Abstract
Recently we reported that CRLK1, a novel calcium/calmodulin-regulated receptor-like kinase plays an important role in regulating plant cold tolerance. Calcium/calmodulin binds to CRLK1 and upregulates its activity. Gene knockout and complementation studies revealed that CRLK1 is a positive regulator of plant response to chilling and freezing temperatures. Here we show that MEKK1, a member of MAP kinase kinase kinase family, interacts with CRLK1 both in vitro and in planta. The cold triggered MAP kinase activation in wild-type plants was abolished in crlk1 knockout mutants. Similarly, the cold induced expression levels of genes involved in MAP kinase signaling are also altered in crlk1 mutants. These results suggest that calcium/calmodulinregulated CRLK1 modulates cold acclimation through MAP kinase cascade in plants.
Key words: calcium, calmodulin, cold stress, MAPK, Arabidopsis, protein phosphorylation
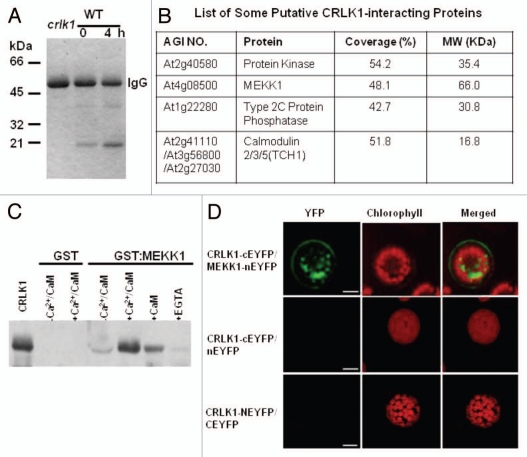
Calcium, a universal second messenger in eukaryotic cells, mediates changes in external and internal signals leading to the physiological responses.1–4 Calcium/calmodulin (Ca2+/CaM)-dependent protein kinases (CaMKs) are very important players in calcium/calmodulin mediated signaling in mammalian cells.5 In plants, Ca2+/CaM-dependent protein phosphorylation was observed more than 25 years ago.6 Several calmodulin-regulated protein kinases have been identified and characterized.7,8 For example, plants have a unique chimeric Ca2+/CaM-dependent protein kinase (CCaMK), which exhibits Ca2+-dependent autophosphorylation and Ca2+/CaM-dependent substrate phosphorylation.9 CCaMK is required for bacterial and fungal symbioses in plants.10–12 Recently, we characterized a novel plant-specific calcium/CaM-regulated receptor-like kinase, CRLK1.13 Ca2+/CaM binds to CRLK1 and stimulates its kinase activity. Functional studies with CRLK1 indicate that CRLK1 acts as a positive regulator in plant response to chilling and freezing temperatures. To further define the CRLK1-mediated signal pathway, we isolated CRLK1 interacting proteins by co-immunoprecipitation using an anti-CRLK1 antibody. Since cold increases the amount of CRLK1 protein, wildtype plants (WT) were treated at 4°C for 1 hr before co-immunoprecipitation. The resulting CRLK1 immunocomplex was separated by SDS-PAGE. We observed several bands of different sizes only in the wild-type but not in the crlk1 knockout mutant plants (Fig. 1A). Furthermore, the intensity of these bands increased upon cold treatment, suggesting that they are the putative partners or associated proteins of the CRLK1 immunocomplex.
Figure 1.
CRLK1 Interacts with MEKK1. (A) One-dimension SDS-PAGE of anti-CRLK1 immunocomplexes from 3-week-old WT or crlk1 plants with or without cold treatment. One mg of total protein was used for immunoprecipitation. (B) A list of putative CRLK1-interacting proteins determined by MALDI-TOF-MS analysis. (C) CRLK1 interacts with MEKK1 as shown by GST pull-down assay. (D) BiFC analysis show that CR LK associates with MEKK1 in vivo. Upper row shows that CRLK and MEKK1 associate both on cell membrane and in endosomes. The middle and last rows are controls. Bar = 10 µm.
To determine the identities of these proteins, mass spectrometric analysis was performed with the total immunocomplex.14 In addition to CRLK1, there were 12 other proteins which matched the Arabidopsis database. Several of them appeared in the pull-down complex from WT, but not from crlk1 mutants. These putative interacting proteins included MEKK1, another unknown protein kinase, a type 2C phosphatase and CaM (Fig. 1B). MEKK1 is one of the 60 putative MAPKKKs in the Arabidopsis genome, and sits on the top of mitogen-activated protein kinase (MAPK) cascade. The MAPK signaling consists of a cascade of three consecutively acting protein kinases, a MAP kinase kinase kinase (MAPKKK), a MAP kinase kinase (MAPKK) and a MAP kinase (MAPK). Plants possess multiple MAPKKKs, MAPKKs and MAPKs, which respond to different upstream signals and activate distinct downstream pathways.15–17 The specific MAPK module responding to lower temperature has been determined in Arabidopsis.18,19 MEKK1, a member of MAPKKKs, specifically interacts and phosphorylates MKK2 and regulates COR genes expression in response to cold stress.19 MEKK1 has been shown to play a role in mediating reactive oxygen species homeostasis.20,21 Therefore we selected MEKK1 from the putative CRLK1 partners for further studies.
CRLK1 Interacts With MEKK1 in vitro and in planta
To confirm the direct interaction between CRLK1 and MEKK1, a well-characterized component in cold signaling, we performed GST pull-down assay (Fig. 1C). The recombinant CRLK1 M29-440 was precipitated by GST:MEKK1, but not by GST alone. However, the intensity of the band was very low, suggesting weak interaction between them. Since CRLK1 is a calcium/CaM-regulated kinase, we investigated the effects of calcium and/or CaM on the interaction between CRLK1 and MEKK1. In the presence of calcium and CaM in the reaction mixture, the interaction between CRLK1 and MEKK1 was dramatically increased as reflected by the intensity of the band (Fig. 1C). These results indicate that the binding of calcium/CaM to CRLK1 increases its affinity to MEKK1.
To address if CRLK1 and MEKK1 associate in vivo and to determine subcellular location of this association, we used Bimolecular Fluorescence Complementation (BiFC) in Arabidopsis protoplasts.22 BiFC vectors carrying CRLK and MEKK1 were co-transfected into protoplasts and observed for the reconstitution of YFP fluorescence. Confocal images showed that CRLK1 associated with MEKK1 both on cell membranes and in intracellular vesicle-like structures (Fig. 1D). Control co-transfections with nEYFP or cEYFP vectors did not display any fluorescence suggesting that the interaction between CRLK1 and MEKK1 is specific (Fig. 1D).
Loss of CRLK1 Altered MAP Kinase Activity and Expression Levels of Genes Involved MAPK Pathway
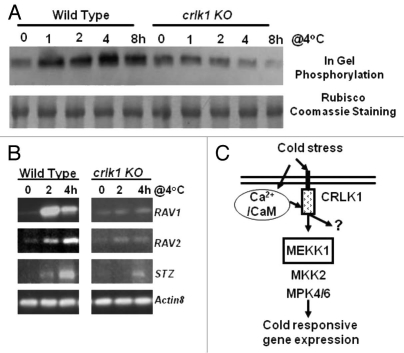
To further study the relationship between CRLK1 and MAPK signaling, we compared the MAPK activity between WT and crlk1 plants in response to cold treatment using in gel phosphorylation assay (Fig. 2A). In WT plants, cold stimulated MAP kinase activity. However, this stimulation was diminished in crlk1 mutants. These results suggest that CRLK1 plays a role in regulating the MAPK cascade during cold signaling.
Figure 2.
CRLK1 regulates the MAPK kinase activity and expression levels of genes affected by MAPK cascade during cold treatment. (A) In gel phosphorylation showed that cold stimulated MAP kinase activity is diminished in crlk1 plants. The in-gel assay of endogenous MAPK was carried out as described.28 Briefly, 50 µg of plant protein was fractioned in a 12.5% SDS-PAGE with 0.25 mg/ml mylein basic protein (MBP). The gel was incubated with protein phosphorylation buffer with [γ32P-ATP], and exposed to X-ray film after washing. Forty micrograms of total proteins were used in the assay. The Coomassie stained RUBISCO band was used to show equal loading. (B) RT-PCR analysis of RAV1, RAV2 and STZ gene expression. Actin8 was used as an internal control. (C) A hypothetical model showing cold stress signal transduction pathway linking calcium/calmodulin, CRLK1 and MAPK cascade.
It has been shown that cold treatment increases the expression of MEKK1.23 To investigate whether CRLK1 affects MEKK1 expression, we compared the RNA level of MEKK1 between WT and crlk1 mutants using semi-quantitative RT-PCR. MEKK1 levels in both WT and crlk1 plants were similar after cold treatment, suggesting that CRLK1 does not regulate MEKK1 at the transcriptional level (data not shown). We further studied the expression of the marker genes such as RAV1, RAV2 and STZ affected by MAPK cascade.19 Their expression levels were lower in crlk1 plants as compared to WT after cold treatment (Fig. 2B). These results are consistent with our earlier observation that CBF and COR genes expression were reduced or delayed in crlk1 knockout plants as compared to wild-type plants during cold treatment.13 It is documented that cold responsive CBF and COR genes expression are regulated by MAPK pathway.19
Accumulating evidence indicates that protein phosphorylation is involved in the pathway connecting the cold-triggered calcium changes and cold acclimation.24,25 However, the protein kinase(s) responsible for inducing cold-regulated genes and activating freezing tolerance is elusive. There are interesting candidates such as an alfalfa MPK, p44mmk4, that is activated within 10 min after being exposed to low temperature.26 Expressing a heterologous tobacco MAPKKK (Nicotiana PK1) enhances freezing tolerance in transgenic maize plants that are normally frost sensitive.27 In Arabidopsis, MEKK1, MKK2, MPK4 and MPK6 have been shown to be involved in cold signal transduction.18,19,23 Our results suggest that calcium/CaM/CRLK1 interacts with MEKK1 and regulates the MAPK cascade during cold stress. Figure 2C is a working model showing the components of CRLK1-mediated cold stress signal transduction pathway linking calcium/calmodulin and MAPK cascade. Further studies on the hierarchy of calcium/CaM/CRLK1 and MAPK will shed light on our understanding of the mechanisms of cold signal transduction pathway(s) in plants.
Acknowledgements
We thank Dr. Shubho Chaudhuri for his help and suggestions and Ms. Y. Chen for her help in the laboratory. This project was supported by the National Research Initiative Competitive Grant no. 2008-35100-04566 from the USDA National Institute of Food and Agriculture; grant no. ISO-0642146 from the National Science Foundation; the Washington State University Agricultural Research Center; Colorado State University Academic Enrichment Program grant 180470; and by a National Science Foundation grant (DBI 0743097).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12225
References
- 1.Poovaiah BW, Reddy AS. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6:47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- 2.Poovaiah BW, Reddy AS. Calcium and signal transduction in plants. CRC Crit Rev Plant Sci. 1993;12:185–211. doi: 10.1080/07352689309701901. [DOI] [PubMed] [Google Scholar]
- 3.Reddy AS. Calcium: silver bullet in signaling. Plant Sci. 2001;160:381–404. doi: 10.1016/s0168-9452(00)00386-1. [DOI] [PubMed] [Google Scholar]
- 4.Trewavas AJ, Malho R. Ca2+ signalling in plant cells: the big network! Curr Opin Plant Biol. 1998;1:428–433. doi: 10.1016/s1369-5266(98)80268-9. [DOI] [PubMed] [Google Scholar]
- 5.Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- 6.Veluthambi K, Poovaiah BW. Calcium-promoted protein phosphorylation in plants. Science. 1984;223:167–169. doi: 10.1126/science.223.4632.167. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Lu Y. Calmodulin-binding protein kinases in plants. Trends Plant Sci. 2003;8:123–127. doi: 10.1016/S1360-1385(03)00013-X. [DOI] [PubMed] [Google Scholar]
- 8.Yang T, Chaudhuri S, Yang L, Chen Y, Poovaiah BW. Calcium/calmodulin upregulates a cytoplasmic receptor-like kinase in plants. J Biol Chem. 2004;279:42552–42559. doi: 10.1074/jbc.M402830200. [DOI] [PubMed] [Google Scholar]
- 9.Patil S, Takezawa D, Poovaiah BW. Chimeric plant calcium/calmodulin-dependent protein kinase gene with a neural visinin-like calcium-binding domain. Proc Natl Acad Sci USA. 1995;92:4797–4801. doi: 10.1073/pnas.92.11.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science. 2004;303:1361–1364. doi: 10.1126/science.1093038. [DOI] [PubMed] [Google Scholar]
- 11.Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GED, et al. A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: Gene identification by transcript-based cloning. Proc Natl Acad Sci USA. 2004;101:4701–4705. doi: 10.1073/pnas.0400595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleason C, Chaudhuri S, Yang T, Munoz A, Poovaiah B, Oldroyd G. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking auto-inhibition. Nature. 2006;441:1149–1152. doi: 10.1038/nature04812. [DOI] [PubMed] [Google Scholar]
- 13.Yang TB, Chaudhuri S, Yang LH, Du LQ, Poovaiah BW. A Calcium/calmodulin-regulated member of the receptor-like kinase family confers cold tolerance in plants. J Biol Chem. 2010;285:7119–7126. doi: 10.1074/jbc.M109.035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phee BK, Shin DH, Cho JH, Kim SH, Kim JI, Lee YH, et al. Identification of phytochrome-interacting protein candidates in Arabidopsis thaliana by coimmunoprecipitation coupled with MALDI-TOF MS. Proteomics. 2006;6:3671–3680. doi: 10.1002/pmic.200500222. [DOI] [PubMed] [Google Scholar]
- 15.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Tena G, Asai T, Chiu W, Sheen J. Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol. 2001;4:392–400. doi: 10.1016/s1369-5266(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 17.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J. 2008;413:217–226. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24:655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 19.Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, et al. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15:141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Nakagami H, Soukupova H, Schikora A, Arsky V, Hirt H. A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem. 2006;281:38697–38704. doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- 21.Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, et al. MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol. 2007;143:661–669. doi: 10.1104/pp.106.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali GS, Prasad KV, Hanumappa M, Reddy AS. Analyses of in vivo interaction and mobility of two spliceosomal proteins using FRAP and BiFC. PLoS ONE. 2008;3:1953. doi: 10.1371/journal.pone.0001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, et al. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monroy A, Dhindsa R. Low-temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25°C. Plant Cell. 1995;7:321–331. doi: 10.1105/tpc.7.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monroy A, Sarhan F, Dhindsa R. Cold-induced changes in freezing tolerance, protein phosphorylation and gene expression (evidence for a role of calcium) Plant Physiol. 1993;102:1227–1235. doi: 10.1104/pp.102.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonak C, Kiegerl S, Ligterink W, Barker P, Huskisson N, Hirt H. Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA. 1996;93:11274–11279. doi: 10.1073/pnas.93.20.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shou H, Bordallo P, Fan J, Yeakley J, Bibikova M, Sheen J, et al. Expression of an active tobacco mitogen-activated protein kinase kinase kinase enhances freezing tolerance in transgenic maize. Proc Natl Acad Sci USA. 2004;101:3298–3303. doi: 10.1073/pnas.0308095100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He P, Shan L, Lin N, Martin G, Kemmerling B, Nurnberger T, et al. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell. 2006;125:563–575. doi: 10.1016/j.cell.2006.02.047. [DOI] [PubMed] [Google Scholar]