. We concluded that defense responses become more efficiently activated against P. indica in 35S::ERF1 plants.
Here, we present data for two other transcription factors belonging to the ERF family within the superfamily AP2/ERF, which contains 147 members in Arabidopsis.6 The AP2/ERF superfamily is defined by the ERF domain, which consists of 60 to 70 conserved amino acids involved in DNA binding. The ERF domain was first identified in the four DNA-binding proteins NtERF1-4 from Nicotiana tabacum,7 which recognize a conserved GCC box present in several ET-inducible genes, e.g., in those for pathogenesis-related (PR) proteins.8,9 Flanking sequences of the GCC box affect the binding of ERFs, thus it is likely that different ERFs regulate different target genes with a conserved GCC sequence in their promters.10
The AP2/ERF superfamily is divided into the RAV, AP2 and ERF families. Among them, the ERF family is the largest with 122 members and is further divided into two subfamilies, the CBF/DREB subfamily also called subgroup A and the ERF subfamily, called subgroup B.11 The roles of the transcription factors of subgroup A are mainly involved in the regulation of abiotic stress responses. For example, DREB1A is induced by low-temperature stress, and DREB2A by dehydration12 and salt stress13 in Arabidopsis. In contrast all transcription factors involved in disease resistance are found in the subgroup B which includes 65 members.6,11 Since many of them are regulated by similar stimuli, a high degree of functional redundancy is expected, therefore, isolation of knock-out mutants for single transcription factor genes encoding members of the B family to discover specific phenotypes is not common. The best studied transcription factor is ERF1, and various overexpressor lines were generated and investigated.5,14 In 2007, Oñate-Sánchez et al.15 showed that ETHYLENE-RESPONSIVE ELEMENT BINDING FACTOR14 (ERF14) plays a key role in defense against several pathogens including Fusarium oxysporum. Here, we demonstrate that ERF14 and another member of the B-family, ERF9, are also involved in the beneficial interaction between Arabidopsis and P. indica.
Tsutsui et al.16 showed in 2009 that DEAR1 (a member of the A family) plays a regulatory role in both freezing tolerance and response to pathogen infection. The authors concluded that DEAR1 mediates the crosstalk between abiotic and biotic stress signalling pathways in plants. ERF9 has a motif in its promoter region which can act as a target of DEAR1.16 ERF9 expression is strongly reduced in DEAR1 overexpressor lines whereas PR-1, -2, -3 and -5 are constitutively upregulated.16 Here, we present evidence that ERF9 might be a negative regulator of PR gene expression. Since the ERF14 and ERF9 mRNA levels are upregulated in P. indica-colonized Arabidopsis roots, both members of the subgroup B were further investigated in this study.
Results
ERF9 and ERF14 seedlings are affected in their growth response to P. indica.
The transcript levels of ERF9 and ERF14 are upregulated in the roots of wild type plants exposed to P. indica for 7 days (Fig. 1A). Therefore, we generated homozygote knock-out lines for ERF9 and ERF14. Since the insertion in ERF14 is in the exon, no transcripts can be detected in a semi-quantitative RT-PCR analysis, whereas ERF9 contains a T-DNA insertion in the 5′-untranslated region and, thus, transcripts are still present in the mutant, but the amount is less than in the wild type (Fig. 1B). Databank analysis reveals that the ERF9 mRNA might be longer than the annotated full length cDNA, therefore, besides a reduction in the mRNA level in the insertion line, also the stability and/or translatability of the ERF9 transcript might be affected.
Figure 1.
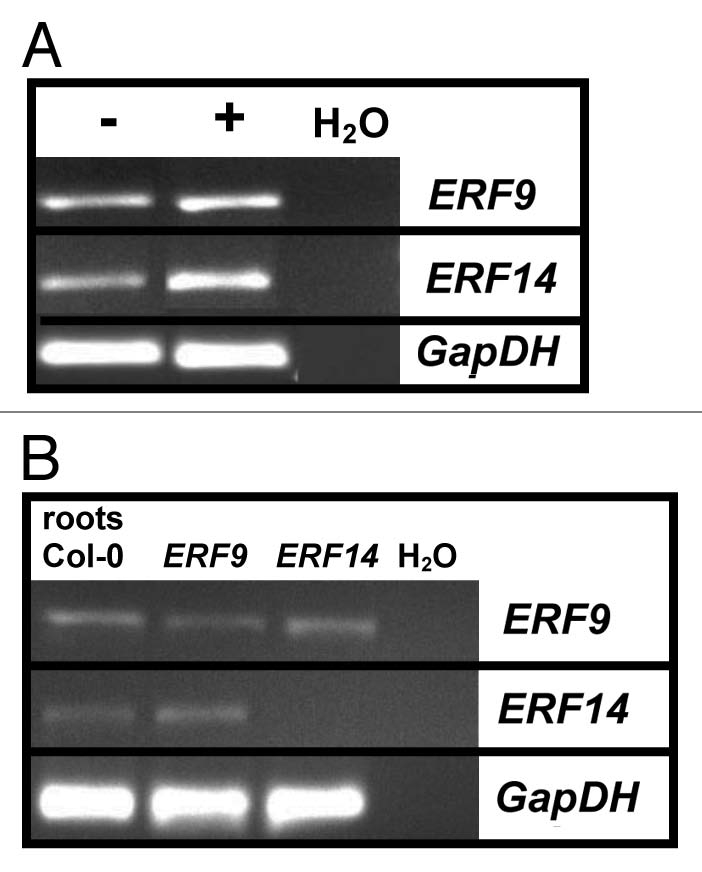
(A) RNA was isolated from Arabidopsis wild type roots seven days after inoculation with P. indica (+) or without (−) prior to RT-PCR analysis. (B) RNA was isolated from the knock-out mutant and Col-0 wild type roots prior to RT-PCR analysis.
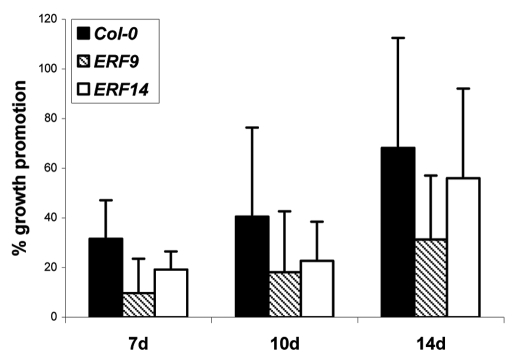
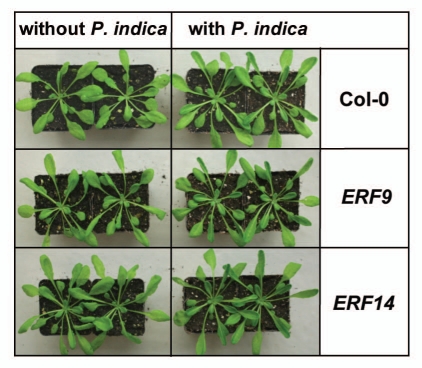
Under our co-cultivation conditions of the two symbionts, growth of wild-type Arabidopsis seedlings is stimulated by P. indica and the seedlings are taller than the uncolonized control seedlings.3 Also growth of the two ERF insertion lines is stimulated by the beneficial fungus, however the stimulatory effect is less compared to wild type seedlings (Fig. 2). The same trend was observed for adult insertion plants transferred to soil (Fig. 3). Although these differences are not significant according to the students T-test, the tendency of the response to the fungus in all individual experiments is similar and comparable to the behaviour of 35S::ERF1,1 for which we could detect significant differences in the response to P. indica. Upregulation of the mRNA levels and the response of the insertion lines to the fungus suggest that ERF9 and ERF14 participate in the beneficial interaction between the two symbionts.
Figure 2.
Root fresh weights of wild-type (Col-0) and mutant seedlings (ERF9/ERF14) 7, 10 and 14 days after inoculation with P. indica. The graph shows percent growth promotion by the fungus. Mean of four independent experiments with SE.
Figure 3.
Plants grown 10 days on MS media were transferred to soil which was either inoculated with or without 1% P. indica mycel. Plants were grown for four weeks under short day conditions.
PR-1 is upregulated in colonized ERF9 and PR-2 in colonized ERF14 seedlings.
Since it is known that ERF14 plays a role in plant defense,15 we tested several marker genes for different defense pathways in Arabidopsis roots. PR-1, PR-2, PR-3 and PR-4 are believed to be involved in systemic acquired resistance (SAR).20 Interestingly, under our growth conditions PR-1 is downregulated in ERF14, irrespective of whether the mutant is grown in the presence or absence of P. indica for 7 days (Fig. 4). PR-1 expression in ERF9 is comparable to wild type seedlings, which may be caused by the residual amount of the ERF9 mRNA in the insertion line (Fig. 1B).
Figure 4.
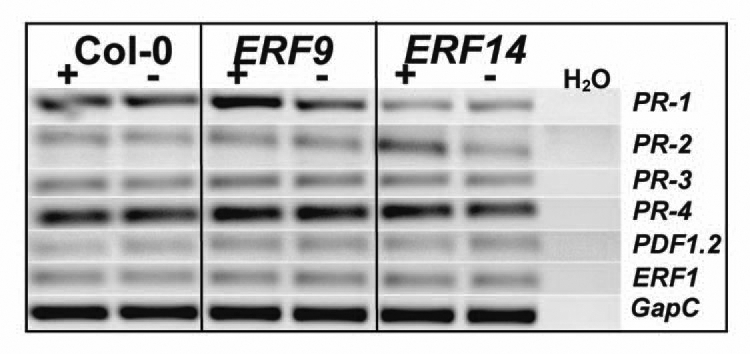
mRNA levels for different defence genes in the roots of wild type (Col-0) and mutant roots (ERF9/ERF14) cocultivated with (+) or without (−) the fungus for seven days.
However, we observe an upregulation of the PR-1 mRNA level in colonized ERF9 roots relative to the uncolonized ERF9 control, similar to the results obtained for 35S::ERF1.1 In contrast, PR-2 is clearly upregulated in colonized ERF14 seedlings relative to the uncolonized ERF14 control, again similar to the results obtained for 35S::ERF1. No regulation of PR-1 is observed in colonized ERF14 and no regulation of PR-2 in colonized ERF9 roots, and both PR genes are also not upregulated in colonized wild-type roots (Fig. 4). These results suggest ERF9 represses P. indica-induced PR-1 and ERF14 repress P. indica-induced PR-2 expression in wild-type roots (cf. Discussion). Although the individual responses are difficult to compare due to different manipulations of the ET signalling pathway in the individual ERF mutants tested, it is reasonable to assume that repression of PR expression in the ERF mutants may be involved in the establishment of a beneficial interaction. The reduced growth response of the ERF mutants to P. indica correlates with the upregulation of PR expression in these mutants.
The PR-3 and PR-4 mRNA level do not respond to P. indica in the two insertion lines (Fig. 4), similar to their regulation in 35S::ERF1.1 Furthermore, PDF1.2 expression is also not regulated in the two insertion lines, although this gene responded strongly to the fungus in the 35S::ERF1 overexpressor. Finally, in none of the ET signalling and ET-related transcription factor mutants analysed, the ERF1 mRNA level responded to P. indica in the roots (Fig. 4).1
Discussion
In the article by Camehl et al.1 we showed that upregulation of ERF1 causes an imbalance of beneficial and nonbeneficial traits in the P. indica/Arabidopsis symbiosis. Here we extend these studies for two other ET-responsive transcription factors, ERF9 and ERF14, by studying the response of corresponding insertion lines to the fungus. Growth of ERF14 and ERF9 is still promoted by P. indica, but less than in wild type (Figs. 2 and 3). Thus, complete (ERF14) or partial (ERF9) inactivation of a single ERF transcription factor results already in an altered response to P. indica. The weaker effects observed in the studies here compared to those reported for the 35S::ERF1 line are probably caused by different experimental manipulations of the ERF protein levels (overexpression vs. knock-out or knock-down), by redundant functions of the ERF proteins which prevents a complete loss of function in insertion lines, or by the fact that ERF9 is only partially inactivated. Nevertheless, all results support the idea that ERFs participate in the interaction between Arabidopsis and P. indica. Furthermore, there are unique features of the investigated transcription factors which have not yet been considered so far.
ERF9 is unique within the ERF family because it contains an “ET response factor-associated amphiphilic repression” (EAR) motif, which might function as a transcriptional repressor.21 Furthermore, a “dehydration-responsive element binding” motif 5′-TACCGACAT-3′)—first identified in the promoter of the drought-responsive gene RD29A from Arabidopsis22—is also present in the ERF9 promoter. This motif binds DREB transcriptional regulators,23 one of them is DEAR1. Tsutsui et al.16 have shown that overexpression of DEAR1 leads to a repression of ERF9 expression. On the other hand PR genes and PDF1.2 are constitutive upregulated in the DEAR1 overexpressor line, which also accumulates endogenous salicylic acid (SA). The authors proposed that DEAR1 functions as a negative transcriptional regulator of the SA- and the ET/JA-induced signalling pathways. The higher ERF9 mRNA level in the P. indica-colonized roots is consistent with conclusions from Tsutsui et al.16 that ERF9 might be involved in PR gene expression. Since relatively little is known about the function of ERF9, a complete knock-out line or an overexpression line for ERF9 would help to understand the role of this putative repressor in both beneficial and pathogenic plant/microbe interactions.
PR-1 and PR-2 expression is differentially regulated in the roots of colonized ERF9 and ERF14 seedlings. PR-1 is upregulated in ERF9 roots after treatment with P. indica, but not in ERF14 and wild-type roots (Fig. 4). Likewise, PR-2 is upregulated in ERF14 roots after treatment with P. indica, but not in ERF9 and wild-type roots. This is consistent with the idea that P. indica stimulates ERF9 (ERF14) gene expression (Fig. 1) in order to repress PR-1 (PR-2) gene expression for establishing a mutualistic interaction (Fig. 4). It is tempting to speculate that such regulatory circuits may be crucial for restricting defense gene activation in beneficial plant/microbe symbioses, in particular, since ERF9 and ERF14 are upregulated in colonized wild-type roots (Fig. 1). ERFs may play a crucial role in beneficial plant/microbe interactions, since this transcription factor family contain both transcriptional activators and repressors.24 It remains to be determined whether selected activation of specific members of this gene family may participate in the decision whether a microbe is accepted as friend or foe. Mutualism or parasitism is directly connected to defense gene expression, which affects microbial growth and root colonization.1,4,25
In addition, PR-1 expression is constitutively downregulated in ERF14, but not in ERF9 roots when compared to the wild-type, independent of fungal colonization (Fig. 4). This clearly defines a stimulatory role for ERF14 in PR-1 expression in the roots. Oñate-Sánchez15 found no difference in PR-1 expression in the leaves of knock-out and the wild type plants and under their conditions PR-1 expression was at the detection limit. In ERF14 overexpressor lines, PR-1 and PR-3 were upregulated in the leaves compared to the wild type. Thus, ERF14 acts directly or indirectly as an activator of PR-1 in both leaves and roots.
PR-1 and PR-2 are specific marker genes for the SA pathway, whereas PR-3 and PDF1.2 are marker genes for ET/JA pathway.5,26 PR-1 and PR-2 are regulated differently in the two ERF mutants whereas they are not regulated in the wild-type. PR-3 and PDF1.2 are not regulated by the fungus in neither the wild type nor the mutants (Fig. 4). Thus, defense gene activation by P. indica cannot be attributed unambiguously to any of the two proposed pathways. Most strikingly, PR-1, but not PR-2 is upregulated in colonized ERF9 and vice versa in colonized ERF14 seedlings, although both genes are believed to be regulated by the SA pathway. PR-1 has antifungal properties but the microbial targets are unkonwn.27 The proposed antimicrobial target of PR-2 is the fungal cell wall component β-1,3 glucan.28 PR-3 and PR-4 have chitinase activity.29 The exact role of the PR proteins and their regulation during the establishment of the beneficial interaction between P. indica and Arabidopsis remains to be determined.
We could lately show that elevation of the ERF1 mRNA level triggers defense gene activation in colonized Arabidopsis roots and both, SA- and ET/JA-regulated defence genes respond to the fungus. We concluded that the activation is not pathway specific.1 The data shown here support this idea. Many responses depend on a cross-talk between the two signalling pathways in pathogenic plant/microbe interactions.30
While ERF1 may function as a putative activator of PR-1, PR-2, PR-5 and PDF1.2 in the beneficial interaction between the two symbionts,1 ERF9/ERF14 may repress PR-1/PR-2 expression. Considering that ERF transcription factors can function as transcriptional activators and repressors of defense genes, it appears that we are only at the beginning to understand the signalling events that occur in both beneficial and pathogenic interactions.
Material and Methods
Growth conditions of plant and fungus.
Wild type (ecotype Columbia) and homozygote T-DNA insertion Arabidopsis seeds (ERF9: SALK_091532O and ERF14: SALK_118494C) were surface-sterilized and placed on Petri dishes containing MS nutrient medium.17 After cold treatment at 4°C for 48 h, plates were incubated for 7 days at 22°C under continuous illumination (100 µmol m−2 sec−1). P. indica was cultured as described previously2,18 on Kaefer medium.19 For solid medium 1% (w/v) agar was included.
Co-cultivation experiments and estimation of plant growth.
Nine days after plating Arabidopsis seeds on MS medium, the seedlings were transferred to nylon disks (mesh size 70 µm) and placed on top of a modified plant nutrient culture medium (5 mM KNO3, 2 mM MgSO4, 2 mM Ca(NO3)2, 0.01 µM FeSO4, 70 µM H3BO3, 14 µM MnCl2, 0.5 µM CuSO4, 1 µM ZnSO4, 0.2 µM Na2MoO4, 0.01 µM CoCl2, 10.5 g l−1 agar, pH 5.6), in Petri dishes. One seedling was used per Petri dish and one fungal plug of 5 mm in diameter was placed at a distance of 1 cm from the roots. The plates were incubated at 22°C under continuous illumination from the side (80 µmol m−2 sec−1). Fresh weights were determined directly after seedlings were removed from the plates.
Experiments on soil.
Arabidopsis plants were cultivated on MS medium as described above for 10 days. The soil was mixed carefully with the mycelium (1%, w/v), which was obtained from liquid cultures after the medium was removed. Cultivation occurred in small plastic pots with Aracon tubes in a temperature-controlled growth chamber at 22°C under long-day conditions (light intensity: 80 µmol m−2 sec−1). The sizes of the plants were daily monitored.
Semiquantitative RT-PCR.
Total RNA was isolated from 10 pooled replicates of Arabidopsis roots with the RNeasy kit from Qiagen according to the protocol provided by the manufacturer. cDNA synthesis was performed with the Omniscript kit from Qiagen. All reactions were repeated with two independent biological replicates.
The transcript levels of ERF9 and ERF14 were tested with the following primer pairs: ERF14 (At1g04370), GGA TCA AGG AGG TCG TAG CAG TGG and TTA TTG CCT CTT GCC CAT GTT G; ERF9 (At5g44210), GCT CCA AGA CAG GCG AAC GGT AGA and CTA AAC GTC CAC CAC CGG TGG A.
Expression of selected defense genes was analysed after 7 days of co-cultivation of P. indica with Arabidopsis roots with the following primer pairs: PR-1 (At2g14610), TGT ATG AGT CTG CAG TTG CC and CAA CTG CAG ACT CAT ACA; PR-2 (At3g57260), ACC ACA CAG CTG GAC AAA TCG and ATG AGC TCG ATG TCA GAG CCA; PR-3 (At3g12500), TCA TGG GGC TAC TGT TTC AAG and TAT TGC TCT ACC GCA TAG ACC; PR-4 (At3g04720), GAC CTC GTG GTC AAG CTT CTT and TTG CTA CAT CCA AAT CCA AGC; PDF1.2 (At5g44420), CTT GTG TGC TGG GAA GAC ATA and AGC ACA GAA GTT GTG CGA GAA and ERF1 (At3g23240), CCT TCC GAT CAA ATC CGT AAG and TCC CGA GCC AAA CCC TAA TAC. For the housekeeping gene GAPC2 (At3g04120) GAG CTG ACT ACG TTG TTG AG and GGA GAC AAT GTC AAG GTC GG were used.
Acknowledgements
Work was supported by the IMPRS of Chemical Ecology Jena and the SFB604. We thank Claudia Röppischer for technical assistance.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12036
References
- 1.Camehl I, Sherameti I, Venus Y, Bethke G, Varma A, Lee J, et al. Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol. 2010;4:1062–1073. doi: 10.1111/j.1469-8137.2009.03149.x. [DOI] [PubMed] [Google Scholar]
- 2.Peškan-Berghöfer T, Shahollari B, Giang PH, Hehl S, Markert C, Blanke V, et al. Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant-microbe interactions and involves early plant protein modifications in the endoplasmatic reticulum and at the plasma membrane. Physiol Plant. 2004;122:465–477. [Google Scholar]
- 3.Oelmüller R, Sherameti I, Tripathi S, Varma A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis. 2009;49:1–17. [Google Scholar]
- 4.Johnson JM, Oelmüller R. Mutualism or parasitism: life in an unstable continuum. What can we lean from the mutualistic interaction between Piriformospora indica and Arabidopsis thaliana? Endocyt Cell Res. 2009;19:81–110. [Google Scholar]
- 5.Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohme-Takagi M, Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995;7:173–182. doi: 10.1105/tpc.7.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyal Y, Meller Y, Lev-Yadun S, Fluhr R. A basic-type PR-1 promoter directs ethylene responsiveness, vascular and abscission zone-specific expression. Plant J. 1993;4:225–234. doi: 10.1046/j.1365-313x.1993.04020225.x. [DOI] [PubMed] [Google Scholar]
- 9.Meller Y, Sessa G, Eyal Y, Fluhr R. DNA-protein interactions on a cis-DNA element essential for ethylene regulation. Plant Mol Biol. 1993;23:453–463. doi: 10.1007/BF00019294. [DOI] [PubMed] [Google Scholar]
- 10.Tournier B, Sanchez-Ballesta MT, Jones B, Pesquet E, Regad F, Latche A, et al. New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett. 2003;550:149–154. doi: 10.1016/s0014-5793(03)00757-9. [DOI] [PubMed] [Google Scholar]
- 11.Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun. 2002;290:998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;20:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakashima K, Shinwari ZK, Sakuma Y, Seki M, Miura S, Shinozaki K, et al. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration- and high-salinity-responsive gene expression. Plant Mol Biol. 2000;42:657–665. doi: 10.1023/a:1006321900483. [DOI] [PubMed] [Google Scholar]
- 14.Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oñate-Sánchez L, Anderson J, Young J, Singh K. AtERF14, a member of the ERF family of transcription factors, plays a nonredundant role in plant defense. Plant Physiol. 2007;143:400–409. doi: 10.1104/pp.106.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsutsui T, Kato W, Asada Y, Sako K, Sato T, Sonoda Y, et al. DEAR1, a transcriptional repressor of DREB protein that mediates plant defense and freezing stress responses in Arabidopsis. J Plant Res. 2009;122:633–643. doi: 10.1007/s10265-009-0252-6. [DOI] [PubMed] [Google Scholar]
- 17.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962;15:473–497. [Google Scholar]
- 18.Verma SA, Varma A, Rexer K-H, Hassel A, Kost G, Sarbhoy A, et al. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia. 1998;90:898–905. [Google Scholar]
- 19.Hill TW, Kaefer E. Improved protocols for Aspergillus medium: trace elements and minimum medium salt stock solutions. Fungal Genet Newsl. 2001;48:20–21. [Google Scholar]
- 20.Van Loon LC, Van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55:85–97. [Google Scholar]
- 21.Otha M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell. 2001;8:1959–1968. doi: 10.1105/TPC.010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang B, Liu JY. A cotton dehydration responsive element binding protein functions as a transcriptional repressor of DRE-mediated gene expression. Biochem Biophys Res Commun. 2006;343:1023–2031. doi: 10.1016/j.bbrc.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Broekaert WF, Delauré SL, De Bolle MF, Cammue BP. The role of ethylene in host-pathogen interactions. Annu Rev Phytopathol. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- 25.Sherameti I, Venus Y, Drzewiecki C, Tripathi S, Dan VM, Nitz I, et al. PYK10, a beta-glucosidase located in the endoplasmatic reticulum, is crucial for the beneficial interaction between Arabidopsis thaliana and the endophytic fungus Piriformospora indica. Plant J. 2008;54:428–439. doi: 10.1111/j.1365-313X.2008.03424.x. [DOI] [PubMed] [Google Scholar]
- 26.Penninckx IA, Thomma BP, Buchala A, Métraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;12:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antoniw JF, Ritter CE, Pierpoint WS, Van Loon LC. Comparison of three pathogenesis-related proteins from plants of two cultivars of tobacco infected with TMV. J Gen Virol. 1980;47:79–87. [Google Scholar]
- 28.Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, et al. Acquired resistance in Arabidopsis. Plant Cell. 1992;4:645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Loon LC. Regulation of changes in proteins and enzymes associated with active defense against virus infection. In: Wood RKS, editor. Active Defense Mechanisms in Plants. New York: Plenum Press; 1982. pp. 247–273. [Google Scholar]
- 30.Leon-Reyes A, Spoel S, De Lange E, Abe H, Kobayashi M, Tsuda S, et al. Ethylene modulates the role of NON EXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 2009;149:1797–1809. doi: 10.1104/pp.108.133926. [DOI] [PMC free article] [PubMed] [Google Scholar]