Abstract
We report, in Arabidopsis thaliana plants, the effect of neutron irradiation on the transcription of a set of genes belonging to different physiological groups: auxin action, senescence, oxidative stress and some aspects of photosynthesis. The results indicated that, in the wild-types, the effect on the ARF1, ARF2 and 19 genes was of downregulation, whereas of the tested AUX/IAA only AUX/IAA7 showed upregulation. Different results were obtained as regards the irradiation of the auxin transport mutants aux1 and eir1 because, in these cases, the ARF genes were upregulated, whereas AUX/IAA7 was downregulated in eir1. On the other hand, the senescence activated genes SAG12 and SAG13, and those connected to oxidative stress were upregulated in the wild-type, but downregulated in aux1. The gene CAB1, connected to photosynthesis, was also downregulated in the wild-type, but upregulated in aux1. Gene expression recovered in many cases almost to the initial condition in a time lapse of 24 hours, even though some effect persisted for a longer time. In particular, the state of juvenility of arf2 was extended by irradiation, whereas, in all the other cases, senescence was accelerated. The research indicates that through mutant selection or genetic engineering a true possibility exists of producing organism more suitable for life in space.
Key words: neutron irradiation, senescence, auxin, oxidative stress, auxin response factors, gene expression
Introduction
The literature regarding the effect of radiation on plants is not very large, even though its volume is increasing in the quest for extended space flights. However most of the available literature, regards the effect of ionising radiation,1,2 and not that of neutrons,3 even though this kind of particles pose a serious problem to the human flights, because so far no significant protection has been found from them.4,5 On the other hand, intensive ionising radiation has been shown to induce variations in the general physiological processes in plants, including respiration, ethylene emission, accumulation of sucrose, and photosynthesis.6–9 In particular, as regards the literature on Arabidopsis, some large scale analyses on the variations of expressed genes after irradiation with ionising agents, have been reported.9–11 In these cases most of the altered genes were involved in cell growth and division, development, signal transduction and stress related responses. In addition, high doses of radiation have been shown to induce the activation of genes involved in the biosynthesis of enzymes that scavenge the toxic accumulation of reactive oxygen species (ROS),12,13 which in turn cause enhanced lipid and protein oxidation,8,14–16 and specific morphology changes in plants.17 On the basis of the presently available data, it appears that the study of the effect of radiation on plants could lead to a better knowledge of the mechanisms that induce general stresses,18 and to the adoption of countermeasures. In this work, we concentrated on studying the effect of radiation on genes activated by the phytohormone auxin, which controls a large part of the morphogenetic processes in plants,19 and, at the same time, of genes involved in the activation of defence against stress and senescence. To overcome stress plants have developed highly-efficient defence systems, involving both enzymatic and non-enzymatic constituents. Whereas lipophylic antioxidants (tocopherols and carotenoids) are active in cell membranes, in the enzymatic ROS-scavenging pathways superoxide dismutase (SOD) converts O2− directly to hydrogen peroxide (H2O2), and catalase (CAT) is one of the main H2O2 scavenging enzymes resulting in its dismutation into water and O2.20–22 The role of ROS in stress biology is complex, involving oxidative damage, as well as signalling responses.15 In general, the antioxidant status is reduced during senescence: the level of ROS is enhanced, many antioxidant enzymes show reduced activity21 and the level of lipid peroxidation increases.23
In this paper we present the first data on the effect of neutron irradiation, administered at three different energy levels on Arabidopsis plants. The effect was measured by real-time PCR on the expression of genes involved in the movement and action of auxin, in senescence, under oxidative stress, and in photosynthesis, both in the wild-type and in two auxin mutants. In addition, we measured the production of thiobarbituric acid reactive substances (TBARS), the chlorophyll content and chlorophyll fluorescence, after exposing plants to neutron irradiation. The results showed notable variations in up or downregulation of the expressed genes, as well as showing the possibility of recovery of the plants within a few days of the irradiation.
Results
The effect of neutron irradiation on plant senescence was studied by treating both Arabidopsis seedlings (15-day-old) and mature plants (35-day-old), from two ecotypes (Col and Ws), and two auxin transport mutants (aux1 and eir1), with different doses of radiation, i.e., 30, 50 and 75 mGy at the Frascati Neutrons Generator (FNG, Frascati, Rome, Italy) for 3 hours. The treatment was replicated five times under the same conditions (see Materials and Methods). Expression profiles of genes involved in specific metabolic pathways was determined by quantitative real-time qPCR analysis. The analyzed genes, about which we report only the effect of irradiation at 50 mGy, behaved similarly at the other dosages employed (30, 75 mGy), can be divided into four different groups: auxin connected (ARF1, ARF2, ARF19, AUX/IAA3, AUX/IAA6, AUX/IAA7, AUX1, EIR1), senescence connected (SAG12 and SAG13), oxidative stress response connected (CAT1 and CAT3, FeSOD1), and photosynthesis connected (CAB1, RUBS-1B).
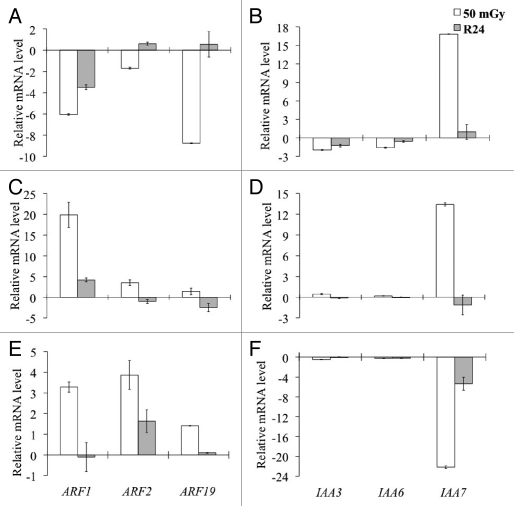
We chose to study particularly the effect of radiation on auxin connected genes, in consideration of the fundamental role played by this hormone in the morphogenesis and metabolism of plants.24 Three of the most significant ARF genes were chosen, i.e., ARF1, ARF2 and ARF19, since a recent study revealed their involvement in various aspect of plant development and physiology.25–27 We also chose three of the most significant AUX/IAA genes, i.e., AUX/IAA3, AUX/IAA6 and AUX/IAA7,28 whose mutants, respectively, are shy1, shy2,29 and axr2.30 In our experiments (Fig. 2), after neutron irradiation, the gene expression of the auxin response factors ARF1, ARF2 and ARF19 was downregulated, in the wild-type, as well as the expression of the auxin-activated genes AUX/IAA3, AUX/IAA6, with the exception of AUX/IAA7, that was consistently upregulated. In contrast, a different response was seen in the auxinic mutant aux1, whose mutated gene was shown to be a facilitator of the auxin influx into cells.31,32 In the aux1 background, in fact, all the auxin response factors analyzed (ARF1, ARF2 and ARF19) were upregulated after the treatment, whereas of the AUX/IAAs only AUX/IAA7 was clearly upregulated.
Figure 2.
Neutrons affect the expression of different auxin and senescence related genes in Arabidopsis plants. To study the effect of neutron radiation, 35-d-old plants, were irradiated with different doses of neutrons (30, 50 and 76 mGy). The expression profile of auxin response factors (ARF1, 3, 19), and auxin activated genes (AUX/IAA3, 6, 7), are shown in wild-type Col plants (A and B), together with that of two Arabidopsis auxin transport mutants: aux1 (C and D), and eir1 (E and F). Note = this assay was performed by a quantitative real time qPCR analysis 1 hour after the end of neutron irradiation at three different doses: 30, 50 and 76 mGy, and after 24 h of recovery (R24, gray bars). Only results obtained at 50 mGy (gray bars) neutron dose are shown. Relative amounts were normalized with respect to ACTIN8 expression level as log2 of the relative mRNA level, calibrated to the not-treated control plants (= 0). Log2 < 0 represents a downregulation, and log2 > 0 represents an upregulation of gene expression. Bars represent the means SD (n = 4–7).
The other Arabidopsis mutant on which the effect of neutron radiation was investigated was eir1/pin2/agr1/wav6. This is considered a mutant of the auxin efflux facilitator protein from the cells.33 Similar to aux1, the mutant eir1 also showed an upregulation of the auxin response factors ARF1, ARF2 and ARF19, whereas AUX/IAA7 showed strong downregulation after irradiation.
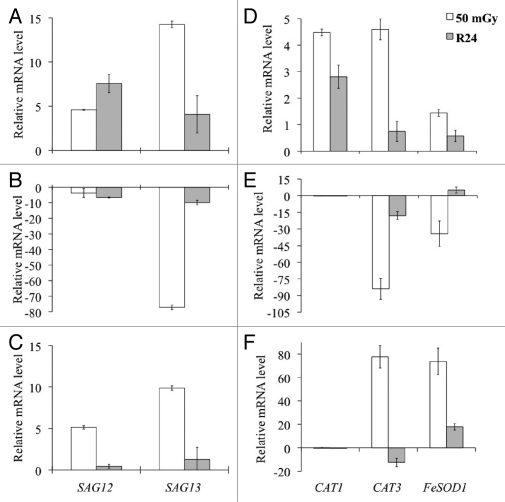
On the other hand, the genes SAG12 and SAG13 (Fig. 3A–C) considered to be involved in senescence processes, were significantly upregulated in their transcription in the wild-type, whereas in the mutant aux1 they were downregulated (especially SAG13). In this case, however, in the mutant eir1 upregulation was seen. The research was then extended to genes involved in processes connected with stress, i.e., CAT1, CAT3 and FeSOD1, which, as reported in Figure 3D–F, are conspicuously upregulated in the wild-type and in the mutant eir1. In contrast in the mutant aux1 all the above considered genes were downregulated.
Figure 3.
Effect of neutron irradiation on senescence and oxidative stress related genes. Relative mRNA transcript of genes bound to the senescence (SAG12 and SAG31) measured after the treatment with 50 mGy absorbed neutron dose (A–C), and 24-h after the recovery from the treatment (R24, grey bars). Measurements were carried out on 35-d-old wild-type Col (A), aux1 (B) and eir1 (C) plants. Relative mRNA transcript of oxidative stress related genes CAT1, CAT3 and FeSOD1 (D–F). For normalization and calibration see Figure 2.
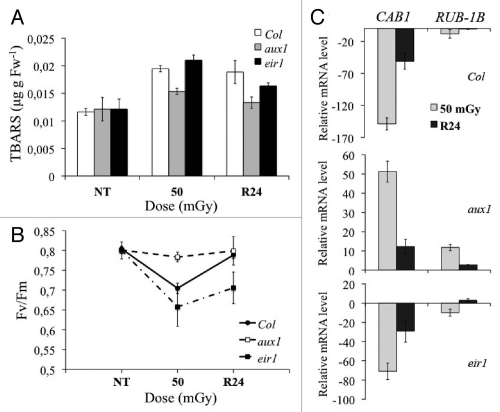
To study the effect of neutron radiation, in addition to the mRNA expression analysis, on the general process of senescence, some specific biochemical tests were performed, namely a test on the lipidic state of the membranes, i.e., a measure of the level of the thiobarbituric acid reactive substances (TBARS) in the wild-type (Fig. 4A), and a test on the efficiency of the photosynthetic system II performed on wild-type Col leaves (Fig. 4B). Wild-type plants displayed an increase of the TBARS content after the treatment at 50 mGy neutron dose with respect to the controls. On the other hand, chlorophyll fluorescence (Fv/Fm ratio) measurements on wild-type leaves showed a significant decrease of the Fv/Fm ratio (ca 15%) compared to the controls (Fig. 4B). These results suggest, that wild-type Arabidopsis plants are negatively affected by neutron radiation at the molecular and physiological level, and that neutrons are able to induce damage even at relatively low doses. In addition, the genes CAB1 and RUBS-1B were downregulated showing, with the exception of aux1, negative effects of irradiation on the photosynthetic apparatus (Fig. 4C).
Figure 4.
Neutron induced physiological alterations in plants. The physiological effect of neutron radiation on 35-d-old Arabidopsis plants. (A) The content of thiobarbituric acid reactive substances (TBARS) in wild-type Col (white bars), aux1 (gray bars) and eir1 (black bars) plants, before and after irradiation with neutron at 50 mGy and after 24 h recovery. (B) Chlorophyll fluorescence (Fv/Fm ratio) measured in control conditions after irradiation with neutrons at 50 mGy, and after recovery from stress (R24). Measurements were done on dark adapted leaves of Col (black circles), aux1 (white squares) and eir1 (black squares) plants. (C) Quantitative mRNA transcript level of photosynthetic apparatus related genes CAB1 and RUB-1B after neutron irradiation at 50 mGy (gray bars), and 24-h after the treatment (black bars) measured in the wild-type Col plants, and in the auxin mutants aux1 and eir1. Expression levels are calibrated with respect to the relative mRNA amount in the untreated control plants. Note: bars represent means ± SD (n = 5–7); NT, untreated plants; 50, 50 mGy of neutron irradiation; R24, 24 hours of recovery after the treatment.
Early recovery from neutron irradiation.
The possibility of recovery from the effects of neutron irradiation was studied in the plants as early as 12 and 24 hours after the treatment. The data showed a consistent recovery of the transcripts in all the considered genes (Figs. 2–4). This however was more evident in the wild-type in the case of ARF1, ARF19, AUX/IAA7, SAG13, CAT3, CAB1. In the mutant aux1 with ARF1, AUX/IAA7, SAG13, CAT3, FeSOD1, CAB1, and in the mutant eir1 regarding ARF1, ARF2, ARF19, AUX/IAA7, CAT3, FeSOD1 and CAB1. The effect was gradual, i.e., more visible at 24 hours than at 12. This seems to indicate that recovery mechanisms, even after exposures of as little as 3 hours were already at work.
Late effects of neutron irradiation.
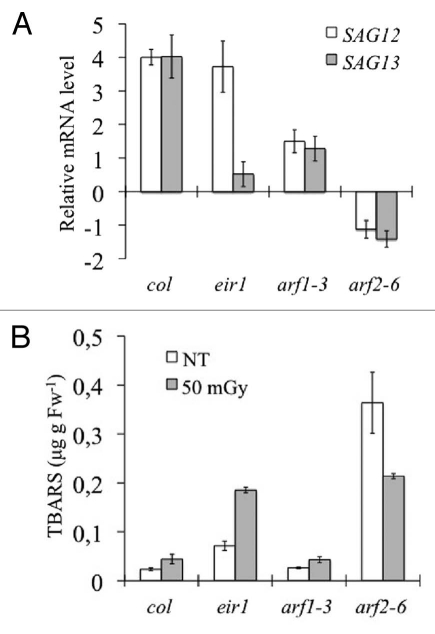
To investigate the late effects of neutron irradiation, both wild-type (Col and Ws), and the auxinic mutants eir1, arf1-3 and arf2-6 plants were irradiated at 50 mGy and left to grow firstly for 20 days, and then for 4 further weeks in the growth chamber together with controls. At the end of the 20 days, the plants were analyzed for the expression of senescence activated genes SAG12 and SAG13, and lipid peroxidation efficiency by measuring the TBARS content (Fig. 5). After 20 days of recovery the irradiated plants showed no apparent specific phenotypic alteration, even though biochemical and molecular parameters suggested increased oxidative stress and senescence. In fact, 20 days after the treatment, SAG12 and SAG13 were significantly upregulated with respect to non-treated plants. This was especially marked in the wild-type, and less in the mutants eir1 (in this case only SAG13), and arf1-3. In contrast, the mutant arf2-6 after recovery showed a downregulation of both the senescence genes SAG12 and SAG13. On the other hand, lipid peroxidation status, displayed a small increase in the wild-type and arf1-3 mutant, whereas this value was larger in the mutant eir1, and especially in the mutant arf2-6.
Figure 5.
Late effects of neutrons on Arabidopsis plants. Determination of the late effects of neutron radiation on wild-type (Col) Arabidopsis plants, the auxin transport mutant eir1, and the auxin response factors arf1-3 and arf2-6. (A) Quantitative qPCR analysis of mRNA expression levels for the senescence activated genes SAG12 (white bars) and SAG13 (gray bars) after irradiation of the plants (absorbed dose 50 mGy). Gene expression is shown as log2 fold change of mRNA level, normalized with ACTIN8 gene, and calibrated with respect to the mRNA level of the respective control plants. For log2 > 0 the gene is upregulated, log2 < 0 is downregulated, and log2 = 0 is unchanged compared to the control level. (B) Quantification of thiobarbituric acid reactive substances (TBARS) in the wild-type (Col), and the mutants eir1, arf1-3 and arf2-6, measured on not-treated control conditions (white bars) and after irradiation with neutrons at a dose of 50 mGy (gray bars). Note: The measurements were done 20 days after the irradiation. Bars represent means SD (n = 4–7); NT = untreated plants.
Four weeks after the irradiation however, all the irradiated plants also started to show phenotype effects (Fig. 6), i.e., an accelerated senescence, shown by the earlier flowering and seed formation with respect to controls. The only exception was the mutant arf2-6 that remained in the rosette condition, i.e., showed an increased delay of senescence after irradiation.
Figure 6.
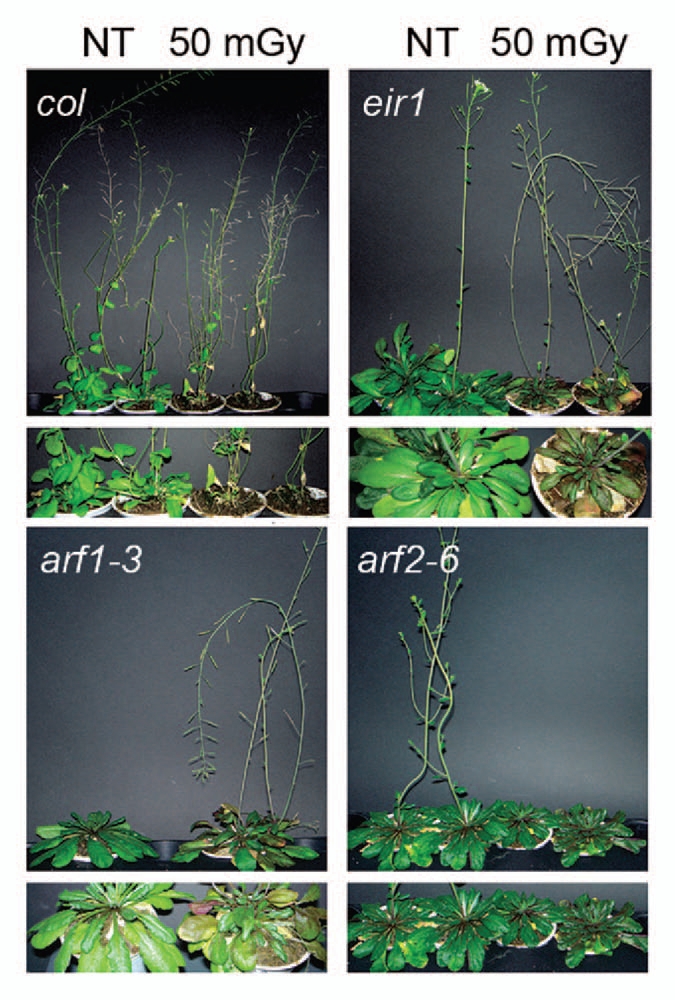
Alteration of the senescence mechanism induced by neutrons. Phenotypical effect of neutrons (at 50 mGy of absorbed dose) on plant senescence 4 weeks after the treatment. Each image represents a comparison between control (NT = untreated plants) and treated (50 mGy) 15-d-old seedlings of the wild-type Col, the auxin transport mutant eir1, and two senescence mutants arf1-3 and arf2-6. The lower pictures represent an enlarged detail of rosettes show immediately above. Note: all pictures are representative of a cultivar of at least 30 plants coming from three independent experiments.
Discussion
After 3 hours of neutron irradiation, the genes chosen by us from the ARF family (ARF1, ARF2 and ARF19), which have been shown to control significant aspects of plant morphogenesis and growth,28,34–36 were notably downregulated, whereas of the genes chosen from the AUX/IAA family (AUX/IAA3, AUX/IAA6, AUX/IAA7), which also are considered to be involved in significant processes,25,34,37 only AUX/IAA7 was clearly upregulated. These general effects appear negative in the wild-type plants, since a downregulation of the genes ARFs can lead to the reduction of the pool of ARF proteins and to the inhibition of the processes controlled by them. In addition, the ARFs proteins, when bound to the AUX/IAAs ones, whose transcription is upregulated at least in AUX/IAA7, cannot activate the auxin response genes as has been reported extensively.38–41 This, at least, can be said on the basis of the knowledge we have today on the functions of these two gene families. On the other hand, we found a significantly different situation when the transcription of the same groups of genes was studied in the background of the two auxin transport mutants aux1 and eir1. In these cases, in fact, the effect was frequently reversed, because the ARFs resulted upregulated in the mutants, whereas AUX/IAA7 was upregulated in aux1, but downregulated in eir1. AUX/IAA7 is a gene, which characterizes the axr2 mutant, it is connected with the action of auxin, and in its mutated form induces dominant strong pleiotropic modifications of plant development, i.e., reduced plant size, suppression of gravitropism in shoots and roots, small anomalous flowers, and an extended life cycle.30 On the other hand, AUX/IAA3 and AUX/IAA6 characterize the mutants shy2 and shy1, whose most significant characteristic is the suppression of the mutation in hy2, which induces elongated hypocotyls in white and red light.29,42,43 These two genes, however, did not show significant variations in transcription both in the wild-type and in the auxinic mutants. Furthermore, no particular phenotype is displayed by ARF1, which was the first of the family to be described,35 even though its action seems particularly connected to that of the gene ARF2.44 The latter, on the contrary, appears to have many functions. It is a suppressor of the mutation hookless and a gene involved in the control of senescence. As said above its mutation confers juvenility to the plants, i.e., extended life cycle.44,45 ARF19 is a gene that has been connected to the action of both auxin and ethylene,46 which complements ARF7/NPH4, involved in phototropism, it resulted downregulated in the wild-type, but upregulated in the mutant eir1.24,46 This result could be an indication that the gene is involved in controlling auxin transport and stress conditions promoted by ethylene. Additional impairment of gene transcription connected to auxin was also seen in the transcript of the auxin transport genes AUX1 and EIR1, both downregulated in the wild-type after irradiation, as well as reciprocally in the mutants (data not shown). What is apparent, therefore, is that the disturbances in the transport of auxin induce significant variations in the expression of the genes ARFs and AUX/IAA7 in the mutants aux1 and eir1.
A specific objective of our research was consequently analysing the expression of genes target of senescence in the irradiated plants, since it is already well known that microgravity and the space environment induce earlier aging, as well as the production of oxidative processes in animals and humans.47,48 In this part of the research what was found (Fig. 3A–C) is that the genes specifically involved in senescence SAG12 and SAG13,49,50 were upregulated in the wild-type. However, again in this case, as in that of the auxin activated genes, a reverse process was seen in the mutant aux1, because both SAG12 and SAG13 were downregulated, whereas in eir1 the effect was like that in the wild-type. This result supports an involvement of auxin transport in the acceleration of senescence seen in the wild-type. Furthermore, in the wild-type, the genes for the catalase CAT1 and CAT3 and for FeSOD1, which are involved in the elimination of free radicals, increased their transcription after irradiation. Result that is in line with the acceleration of the stress and senescence processes induced by radiation.21 However, the genes controlling the oxidative processes were downregulated in aux1 and, since plants with low catalase activity show less severe stress symptoms,51 from these data again emerges a clear difference between the wild-type and the two auxinic mutants (at least for aux1). These observations, considering that the two mutants show only modest defects, apparently not serious for the life of the plants, lead us to think that they could be grown in space more successfully than the wild-type plants, and that a defect in auxin transport in roots can be advantageous in some cases.
Furthermore, an increase of TBARS, i.e., of thiobarbituric acid (Fig. 4A), revealed, especially in the wild-type, damage to the plasmatic membrane, a process bound to senescence. The maximum photochemical efficiency of Photosystem II (PSII), determined from the ratio of variable Fv to maximum Fm fluorescence Fv/Fm = (Fm − F0)Fm, was consistently lowered after the course of the neutron irradiation experiments in the wild-type and eir1 (Fig. 4B). This indicates possible damage of the photosynthetic process in the chloroplasts, and suggests that neutron irradiation induces lipid peroxidation associated with the damage and degradation of PSII. In fact, measurement of two genes connected with photosynthesis (Fig. 4C) i.e., RUBS-1B and CAB1, chosen to check the general effect of irradiation on the process, showed in the case of CAB1 and in the wild-type, as well as in eir1, a general decline in transcription, whereas in aux1 upregulation was seen. This indicates that in aux1 again the irradiation has opposite effects with respect to the wild-type and frequently also with respect to eir1. No large variation in transcription was seen by contrast in the case of RUBS-1B.
Regarding the recovery over a short period of time, as mentioned previously, we measured a remarkable return to pre-treatment status in all the genes considered (Figs. 2–4), whereas in the case of recovery over a long period of time some effects of irradiation persisted. In fact, after 20 days from the irradiation (Fig. 5) both SAG12 and SAG13 showed still a consistent upregulation in the wild-type, whereas in the mutants arf1-3 and eir1 (as regards SAG13) the transcription values were low, and especially in arf2-6 the senescence genes were clearly downregulated, showing juvenility. However, the data relative to the level of TBARS (Fig. 5B) showed a value particularly high in arf2-6, a fact that indicates that some negative effects of the irradiation persisted even in the most juvenile of the mutants.
The data collected four weeks after the irradiation, on the other hand, showed an accelerated senescence in the adult plants (Fig. 6), with the exception of arf2-6, in which case the juvenility was even increased by the irradiation. This gene, controlled by auxin in its activity, as said, is already known as a trigger of juvenility when mutated, probably as a consequence of the fact that it seems to control the switching between different and progressive morphological stages in the plant.24,44,45 Should further testing reveal that the plants mutated in ARF2 are healthy and reproductive, the manipulation of this gene could become a useful technique to apply in the production of plants suited to space flights.
Furthermore, of the two mutants involved in auxin transport, aux1 was particularly interesting in that its reaction to the aging and stressing agents (i.e., neutron irradiation) was almost opposite to those in the wild-type. This observation could indicate that, by manipulating the transport of auxin, it might be possible to increase the resistance of the plants to stress. Perhaps the transport of auxin may also be one of the keys to senescence in plants.
Materials and Methods
Plant material and growth conditions.
Seeds of Arabidopsis thaliana plants, ecotypes Ws (Wassilewskjia) and Col (Columbia), as well as the mutants aux1, eir1, arf1-3 and arf2-6, were provided by the Nottingham Arabidopsis Stock Centre (NASC, Nottingham, UK). Before plating, the seeds were sterilized for 10 minutes in a solution containing 50% commercial bleach and 0.01% SDS, followed by four washes with sterile distilled water. Seeds were plated in Petri dishes, on a growth medium made up of 1.5% agar, 1% sucrose, and 0.5X MS basal medium enriched with Gamborg's vitamins and adjusted to pH 5.7 with NaOH and then transferred to 7 cm pots on a sterile mixture made up of 40% sand, 35% turf and 25% soil. The plants were grown by keeping them at first in short day (8/16 h light/dark photoperiod) to promote rosette growth, and after three weeks in long day (16/8 h light/day photoperiod). Growth chamber conditions were: 150 µmol m−2 s−1 light intensity, 23/19°C day/night air temperature, relative humidity ca. 65%.
Neutron exposure facility and experimental set-up.
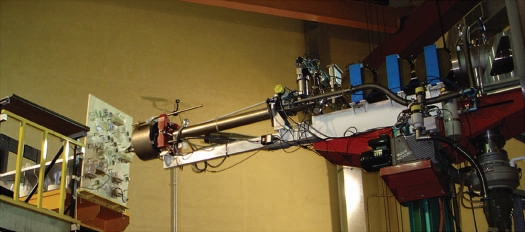
For all the neutrons irradiation experiments Arabidopsis 35-d-old plants (wild-type Ws, col, and the mutants aux1, and eir1, arf1-3 and arf1-6) were used. Treatments were carried out at the Frascati Neutron Generator (FNG), a research center of the National Agency for New Technologies, Energy and the Environment (ENEA) in Rome, Italy. To generate the neutron field a deuterion beam was accelerated up to 300 keV inside an accelerating pipe and focused onto a target plate containing tritium. A nuclear fusion reaction occurs between deuterium and tritium ions, resulting in the production of α particles and neutrons with a kinetic energy of 14 MeV. The produced particle fluxes are constant on a generic spherical surface surrounding the neutron source. A wood panel (Fig. 1) was placed perpendicular to the direction of the accelerating pipe, and the iso-flux curves described on the panel were represented by circumferences having as a center the intersection point between the panel itself and the horizontal axis of the accelerating pipe. The plants were placed along three circumferences, each corresponding to a different total neutron dose: 30, 50 and 75 mGy. During each radiation session the plants were irradiated in the dark at 23°C and 60% relative humidity for an uninterrupted period of ∼3 hours at a constant rate. The absorbed doses in this experimental configuration were calculated by using the Monte Carlo N-Particle transport code (MNCP). Several plants used as control were housed in a separate room completely shielded against the neutron radiation, and kept at the same environmental conditions of the irradiated samples.
Figure 1.
Experimental system used for neutron irradiation of Arabidopsis plants. Arabidopsis plants (15-day-old seedlings in Petri dishes and 35-day-old plants in pots) were mounted on a vertical panel and irradiated with neutrons at the Frascati Neutron Generator (FNG). The distances between plant samples and the generator was selected to deliver three different absorbed doses of neutrons: 30, 50 and 76 mGy.
After the treatments, three samples from five plants (control and irradiated) of each ecotype or mutants were collected and stored at −80°C for further analysis. Leaves from both irradiated and control plants were detached, immediately immersed in RNALater solution (Sigma, CA, USA), and stored for further molecular analysis.
Late effects of neutrons on Arabidopsis plants were assayed by growing seedlings of the wild-type Col and the mutants eir1, arf1-3 and arf2-6 for two weeks in Petri dishes; then plants were irradiated with neutrons and recovered, transferring them to the growth chamber, together with the control plants, for 4 more weeks. Three weeks after the recovery period, leaf samples of both irradiated and control plants were collected and stored at −80°C.
Finally, at the end of the recovery period, images of the plants were recorded using a digital camera. For each experiment, only results obtained with the 50 mGy absorbed irradiation dose are presented. Only insignificant qualitative or quantitative differences were noted in the data collected at the other two irradiation doses (30 and 75 mGy).
Chlorophyll fluorescence measurement and lipid peroxidation assay.
Damage to the photosynthetic apparatus after neutron radiation was assessed by measuring the photosynthetic efficiency of the photosystem II (PSII), using a portable chlorophyll fluorometer MINI-PAM (Waltz, Effeltrich, Germany). The chlorophyll fluorescence, given as the Fv/Fm ratio, was measured under pre-stressed conditions and one hour after the neutron irradiation after 30 minutes of dark adaptation.
Lipid peroxidation was determined using the thiobarbituric acid reactive substances (TBARS) content, according to Heath & Packer.52 Three different replicates from at least three plants (control and treated) were used for each ecotype. Leaf material (0.1 g) was homogenized in 1.2 ml 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000 g for 30 min and 0.5 ml of the supernatant was added to 1 ml 0.5% (w/v) thiobarbituric acid (TBA) in 20% TCA. The mixture was incubated in a boiling water bath for 30 min, and then quickly cooled on ice. Absorption of the solution was recorded at 532 nm. The value for non-specific absorption at 600 nm was subtracted. For calculation of the content of MDA-TBA complex the molar extinction coefficient of 155,000 was used.
Quantitative real time qPCR analysis.
Total RNA was extracted from 50 mg of each leaf sample using the TRIZOL reagent (Invitrogen, CA), following the manufacturer's instructions. Two µg of total RNA was reverse transcribed using the Super Script III (Invitrogen) reverse transcriptase to synthesize the first-strand cDNA mixture, which was used for the polymerase chain reactions (PCR). For each point of the experiments at least three leaves (for total RNA extraction from 35-d-old plants), harvested from 3 different plants or 100 seedlings (for total RNA extraction from 15-d-old plants), were used for all ecotypes and mutants. Real time quantitative polymerase chain (qPCR) reactions were performed using an Mx3000P® (Stratagene, CA) system following the protocol described in Fortunati et al.53 Conditions for all qPCR reactions were: 10 min at 95°C and 35 three-temperature cycles (30 sec at 95°C, 45 sec at 57°C and 1.2 min at 72°C). For the complete list of primers used in qPCR reactions see Table 1. All qPCR data were analyzed using the MxPro v 4.0 software (Stratagene).
Table 1.
List of qPCR primer pairs used for the gene expression experiments
| Gene name | Primer ID | Sequence |
| ARF1 | ARF1 Fw1 | 5′-ATGCCTGTGCTGGACCTCTTGTAAC-3′ |
| ARF1 Bw1 | 5′-CCTCGGAAAATGTGCCTAAAATGCC-3′ | |
| ARF2 | ARF2 Fw1 | 5′-CTCGTCGTCGGTATTCAAGCGTC-3′ |
| ARF2 Bw1 | 5′-ATCTGTTGTTCTGCCGCCTGGTTCG-3′ | |
| ARF19 | ARF19 Fw1 | 5′-CCTCCTGTGGGAAGTCTTGTGGTTTAC-3′ |
| ARF19 Bw1 | 5′-GCTCCAACCTGTGGTAAGCAAGTG-3′ | |
| IAA3 | IAA3 Fw1 | 5′-AAACAGAGCTGAGGCTGGGATTA-3′ |
| IAA3 Bw1 | 5′-AGAAACCCGACAACCCAAGCA-3′ | |
| IAA6 | IAA6 Fw1 | 5′-TTCGATTGGGTCTTCCAGGAGATA-3′ |
| IAA6 Bw1 | 5′-ATCTTGCTGGAGACCAAAACCA-3′ | |
| IAA7 | IAA7 Fw1 | 5′-AAGCAGTTGAGAGTCCTGCCAAAT-3′ |
| IAA7 Bw1 | 5′-CCGTCATATTGTTGATCATTGATGC-3′ | |
| SAG12 | SAG12 Fw1 | 5′-GATGAAGGCAGTGGCACACCAACCG-3′ |
| SAG12 Bw1 | 5′-GACATCAATCCCACACAAACATACACA-3′ | |
| SAG13 | SAG13 Fw1 | 5′-CTGGTGGCTCTAAAGGCATCGGGTC-3′ |
| SAG13 Bw1 | 5′-CTTATGTTGTCGCTCGCCCATTCGC-3′ | |
| CAB1 | CAB1 Fw1 | 5′-TGAAGGCTACAGAGTCGCAGGAA-3′ |
| CAB1 Bw1 | 5′-GCTCTCTTTCTCCTCTCACACTCAC-3′ | |
| RUB-1B | RUB-1B Fw1 | 5′-CATCACAAGCAATGGGGGAAGAG-3′ |
| RUB-1B Bw1 | 5′-AACCTTCAGTTTTTTCACTTT-3′ | |
| CAT1 | CAT1 Fw1 | 5′-CTGCTCTGGAAATCGTGAGAAGTGC-3′ |
| CAT1 Bw1 | 5′-AGAAACCAAACCGTAAGAGGAGCATA-3′ | |
| CAT3 | CAT3 Fw1 | 5′-CACTCAGAGACATCGCCTTGGACCG-3′ |
| CAT3 Bw1 | 5′-CGTGGGTGAGACGTGGCTCCGATAG-3′ | |
| FeSOD1 | FeSOD Fw1 | 5′-TCCAGAACCGAAGACCAGATTACAT-3′ |
| FeSOD Bw1 | 5′-CTTGACACACACAAAACGCACACAC-3′ | |
| ACT8 | ACT8 Fw1 | 5′-ATGAAGATTAAGGTCGTGGCA-3′ |
| ACT8 Bw1 | 5′-TCCGAGTTTGAAGAGGCATC-3′ |
Acknowledgements
The authors thank Dr. Maria Teresa Giardi of the Institute of Cristallography—National Research Council (IC-CNR), Rome, Italy, for useful discussions and coordination of the space research group. We are also grateful to the Italian Space Agency (ASI) for financial support.
Abbreviations
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid reactive substances
- qPCR
quantitative polymerase chain reaction
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11768
References
- 1.Todd P. Overview of the spaceflight radiation environment and its impact on cell biology experiments. J Grav Physiol. 2001;11:11–16. [PubMed] [Google Scholar]
- 2.Tim JH, Moon YR, Kim JS, Lee JW. Transcriptomic profile of Arabidopsis rosette leaves during the reproductive stage after exposure to ionizing radiation. Rad Res. 2007;168:267–280. doi: 10.1667/RR0963.1. [DOI] [PubMed] [Google Scholar]
- 3.Ngo FQ, Schroy CB, Jia XL, Kalvakolanum I, Roberts WK, Blue JW, et al. Basic radiobiological investigations of fast neutrons. Rad Res. 1991;128:94–102. [PubMed] [Google Scholar]
- 4.Benton ER, Benton EV. Space radiation dosimetry in low-Earth orbit and beyond. Nuclear Instruments Met & Phys Res B. 2000:25–294. doi: 10.1016/s0168-583x(01)00748-0. [DOI] [PubMed] [Google Scholar]
- 5.Tanner RJ, Bolognese-Milsztajn T, Boschung M, Coeck M, Curzio G, d'Errico F, et al. Achievements in workplace neutron dosimetry in the last decade; lessons learned from the EVIDOS project. Rad Prot Dos. 2007;126:471–476. doi: 10.1093/rpd/ncm095. [DOI] [PubMed] [Google Scholar]
- 6.Wada H, Koshiba T, Matsui TM. Involvement of peroxidase in differential sensitivity to γ-radiation in seedlings of two Nicotiana species. Plant Science. 1998;132:109–119. [Google Scholar]
- 7.Cho HS, Lee HS, Pai HS. Expression patterns of diverse genes in response to gamma-irradiation in Nicotiana tabacum. J Plant Biol. 2003;43:82–87. [Google Scholar]
- 8.Kim JH, Chung BY, Kim JS, Wi SG. Effect of in plant gamma irrradiation on growth, photosynthesis and antioxidative capacity in red pepper (Capsicum annuum L.) plants. J Plant Biol. 2005;48:47–56. [Google Scholar]
- 9.Nagata T, Yamada H, Du Z, Todoriki S, Kikuchi S. Microarrays analysis of genes that respond to gamma irradiation in Arabidospsis. J Agricult Food Chem. 2005;53:1022–1030. doi: 10.1021/jf0486895. [DOI] [PubMed] [Google Scholar]
- 10.Kranz AR. Heavy ion and cosmic radiation effects in different targets of the Arabidopsis seed. Acta Astron. 1994;33:201–210. doi: 10.1016/0094-5765(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 11.Sahr T, Voigt G, Scimmack W, Paretzke T, Ernst D. Low level radiocesium exposure alters gene expression in roots of Arabidopsis. New Phytol. 2005;168:141–148. doi: 10.1111/j.1469-8137.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller MW. Radiation hormesis in plants. Health Phys. 1985;2:607–616. doi: 10.1097/00004032-198705000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Nagata T, Todorichi S, Hayashi Y, Shibata M, Mori H. Gamma-radiation induces leaf trichome formation in Arabidopsis. Plant Physiol. 1999;120:113–119. doi: 10.1104/pp.120.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landry LG, Chapple CC, Last RL. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 1995;109:1159–1166. doi: 10.1104/pp.109.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittler R. Oxidative stress, antioxidants and stress tolerance. TIPS. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 16.Yannarelli GG, Noriega GO, Batle A, Tomaro ML. Heme oxygenase upregulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta. 2006;224:1154–1162. doi: 10.1007/s00425-006-0297-x. [DOI] [PubMed] [Google Scholar]
- 17.Hectors K, Prinsen E, De oen W, Jansen MAK, Guisez Y. Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 2007;175:255–270. doi: 10.1111/j.1469-8137.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- 19.Woodward AW, Bartel B. Auxin: regulation, action and interaction. Ann Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corpas FJ, Palma JM, Sandalio LM, Lopez-Huertas E, Romero-Puertas C, Barroso JB. Purification of catalase from pea leaf peroxisomes: identification of five different isoforms. Free Rad Res. 1999;31:235–241. doi: 10.1080/10715769900301561. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann P, Heinlein C, Orendi G, Zentgraf U. Senescence specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell & Environ. 2006;29:1049–1060. doi: 10.1111/j.1365-3040.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- 22.Du YY, Wang PC, Chen J, Song CP. Comprehensive functional analysis of Catalase gene family in Arabidopsis thaliana. J Integrat Plant Biol. 2008;50:1318–1326. doi: 10.1111/j.1744-7909.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 23.Vanacker H, Sandalio L, Jimenez A, Palma JM, Corpas FJ, Meseguer V, et al. Roles for redox regulation in leaf senescence of pea plants grown on different sources of nitrogen nutrition. J Exp Bot. 2006;57:1735–1745. doi: 10.1093/jxb/erl012. [DOI] [PubMed] [Google Scholar]
- 24.Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, et al. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005;43:118–130. doi: 10.1111/j.1365-313X.2005.02432.x. [DOI] [PubMed] [Google Scholar]
- 25.Liscum E, Reed JW. Genetics of AUX/IAAs and ARFs action in plants growth and development. Plant Mol Biol. 2002;49:387–400. [PubMed] [Google Scholar]
- 26.Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell. 2005;17:444–463. doi: 10.1105/tpc.104.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okushima Y, Mitina I, Quach HL, Theologis A. AUXIN RESPONSE FACTOR 2 (ARF2): a pleiotropic developmental regulator. Plant J. 2005;4:29–46. doi: 10.1111/j.1365-313X.2005.02426.x. [DOI] [PubMed] [Google Scholar]
- 28.Dharmasiri N, Estelle M. Auxin signaling and regulated protein degradation. TIPS. 2004;9:302–308. doi: 10.1016/j.tplants.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Tian Q, Reed JW. Control of auxin regulated root development by Arabidopsis thaliana SHY2/IAA3 gene. Develop. 1997;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- 30.Nagpal P, Walker LM, Joung JC, Sonawala A, Timpte C, Estelle M, et al. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000;123:563–574. doi: 10.1104/pp.123.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;16:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- 32.Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, et al. aux1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1998;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagen G, Guilfoyle TJ. Rapid induction of selective transcription by auxin. Mol Cell Biol. 1985;5:1197–1203. doi: 10.1128/mcb.5.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulmasov T, Hagen G, Guilfoyle TJ. ARF1, a transcription factor that binds to auxin response elements. Science. 1997;20:1865–1868. doi: 10.1126/science.276.5320.1865. [DOI] [PubMed] [Google Scholar]
- 36.Gray WM, Kempiski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCF (TIR1) dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- 37.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;26:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 38.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiwari SB, Hagen G, Guilfoyle TJ. The role of auxin response factor domains in auxin-responsive transcription. Plant Cell. 2003;15:533–543. doi: 10.1105/tpc.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kepinski S, Leyser O. The Arabidopsis F-Box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 41.Guilfoyle TJ, Hagen G. Auxin response factors. Curr Op Plant Biol. 2007;10:453–460. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Kim BC, Soh MS, Hong SH, Furuya M, Nam HG. Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor of mutation of hy2. Plant J. 1996;9:441–456. doi: 10.1046/j.1365-313x.1996.09040441.x. [DOI] [PubMed] [Google Scholar]
- 43.Soo MS, Hong SH, Kim BC, Vizir I, Park DH, Choi G, et al. Regulation of both light and auxin-mediated development by the Arabidopsis IAA3/SHY2 gene. J Plant Biol. 1991;42:239–246. [Google Scholar]
- 44.Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Develop. 2005;132:4563–4574. doi: 10.1242/dev.02012. [DOI] [PubMed] [Google Scholar]
- 45.Schruff MC, Spielmann M, Tiwari S, Adams S, Fenby N, Scott RJ. The AUXIN RESPONSE FACTOR2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Develop. 2005;133:251–261. doi: 10.1242/dev.02194. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Dai X, Zhao Y. A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiol. 2006;140:899–908. doi: 10.1104/pp.105.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serova LV. Microgravity and aging in animals. J Grav Physiol. 2001;8:137–138. [PubMed] [Google Scholar]
- 48.Biolo G, Heer M, Narici M, Strollo F. Microgravity as a model of aging. Curr Op Clin Nutr Met. 2003;6:31–40. doi: 10.1097/00075197-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Navabpour S, Morris K, Allen R, Harrison E, A-H-Mackerness S, Buchanan-Wollaston V. Expression of senescence-enhanced genes in response to oxidative stress. J Exp Bot. 2003;54:2285–2292. doi: 10.1093/jxb/erg267. [DOI] [PubMed] [Google Scholar]
- 50.Wagstaff C, Yang TJ, Stead AD, Buchanan-Wollaston V, Roberts JA. A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J. 2009;57:690–705. doi: 10.1111/j.1365-313X.2008.03722.x. [DOI] [PubMed] [Google Scholar]
- 51.Rizhsky L, Liang H, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002;130:1143–1151. doi: 10.1104/pp.006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem & Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 53.Fortunati A, Barta C, Brilli F, Centritto M, Zimmer I, Schnitzler JP, et al. Isoprene emission is not temperature-dependent during and after severe drought-stress: a physiological and biochemical analysis. Plant J. 2008;55:687–697. doi: 10.1111/j.1365-313X.2008.03538.x. [DOI] [PubMed] [Google Scholar]