Abstract
Numerous fungal and oomycete pathogens penetrate the plant cell wall and extract nutrition from the host cells by a feeding structure called the haustorium. We recently revealed that the Arabidopsis resistance protein RPW8.2 is specifically targeted to the extrahaustorial membrane (EHM) for activation of haustorium-targeted resistance to powdery mildew pathogens. Consistent with its EHM-localization, RPW8.2 contains a putative transmembrane (TM) domain at its N-terminus. Here, we show that translational fusion of YFP to the N-terminus of RPW8.2 results in localization of YFP-RPW8.2 to both the plasma membrane and the EHM, and loss of RPW8.2's defense function. We also show that deletion of the TM domain results in mis-localization of the RPW8.2-YFP fusion protein and extremely low levels of accumulation. These results indicate that an intact N-terminal TM domain is necessary for EHM-specific localization and defense function of RPW8.2. In addition, we show that when expressed from the strong constitutive 35S viral promoter, RPW8.2 accumulates at low levels in the EHM insufficient to activate resistance, highlighting the importance of strong spatiotemporal expression of RPW8.2 from its native promoter. Taken together, our results indicate that accurate and adequate spatiotemporal expression and localization of RPW8.2 is key to activation of resistance at the host-pathogen interface.
Key words: Arabidopsis, RPW8.2, resistance, powdery mildew, haustorium, extrahaustorial membrane, host-pathogen interface, protein localization
In order to establish successful colonization on plant hosts, a haustorium-forming fungus such as powdery mildew must conquer two spatio-temporally interconnected layers of host resistance: pre-invasion (penetration) resistance and post-invasion resistance.1 Pre-invasion resistance protects plants from non-adapted pathogens by blocking their entry into the host cell.2–4 One common induced cellular defense response at this resistance level is the deposition of defense chemicals, including callose (β-1,3-glucan) at the site of penetration, resulting in cell wall apposition, a subcellular structure also known as a papilla.5–7 It has been reported that a syntaxin encoded by PENETRATION 1 (PEN1) is required for the timely assembly of the papilla,8 which is consistent with PEN1's role in pre-invasion resistance.2 Once the fungus penetrates the plant cell wall, it will have to overcome the second layer of resistance, i.e., post-invasion resistance, to develop a functional haustorium in close contact with the host cell cytoplasm for successful colonization. Hypersensitive response (HR) manifested as rapid collapse of the invaded cell is often associated with post-invasion resistance.9–11 Another cellular defense response to haustorial invasion is the formation of an encasement of the haustorial complex (EHC).12–16 Like the papilla, the EHC is also enriched for callose and thought to be formed via extension from the papilla by rim-growth.17
We have recently reported that RPW8.2-mediated broad-spectrum powdery mildew resistance is associated with both HR and an enhancement of EHC formation.18 Most strikingly, we found that the RPW8.2-YFP fusion protein expressed from its native promoter (NP) is specifically targeted to the extrahaustorial membrane (EHM), suggesting that RPW8.2 functions at the host-pathogen interface to activate post-invasion resistance. How RPW8.2 is targeted to the EHM and directs host defense to the host-pathogen interface remains to be an open question.
YFP-RPW8.2 is Non Functional and is Localized to Both the Plasma Membrane and the Extrahaustorial Membrane
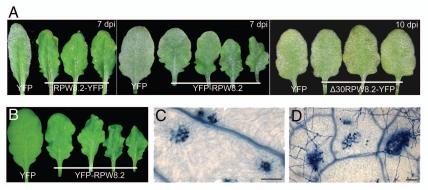
The RPW8.2 protein, as other members of the small RPW8 protein family, contain a predicated N-terminal transmembrane domain (TM) (amino acid 1 to 22) or signal peptide.19,20 To investigate the role of the putative TM domain, we translationally fused YFP to the N-terminus of RPW8.2 and the NP::YFP-RPW8.2 construct was introduced into the mildew-susceptible, RPW8.2-null Col-0 background. We found that the majority (>75%) of transgenic lines expressing NP::YFP-RPW8.2 exhibited a wavy leaf phenotype which had not been observed in plants expressing NP::RPW8.2-YFP (Fig. 1A and B). Unlike Col-0 lines transgenic for NP::RPW8.2-YFP (of which, ∼5–10% showed spontaneous HR-like cell death), none of the Col-0 lines expressing NP::YFP-RPW8.2 developed HR-like lesions visible to the naked eye. We then evaluated the disease reaction phenotypes of these NP::YFP-RPW8.2 transgenic lines in response to infection by Golovinomyces cichoracearum (Gc) UCSC1, the causal agent of powdery mildew diseases in Arabidopsis and many other plant species.21 Except for a few (4/35) transgenic lines that showed some slightly reduced susceptibility (Fig. 1A), we found the majority were as susceptible to Gc UCSC1 as Col-0 (data not shown). These results suggest that unlike RPW8.2-YFP which largely retains the function of the native RPW8.2 protein,18 YFP-RPW8.2 is largely nonfunctional in defense. We performed trypan blue staining with Arabidopsis leaves prior to inoculation and 3 days post-inoculation (dpi) of Gc UCSC1 to see if there is any cell death in the lines transgenic for NP::YFP-RPW8.2. We found that there were some small clusters of cell death on both uninoculated and inoculated wavy leaves, and the cell death was not tightly associated with fungal invasion (Fig. 1C and D). A close microscopic examination of the spontaneous cell death lesions revealed that some surrounding epidermal and mesophyll cells had irregular shapes and sizes, resulting in contraction of the neighboring areas. This may explain the curly, wavy leaf phenotype.
Figure 1.
Leaf phenotypes of transgenic plants expressing different RPW8.2 constructs. (A) Representative powdery mildew-infected leaves of independent Col-gl lines expressing the indicated DN A constructs. Pictures were taken at 7 or 10 days post-inoculation (dpi) with Golovinomyces cichoracearum UC SC1. (B) Representative wavy leaves from four independent Col-gl lines expressing YFP-RPW8.2 and a normal leaf from a Col-gl line expressing YFP. (C and D) Cell death in Col-gl lines expressing YFP-RPW8.2 prior to inoculation (C) or at 3 dpi (D). Leaf sections from respective plants were stained with trypan blue. Dead plant cells and vascular veins, and fungal mycelia were stained blue. Scale bar, 100 µm.
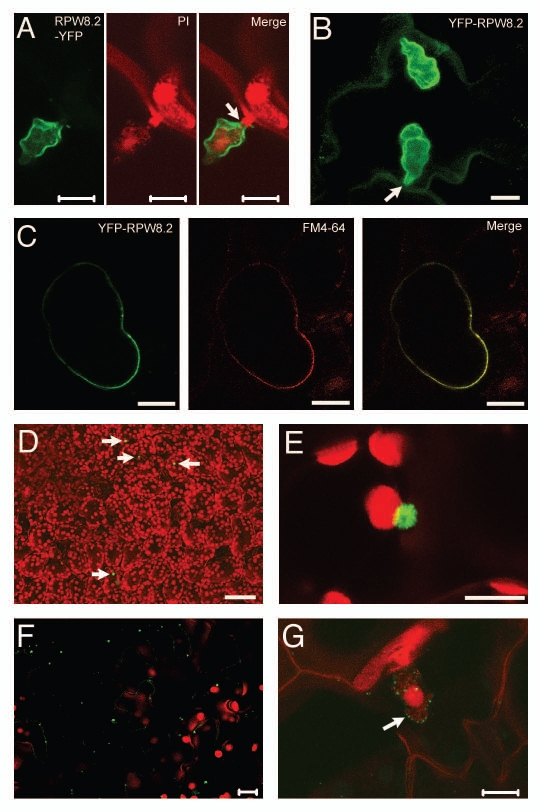
We then questioned if YFP-RPW8.2 is correctly targeted to the EHM induced by Gc UCSC1. Interestingly, unlike RPW8.2-YFP, which is highly specifically targeted to the EHM (Fig. 2A; Wang et al., 2009), fluorescent signals from YFP-RPW8.2 was found to be distributed in both the EHM and the cytoplasm or plasma membrane (PM) (Fig. 2B). To make sure if YFP-RPW8.2 is indeed localized at the PM, we treated a leaf section with the endomembrane dye FM4-64 and performed cytoplasmolysis. As shown in Figure 2C, YFP-RPW8.2 was nicely co-localized with the FM4-64 stained PM, indicating that YFP-RPW8.2 is not only targeted to the EHM, but also distributed at the PM. Apparently, this decrease in localization specificity could result in a reduced efficacy of YFP-RPW8.2 in triggering EHM-directed defense against powdery mildew compared to RPW8.2-YFP or the native RPW8.2 protein. However, given the seemingly preferential EHM-localization of YFP-RPW8.2 versus its PM-localization in the same cells (Fig. 2B), one would not expect a nearly complete loss of resistance in plants expressing YFP-RPW8.2. Therefore, it is possible that, in addition to a compromised EHM-targeting specificity, YFP fusion at the N-terminus of RPW8.2 may result in (i) change of RPW8.2's membrane orientation/association due to masking the N-terminal domain as a potential signal peptide for co-translational translocation at the endoplamic reticulum, or (ii) alteration of the protein conformation that renders RPW8.2 nonfunctional, or (iii) disruption of RPW8.2's interaction with other proteins, which is required for its defense function, via the N-terminal domain.
Figure 2.
Laser scanning confocal microscopic images showing subcellular localization of different YFP-tagged RPW8.2 proteins. (A) RPW8.2-YFP (green) expressed from the native promoter is specifically localized to the extrahaustorial membrane encasing the haustorium (stained red by 0.5% propidium iodide). (B) YFP-RPW8.2 expressed from the native promoter is localized to both the plasma membrane and the extrahaustorial membrane. The arrow indicates the fungal penetration site. (C) Cytoplasmolysis of YFP-RPW8.2 expressing cells showing YFP-RPW8.2 is indeed localized to the plasma membrane stained red by the fluorescent dye FM4-64. (D and E) Low-level accumulation of Δ30RPW8.2-YFP expressed from the native promoter in punctate spots (arrows). (F and G) Typical low-level accumulation of RPW8.2-YFP expressed from the 35S promoter in unchallenged epidermal cells (F) or in a representative challenged epidermal cell at 2 dpi (G). The arrow indicates a haustorium decorated with RPW8.2-YFP in punctate spots presumably at the extrahaustorial membrane. All pictures are Z-stack of 15–25 images. Scale bars, 10 µm for all except D (50 µm).
Adequate Expression and EHM-Accumulation of RPW8.2 is Required for Activation of Resistance
To test if overexpression of RPW8.2 could improve resistance, we expressed both YFP-RPW8.2 and RPW8.2-YFP from the strong constitutive viral 35S promoter. Quite counterintuitively, we found that both YFP-RPW8.2 and RPW8.2-YFP were expressed at very low levels prior to and after Gc UCSC1 infection with protein localization patterns similar to those expressed from the native promoter. For example, RPW8.2-YFP expressed from the 35S promoter was found in punctate spots along cytoplasmic streams in unchallenged epidermal cells of some transgenic lines (Fig. 2F). After powdery mildew infection, RPW8.2-YFP was mostly found in punctate spots presumably at the EHM (Fig. 2G). Not surprisingly, all transgenic lines (>15 examined) expressing either of the two constructs from the 35S promoter were generally susceptible to the pathogen (data not shown). Based on these observations, we concluded that an adequate dosage of the functional, EHM-localized RPW8.2 protein is essential for inducing interface-focused resistance to powdery mildew. Our results also confirmed our previous findings at the protein level that the native promoters of RPW8.1 and RPW8.2 are required for salicylic acid-dependent feedback amplification of RPW8.1 and RPW8.2 in cells invaded by powdery mildew.22
The N-Terminus of RPW8.2 is Indispensable for its EHM Localization and Resistance Function
To further clarify the importance of the TM domain of RPW8.2, we translationally fused two RPW8.2 mutants in which the N-terminal TM domain was truncated from amino acids 2 to 22 (Δ22RPW8.2) or from 2 to 30 (Δ30RPW8.2) with YFP at the C-terminus of RPW8.2. We individually introduced these two constructs into Col-0 and found that all Col-0 transgenic lines (>15) expressing Δ22RPW8.2-YFP or Δ30RPW8.2-YFP from the RPW8.2 native promoter were fully susceptible to Gc UCSC1 (Fig. 1A). Examination of infected leaves by confocal microscopy showed that these fusion proteins were rarely detectable in uninoculated or infected leaves. In a few mesophyll cells where YFP signals were detectable, we observed some fluorescent spots in the size of ∼3 µm as shown in Figure 2D and E. We have never detected any YFP signals in the EHM, indicating a complete loss of EHM-localization and accumulation for these two TM-truncated RPW8.2 fusion proteins. Based on these results, we speculate that the N-terminal TM domain of RPW8.2 is not only important for the membrane anchorage of RPW8.2 for EHM-targeting but also plays a role for RPW8.2 accumulation by stabilizing the protein and/or contributing to the function of RPW8.2 in triggering the SA-dependent feedback amplification of RPW8.2.
Taken together, our results support a conclusion that adequate, spatially correct and temporally amplifiable RPW8.2 is critical to RPW8.2-mediated, EHM-focused resistance against haustorial invasion from powdery mildew pathogens.
Acknowledgements
This project was supported by National Science Foundation Grant IOS-0842877 to S.X.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12230
References
- 1.Thordal-Christensen H. Fresh insights into processes of nonhost resistance. Curr Opin Plant Biol. 2003;6:351–357. doi: 10.1016/s1369-5266(03)00063-3. [DOI] [PubMed] [Google Scholar]
- 2.Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature. 2003;425:973–977. doi: 10.1038/nature02076. [DOI] [PubMed] [Google Scholar]
- 3.Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, et al. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science (New York, NY) 2005;310:1180–1183. doi: 10.1126/science.1119409. [DOI] [PubMed] [Google Scholar]
- 4.Stein M, Dittgen J, Sanchez-Rodriguez C, Hou BH, Molina A, Schulze-Lefert P, et al. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell. 2006;18:731–746. doi: 10.1105/tpc.105.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushnell WR, Bergquist SE. Aggregation of host cytoplasm and formation of papillae and haustoria in powdery mildew of barley. Phytopathology. 1975;65:310–318. [Google Scholar]
- 6.Huckelhoven R, Fodor J, Preis C, Kogel KH. Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant physiol. 1999;119:1251–1260. doi: 10.1104/pp.119.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aist JR. Papillae and related wound plugs of plant cells. Ann Rev Phytopatho. 1976;14:145–163. [Google Scholar]
- 8.Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell. 2004;15:5118–5129. doi: 10.1091/mbc.E04-02-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thordal Christensen H, Zhang ZG, Wei YD, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- 10.Huang CC, Groot T, Meijer-Dekens F, Niks RE, Lindhout P. The resistance to powdery mildew (Oidium lycopersicum) in Lycopersicon species is mainly associated with hypersensitive response. Eur J Plant Pathol. 1998;104:399–407. [Google Scholar]
- 11.Niks RE, Dekens RG. Prehaustorial and posthaustorial resistance to wheat leaf rust in diploid wheat seedlings. Phytopathology. 1991;81:847–851. [Google Scholar]
- 12.Ehrlich HG, Ehrlich MA. Electron microscopy of sheath surrounding haustorium of Erysiphe graminis. Phytopathology. 1963;53:1378–1380. [Google Scholar]
- 13.Perumalla CJ, Heath MC. Effect of callose inhibition on haustorium formation by the cowpea rust fungus in the non-host, bean plant. Physiol Mol Plant Pathol. 1989;35:375–382. [Google Scholar]
- 14.Skalamera D, Heath MC. Changes in the plant endomembrane system associated with callose synthesis during the interaction between cowpea (Vignaunguiculata) and the cowpea rust fungus (Uromycesvignae) Can J Bot. 1995;73:1731–1738. [Google Scholar]
- 15.Silva MC, Nicole M, Rijo L, Geiger JP, Rodrigues CJ. Cytochemical aspects of the plant-rust fungus interface during the compatible interaction Coffea arabica (cv. Caturra) Hemileia vastatrix (Race III) Int J Plant Sci. 1999;160:79–91. [Google Scholar]
- 16.Soylu EM, Soylu S, Mansfield JW. Ultra-structural characterisation of pathogen development and host responses during compatible and incompatible interactions between Arabidopsis thaliana and Peronospora parasitica. Physiol Mole Plant Pathol. 2004;65:67–78. [Google Scholar]
- 17.Meyer D, Pajonk S, Micali C, O'Connell R, Schulze-Lefert P. Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J. 2009;57:986–999. doi: 10.1111/j.1365-313X.2008.03743.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Wen Y, Berkey R, Xiao S. Specific targeting of the Arabidopsis resistance protein RPW8.2 to the interfacial membrane encasing the fungal Haustorium renders broad-spectrum resistance to powdery mildew. Plant Cell. 2009;21:2898–2913. doi: 10.1105/tpc.109.067587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao S, Ellwood S, Calis O, Patrick E, Li T, Coleman M, et al. Broad-spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8. Science (New York, NY) 2001;291:118–120. doi: 10.1126/science.291.5501.118. [DOI] [PubMed] [Google Scholar]
- 20.Xiao S, Emerson B, Ratanasut K, Patrick E, O'Neill C, Bancroft I, et al. Origin and maintenance of a broad-spectrum disease resistance locus in Arabidopsis. Mol Biol Evo. 2004;21:1661–1672. doi: 10.1093/molbev/msh165. [DOI] [PubMed] [Google Scholar]
- 21.Micali C, Göllner K, Humphry K, Consonni CRP. The Arabidopsis Book 2008. Published online by: The American Society of Plant Biologists; The powdery mldew disease of Arabidopsis: A paradigm for the interaction between plants and biotrophic fungi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao S, Brown S, Patrick E, Brearley C, Turner JG. Enhanced transcription of the Arabidopsis disease resistance genes RPW8.1 and RPW8.2 via a salicylic acid-dependent amplification circuit is required for hypersensitive cell death. Plant Cell. 2003;15:33–45. doi: 10.1105/tpc.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]