Abstract
Considerable evidence has appeared over the past few years that nitric oxide (NO) is an important anoxic metabolite and a potent signal molecule in plants. Several pathways operative in different cell compartments, lead to NO production. Mitochondria, being a major NO producing compartment, can generate it by either nitrite reduction occurring at nearly anoxic conditions or by the oxidative route via nitric oxide synthase (NOS). Recently we compared both pathways by ozone collision chemiluminescence and by DAF fluorescence. We found that nitrite reduction to NO is associated with the mitochondrial membrane fraction but not with the matrix. In case of the nitric oxide synthase pathway, an L-arginine dependent fluorescence was detected but its response to NOS inhibitors and substrates was untypical. Therefore the existence of NOS or NOS-like activity in barley root mitochondria is very doubtful. We also found that mitochondria scavenge NO. In addition, we found indirect evidence that mitochondria are able to convert NO to gaseous intermediates like NO2, N2O and N2O3.
Key words: nitrate reductase, nitric oxide synthase, nitric oxide, mitochondria, DAF fluorescence
Mitochondria are known as powerhouses of the cell. These organelles harbour the citric acid cycle and electron transport chain. Almost all the eukaryotic mitochondria share these basic functions. In addition to the energy generation, mitochondria are one of the major producers of reactive oxygen species1 and involved in retrograde signalling.2 Recent evidence suggests that mitochondria are one of the major producers of nitric oxide (NO) in plants.3,4 Since nitric oxide has gained high importance, this novel property of mitochondria stimulated interest in NO signalling research.
Eukaryotic mitochondria may produce NO by two distinct pathways. One is an oxidative pathway which uses L-arginine as a substrate and produces NO and citrulline7 and the other is a reductive pathway which uses nitrite as a substrate and produces NO at low oxygen conditions.5,6
L-Arginine Dependent NO Production
In animal systems, several nitric oxide synthases are well characterized: these are eNOS (endothelial NOS), iNOS (inducible NOS), and nNOS (neuronal NOS). eNOS and nNOS are constitutively expressed7 whereas iNOS is inducible in certain physiological conditions. In addition to these NOS enzymes, existence of mitochondrial NOS has been suggested in animal systems. All nitric oxide synthases use L-Arg as a substrate and NAD(P)H and O2 as co-substrates to produce NO and citrulline. NOS activity was investigated and confirmed in animal mitochondria8 but there are still many controversies on the existence of NOS in animal mitochondria.9 In plants as well, there are several reports suggesting the existence of NOS-like activities in various developmental and physiological stressful situations.10 NOS activity was shown in peroxisomes from pea plants11 and also in mitochondria from Arabidopsis.12 However, the existence of NOS in Arabidopsis, AtNOS1, has recently been challenged,13,14 Therefore, AtNOS1 was renamed to AtNOA1, for NITRIC OXIDE ASSOCIATED PROTEIN1,15 and showed that the protein can hydrolyse GTP to GDP.
Recently we made an attempt to measure NOS dependent NO production from barley root mitochondria in air by supplying L-arginine, NADPH, Ca2+, calmodulin, tetrahydrobiopterin (BH4), FMN and FAD.16 We used the chemiluminescence method to measure NO. This method is based on the reaction of nitric oxide with ozone which generates the chemiluminiscence signal. By this method we could not detect any NO production from mitochondria in aerobic conditions. There is a possibility that the produced NO was rapidly oxidized to nitrite and nitrate. In order to check for any potential oxidation of NO to NO2− and NO3−, we employed another approach called ‘indirect chemiluminescence method’. This method is based on reduction of the products of NO oxidation such as NO2− and NO3− in acidic VCl3 solution, followed by normal chemiluminescence detection of NO formed. By using the direct and indirect chemiluminescence methods, we could not find any aerobic arginine-dependent NO generation by mitochondria, indicating that there is no NOS like activity in these organelles.
In order to check the reliability of our method, we tested NO production by using an inducible nitric oxide synthase (recombinant) from mouse. With this purified iNOS we could measure NO and the NO production rates were in the range of 4 nmoles/unit enzyme/hr. We also estimated the potential oxidation of NO to nitrate and nitrite by ‘indirect chemiluminescence’ and we could estimate that two thirds of the NO formed were rapidly oxidized.
To confirm our findings we also estimated a potential L-arginine based NO emission by the widely used DAF fluorescence method. First we measured NO from mitochondria after incubating them with various NOS cofactors and substrates. Mitochondria always produced DAF fluorescence which slightly increased in the presence of L-arginine and was partly impaired by the NO scavenger cPTIO. This would be normally interpreted to indicate some NOS like activity, but unfortunately the fluorescence was insensitive to the L-arginine analogues L-NAME and L-NIL, which are potent NOS inhibitors. It was also surprising that fluorescence was diminished in the presence of NADPH which is actually required as co-substrate for NOS. We concluded that there is probably no NOS-like activity in plant mitochondria.
Nitrite-Dependent NO Production
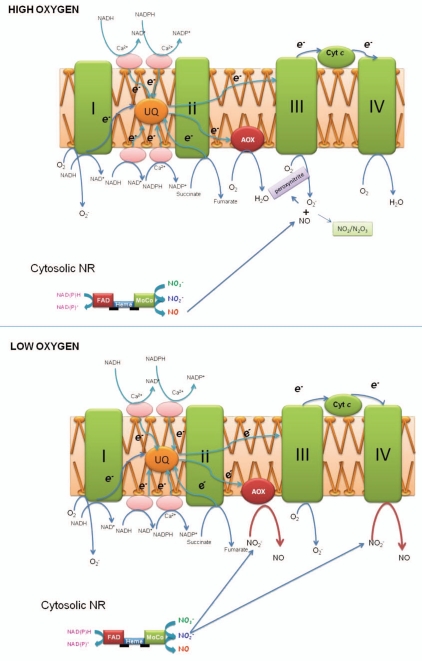
Nitrite acts as a main substrate for NO synthesis in plants.3 The major enzyme in this pathway is nitrate reductase (NR), which catalyses the reduction of nitrate to nitrite using NAD(P)H as an electron donor and provides nitrite to other NO producing sites such as mitochondria (Fig. 1, lower). Under oxygen free conditions, mitochondria from plants, like from animals, produce nitric oxide from nitrite in the presence of NADH.3,4 The site of nitrite to NO reduction is the terminal cytochrome c oxidase of mitochondrial electron transport chain.4,6 Interestingly this property is detected only in root mitochondria.4 This pathway can lead to a limited amount of ATP production during anoxia.17 If the nitrite reduction site would lie in the electron transport chain of the membranes, all plant mitochondria should be able to produce NO from nitrite. If matrix components would be involved, this might explain the above difference in leaf and root mitochondria. Therefore in our recent investigation we separated root mitochondria into membrane and matrix fractions and checked them for NO production. Interestingly, only the membrane fraction was capable to produce NO. Application of the detergent Triton-X 100 abolished NO production from the membrane, suggesting that membrane integrity is essential in this process.
Figure 1.
Upper: Scavenging of nitric oxide by plant mitochondrial electron transport chain components when oxygen is present. In these conditions nitrate reductase produces NO and superoxide produced in mitochondria reacts with NO and forms peroxynitirte and also NO can easily react with oxygen to form gaseous intermediates like N2O3 and NO2. Lower: Reduction of nitrite to NO by the electron transport chain of plant mitochondria in hypoxic conditions. According to the inhibitory effects of myxothiazol and (complex III) and cyanide (IV) salicylhydroxamic acid (AOX), both terminal oxidases appear to possess nitrite: NO reducing activity.
Do Mitochondria Scavenge NO?
Due to the lipophilicity of NO, NO concentrations in the lipid phase will be several orders of magnitude higher than in the water phase, which might favour catalysis of NO oxidation. In order to check whether mitochondria are able to oxidize NO, we used iNOS in mixture with mitochondrial membranes. NO emission from 5 units of iNOS (without mitochondria added) was 4 nmol h−1 and the products of NO oxidation (nitrate plus nitrite) were about of 15.2 nmol h−1. When the mitochondrial membranes were injected into a sample of iNOS reaction mixture, the direct NO emission decreased by almost 2/3, yet the amount of (nitrite + nitrate) was increased only slightly, suggesting that mitochondria accelerate conversion of NO to gaseous forms like NO2 or N2O. There is also possibility that mitochondria produce peroxynitrite (ONOO−) in normoxic and hypoxic conditions from the reaction of superoxide (O2−) and NO (Fig. 1, upper). In animal systems it has been shown that isolated cytochrome c oxidase metabolizes NO to N2O at low oxygen tensions, and to NO2 at higher oxygen tensions.18
Conclusions
The anoxic nitrite-dependent NO emission by plant mitochondria is attributed to the membrane but not to the matrix. There is a high probability that mitochondria oxidize NO to gaseous intermediate such as NO2 and N2O3 and also they can reduce N2O. Plant mitochondria are unable to produce NO from L-Arg suggesting that they do not contain a NOS-like activity.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 567) to WMK and by the Natural Sciences and Engineering Research Council of Canada to AUI.
Abbreviations
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAF
2,4,5-diaminofluorescein
- DAF-2DA
4,5-diaminofluorescein diacetate
- NO
nitric oxide
- NOS
nitric oxide synthase
- NR
nitrate reductase
- SHAM
salicylhydroxamic acid
- AOX
alternative oxidase
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12229
References
- 1.Møller IM. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- 2.Rhoads DM, Subbaiah CC. Mitochondrial retro-grade regulation in plants. Mitochondrion. 2007;7:177–194. doi: 10.1016/j.mito.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Planchet E, Gupta KJ, Sonoda M, Kaiser WM. Nitric oxide emission from tobacco leaves and cell suspensions: rate-limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 2005;41:732–743. doi: 10.1111/j.1365-313X.2005.02335.x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot. 2005;56:2601–2609. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser WM, Gupta KJ, Planchet E. Higher plant mitochondria as a source for NO. In: Lamattina L, Polacco JC, editors. Nitric Oxide in Plant Growth. Plant Cell Monographs. Vol. 6. Berlin: Springer; 2007. pp. 1–14. [Google Scholar]
- 6.Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- 7.Daff S. NO synthase: Structures and mechanisms. Nitric Oxide. 2010 doi: 10.1016/j.niox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. J Biol Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 9.Lacza Z, Pankotai E, Csordás A, Gero D, Kiss L, Horváth EM, et al. Mitochondrial NO and reactive nitrogen species production: does mtNOS exist? Nitric Oxide. 2006;14:162–168. doi: 10.1016/j.niox.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Corpas FJ, Palma JM, Del Río LA, Barroso JB. Evidence supporting the existence of L-arginine-dependent nitric oxide synthase activity in plants. New Phytol. 2009;184:9–14. doi: 10.1111/j.1469-8137.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 11.Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM, et al. Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem. 1999;274:36729–36733. doi: 10.1074/jbc.274.51.36729. [DOI] [PubMed] [Google Scholar]
- 12.Guo FQ, Crawford NM. Arabidopsis nitric oxide synthase 1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell. 2005;17:3436–3450. doi: 10.1105/tpc.105.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford NM, Galli M, Tischner R, Heimer YM, Okamoto M, Mack A. Plant nitric oxide synthase: back to square one. Trends Plant Sci. 2006;11:526–527. [Google Scholar]
- 14.Zemojtel T, Frohlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, et al. Plant nitric oxide synthase: a never-ending story? Trends Plant Sci. 2006;11:524–525. doi: 10.1016/j.tplants.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF. AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J Biol Chem. 2008;283:32957–32967. doi: 10.1074/jbc.M804838200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta KJ, Kaiser WM. Production and scavenging of nitric oxide by barley root mitochondria. Plant Cell Physiol. 2010;51:576–584. doi: 10.1093/pcp/pcq022. [DOI] [PubMed] [Google Scholar]
- 17.Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta. 2007;226:465–474. doi: 10.1007/s00425-007-0496-0. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe MA, Cooper CE. Reactions of nitric oxide with mitochondrial cytochrome c: a novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem J. 1998;332:9–19. doi: 10.1042/bj3320009. [DOI] [PMC free article] [PubMed] [Google Scholar]