Abstract
Tomato fruit growth is characterized by the occurrence of numerous rounds of DNA endoreduplication in connection to cell expansion and final fruit size determination. Endoreduplication occurs as an impairment of mitosis, which can originate from the selective degradation of M-phase-specific cyclins via the ubiquitin-mediated proteolytic pathway, requiring the E3 ubiquitin ligase Anaphase Promoting Complex/Cyclosome (APC/C). In plants CCS52A is the ortholog of CDH1/FZR proteins from yeast, drosophila and human, belonging to the WD40-repeat protein family. During fruit development, the SlCCS52A gene expression is specifically associated to endoreduplication in tomato. Altering SlCCS52A expression in either negative or positive manner impacts the extent of endoreduplication in fruit and affects fruit size. When SlCCS52A is down-expressed endoreduplication is impaired during fruit growth leading to reduced fruit growth. However when SlCCS52A is overexpressed, endoreduplication is initially delayed, accounting for the altered final fruit size, but resumes and is even enhanced leading to fruit growth recovery, pointing at the physiological role of endoreduplication in growth induction during tomato fruit development.
Key words: anaphase promoting complex, CCS52A, endoreduplication, fruit development, growth, tomato
Endoreduplication represents the most common mode of cell endopolyploidization in plants and is estimated to occur in over 90% of Angiosperms.1,2 This process is an endonuclear DNA stock duplication leading to the production of chromosomes with 2n chromatids without any change in chromosome number.3,4 As a consequence hypertrophying nuclei arise from successive cycles of DNA replication.
In fruits of Cucurbitaceae and Solanaceae, mesocarp cells commonly undergo six rounds of DNA duplication (endocycle), and the highest ploidy levels for these cells being reached in tomato fruits where eight endocycles (up to 512C) can be observed.5 This high level of endopolyploidy in tomato fruits and the numerous data reported on this process in this species,6–9 makes it an outstanding model for studying endopolyploidization and its physiological role during fruit development.
Elucidating the Functional Role of Endoreduplication during Fruit Growth
The frequent positive correlations between endoreduplication and cell size in many different plant species, organs and cell types3,10,11 are commonly interpreted as endoreduplication being the driver for cell expansion. The successive rounds of DNA synthesis during endoreduplication induce a consequent hypertrophy of the nucleus, thus influencing the final size of the cell which therefore may adjust its cytoplasmic volume with respect to the DNA content of the nucleus (according to the “karyoplasmic ratio” theory10). In tomato, the evidence for such a positive correlation between cell size and ploidy level was indeed provided during fruit development.8 In a recent survey5 we showed that the ability to develop large cells in various fleshy fruit species is not restricted to endopolyploidizing fruits. Nevertheless when endopolyploidizing fruits are compared, it appeared that the largest cells are present in fruits which undergo the highest number of endocycles (e.g., tomato, pepper, melon), thus suggesting that endoreduplication might be necessary for plant cells to reach very large sizes.
If obviously endoreduplication does not seem to be always necessary for cell expansion, it participates in modulating the rate or organ growth and/or cell expansion. A clear correlation exists in tomato between the mean ploidy level in fully developed pericarp and final fruit size and weight.8 Endoreduplication influences not only the rate of cell expansion but also the rate of fruit development in species exhibiting rapid fruit development. Indeed endoreduplication never occurs in fruit from species where fruit development lasts for a very long period of time (over 17 weeks), while fruits developing rapidly (in less than 10 weeks) undergo several rounds of endocycle (from 3 to 8).5
The APC/C Activator CCS52A Controls Endoreduplication in Tomato
The commitment towards endoreduplication requires the loss of mitotic Cyclin-Dependent Kinase/Cyclin (CDK/CYC) complex activity which can occur upon the selective proteolytic destruction of the cyclin subunits via the ubiquitin proteasome pathway. The ubiquitin-dependent proteolysis of mitotic cyclins requires the involvement of a specific E3-type ubiquitin ligase named the Anaphase-Promoting Complex/Cyclosome (APC/C). In plants the APC/C is activated by the CCS52A protein, the homologue of mammalian CDH1 and Drosophila FZR, which binds to cyclins as to drive them towards the degradation process by the 26S proteasome.12 Alike its eukaryotic counterparts, CCS52A was found to promote the onset and progression of endoreduplication in different plant organs,13,14 and therefore the functional role of CCS52A was investigated during tomato fruit development.
Transgenic plants aimed at downexpressing SlCCS52A using the CaMV 35S promoter were generated. The Pro35S:Slccs52AAS plants displayed smaller fruits than wild-type plants. The ploidy level of the Pro35S:Slccs52AAS was affected towards lower levels, in correlation with a decrease in mean cell size and an increase in cell number.15 Using protoplasts prepared from Pro35S:Slccs52AAS leaves and a luciferase gene reporter assay, we were able to demonstrate that the phenotype observed in Pro35S:Slccs52AAS fruits originates from a decrease in the APC/CCCS52A activity targeting cyclin proteins for further proteasome destruction, thus resulting in a higher stabilization of cyclins and consequent enhanced cell division activity.
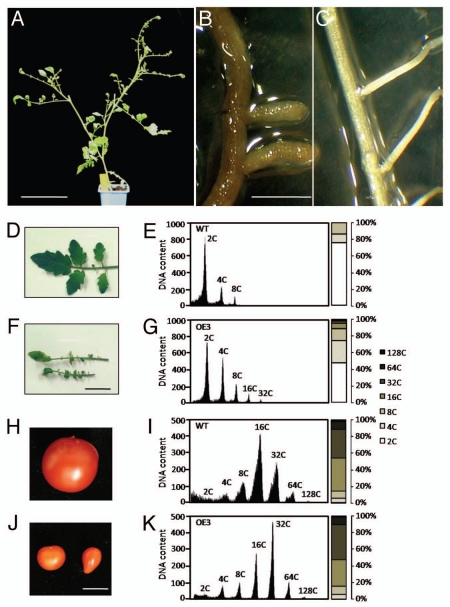
Gain-of-function transgenic tomato plants overexpressing SlCCS52A were also generated (Pro35S:SlCCS52AOE). In the most extreme case, the phenotype of the Pro35S:SlCCS52AOE line OE3 exhibited deep effects on the whole plant development and morphology (Fig. 1A). The plant phenotype was characterized by the appearance of an under-developed root system, with aborted lateral roots (Fig. 1B), under-developed (small and curly) leaves (Fig. 1F), resembling those of Arabidopsis plants overexpressing CDK inhibitors.16,17 Interestingly the ploidy level in these plants was increased towards high DNA levels (Fig. 1G), suggesting the promoting effect of the SlCCS52A overexpression on endoreduplication. The Pro35S:SlCCS52AOE line OE3 hardly produced viable flowers and when fruits possibly developed, they were of very small size, irregular shape (Fig. 1J) with higher levels of endoredupication (Fig. 1K). All these data suggested that cell division was deeply impaired in these plants.
Figure 1.
SlCCS52A overexpression affects organ growth in Pro35S:SlCCS52AOE line OE3. The whole plant development is affected leading to dwarf phenotype (A). The comparison of Pro35S:SlCCS52AOE line OE3 (B) and wild-type (C) root system demonstrated that primary and secondary root growth is impaired in the transgenic lines. The endoreduplication process is increased in both leaves (G) and fruits (K) of the Pro35S:SlCCS52AOE line OE3 when compared to that of the wild-type plants (E and I) respectively. Such modifications led to abnormal leaves (F) and fruits (J) development compared to wild-type (D and H).
Additional SlCCS52A overexpressing plants were analyzed for which the overall phenotypes were less affected allowing subsequent analyses, especially dealing with fruit growth characteristics.15 Fruits from these Pro35S:SlCCS52AOE lines displayed a slower kinetics of fruit growth. However, they tended to reach almost the size of wild type fruit at the end of the growing period (35 DPA). To explain these data, the effects of a SlCSS52A overexpression during the very early fruit development are likely to affect the cell division process as well as the endoreduplication-driven cell expansion, thus leading to very small fruits, similarly to the extreme line OE3. Thereafter and in accordance with the functional specificity of SlCSS52A in the control of endoreduplication, fruit growth is then accelerated during the endoreduplication-driven cell expansion phase to recover optimum final fruit sizes close to that of wild-type fruits. A kinetic study of the appearance of high polyploid nuclei during fruit growth in these Pro35S:SlCCS52AOE lines did support this hypothesis.15
Conclusions
The functional analysis of endoreduplication-promoting genes such as WEE1,18 and CCS52A15 thus demonstrated the physiological role of endoreduplication in fleshy fruit growth, since a reduction in cell size originating from a decrease in DNA ploidy levels impacts whole fruit development and final fruit size. Although the correlation between cell size and endoreduplication is obvious, which process triggers the other is still a matter of debate. Since endoreduplication corresponds to successive rounds of DNA duplication in the absence of mitosis, it is therefore likely that a minimal cell size must be required to commit to the following round of DNA replication, thus implying cell growth. WEE1 is likely to control the endocycle G phase length as to allow this sufficient cell growth prior to commitment to the next nuclear DNA amplification,18 and accordingly the doubling of the DNA quantity can in turn promote cell enlargement, according to the “karyoplasmic ratio” theory.10 At the organ level, manipulating endoreduplication through CCS52A overexpression highlights the function of endoreduplication as an ultimate driving force for fruit growth, and even an enhancer of cell growth rate facilitating and/or accelerating fruit growth or bigger fruit size,15 in accordance with our previous hypothesis stating that endoreduplication is a way to get quickly big fruits.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12222
References
- 1.Nagl W. DNA endoreduplication and polyteny understood as evolutionary strategies. Nature. 1976;261:614–645. doi: 10.1038/261614a0. [DOI] [PubMed] [Google Scholar]
- 2.D'Amato F. Role of polyploidy in reproductive organs and tissues. In: Johri BM, editor. Embryology of Angiosperms. New York: Springer-Verlag; 1984. pp. 519–566. [Google Scholar]
- 3.Joubès J, Chevalier C. Endoreduplication in higher plants. Plant Mol Biol. 2000;43:735–745. doi: 10.1023/a:1006446417196. [DOI] [PubMed] [Google Scholar]
- 4.Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: more for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 5.Bourdon M, Frangne N, Mathieu-Rivet E, Nafati M, Cheniclet C, Renaudin JP, Chevalier C. Endoreduplication and growth of fleshy fruits. In: Lüttge U, et al., editors. Progress in Botany. Vol. 71. Heidelberg: Springer-Verlag; 2009. pp. 101–132. [Google Scholar]
- 6.Bergervoet JHW, Verhoeven HA, Gilissen LJW, Bino RJ. High amounts of nuclear DNA in tomato (Lycopersicon esculentum Mill.) pericarp. Plant Sci. 1996;116:141–145. [Google Scholar]
- 7.Joubes J, Phan TH, Just D, Rothan C, Bergounioux C, Raymond P, et al. Molecular and biochemical characterization of the involvement of cyclin-dependent kinase A during the early development of tomato fruit. Plant Physiol. 1999;121:857–869. doi: 10.1104/pp.121.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheniclet C, Rong WY, Causse M, Bolling L, Frangne N, Carde JP, et al. Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiol. 2005;139:1984–1994. doi: 10.1104/pp.105.068767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevalier C. Cell cycle control and fruit development. In: Inzé D, editor. Cell cycle control and plant development. Vol. 32. Oxford: Blackwell Publishing Ltd. Annual Plant Reviews; 2007. pp. 269–293. [Google Scholar]
- 10.Sugimoto-Shirasu K, Roberts K. “Big it up”: endoreduplication and cell-size control in plants. Curr Opin Plant Biol. 2003;6:544–553. doi: 10.1016/j.pbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Kondorosi E, Kondorosi A. Endoreduplication and activation of the anaphase-promoting complex during symbiotic cell development. FEBS Lett. 2004;567:152–157. doi: 10.1016/j.febslet.2004.04.075. [DOI] [PubMed] [Google Scholar]
- 12.Capron A, Okrész L, Genschik P. First glance at the plant APC/C, a highly conserved ubiquitin-protein ligase. Trends Plant Sci. 2003;8:83–89. doi: 10.1016/S1360-1385(02)00028-6. [DOI] [PubMed] [Google Scholar]
- 13.Cebolla A, Vinardell JM, Kiss E, Olah B, Roudier F, Kondorosi A, et al. The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J. 1999;18:4476–4484. doi: 10.1093/emboj/18.16.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanstraelen M, Baloban M, Da Ines O, Cultrone A, Lammens T, Boudolf V, et al. APC/CCCS52A complexes control meristem maintenance in the Arabidopsis root. Proc Natl Acad Sci USA. 2009;106:11806–11811. doi: 10.1073/pnas.0901193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu-Rivet E, Gévaudant F, Sicard A, Salar S, Do PT, Mouras A, et al. The functional analysis of the Anaphase Promoting Complex activator CCS52A highlights the crucial role of endoreduplication for fruit growth in tomato. Plant J. 2010;62:727–741. doi: 10.1111/j.1365-313X.2010.04198.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Zhou Y, Gilmer S, Whitwill S, Fowke LC. Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J. 2000;24:613–623. doi: 10.1046/j.1365-313x.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 17.De Veylder L, Beeckman T, Beemster GT, Krols L, Terras F, Landrieu I, et al. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell. 2001;13:1653–1668. doi: 10.1105/TPC.010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez N, Gévaudant F, Hernould M, Chevalier C, Mouras A. The cell cycle-associated protein kinase WEE1 regulates cell size in relation to endoreduplication in developing tomato fruit. Plant J. 2007;51:642–655. doi: 10.1111/j.1365-313X.2007.03167.x. [DOI] [PubMed] [Google Scholar]