Abstract
Plant association with arbuscular mycorrhizal (AM) fungi is usually regarded as mutualistic. However, this positive effect could disappear if the benefit of the fungal-plant association changes with colonization density. In order to test the conditionality of this interaction, we evaluated plant performance and tolerance to defoliation across five levels of commercial AM fungal inoculum concentrations. Additionally, we evaluated if plant performance and tolerance were similarly affected by a whole soil community collected under a native congener. Along the gradient of inoculation, plant performance exhibited a peak at intermediate inoculum concentration, indicating the presence of an optimum level of AM fungal concentration that maximized AM fungal benefit. Root colonization by fungal hyphae increased linearly across the experimental inoculation gradient. Paralleling root colonization, plant tolerance to defoliation decreased linearly along the inoculum gradient. Plant performance was similar under the whole soil and commercial treatments. Our results show a negative correlation between tolerance to defoliation and AM fungal inoculum concentration, indicating that AM fungi colonization could constrain the evolution of plant tolerance to herbivory.
Key words: compensation, defences, ecological interactions, herbivory, multitrophic interactions, mycorrhizal fungi, tolerance
Arbuscular mycorrhizal (AM) fungi occur in all ecosystems of the world and associate with the roots of about 70% of all vascular plants.1 This association is typically regarded as mutualistic, because there is a bidirectional transfer of nutrients between the host plant and its fungal partners. Carbon compounds are passed from the plant to the fungus and, in return, there is a transfer of mineral nutrients, principally nitrate and phosphate.2 However, this association also entails costs. The amount of carbon allocated to AM fungi is estimated to range from 4% to 20% of a plant's total carbon budget.2 Throughout the literature, there are examples of the conditionality of this relationship exemplified by a continuum of the effects of AM fungal colonization on hosts from positive, through null to negative.3–5 Moreover, it has been suggested that the benefit of a plant associating with fungal symbionts depends not only on the identity of AM fungi4 and plant genotypes6 but also on hyphal colonization density in roots.7 In a recent greenhouse study, we examined components of the conditionality of plant interactions with soil biota.8 We were interested in knowing how the performance and tolerance to defoliation of the annual plant Datura stramonium varied along a concentration gradient of commercial AM fungal inoculum containing four Glomus species (Mycorrhizal Applications, Grants Pass, OR USA).
We found a curvilinear relationship between AM fungal inoculum concentration and plant performance, as predicted by previous models.7 The quadratic decelerating function between inoculum concentration and plant performance indicates an optimum level of AM fungal concentration (1/24th total pot volume) that maximizes AM fungal benefit (Fig. 1A). This result suggests that, in D. stramonium, positive associations between AM fungi and plant fitness may not be proportional and, that at high colonization densities, mycorrhizae may have detrimental effects, perhaps by competing with plants for nutrients, or by interfering with other essential interactions.4,5 We also found, from root examination, that hyphal colonization of roots increased linearly with AM fungi inoculum concentration. Moreover, we found that tolerance to herbivory decreased linearly with increasing AM fungal inoculum concentration (r2 = −0.40; F1,27 = 5.89; p = 0.0222; Fig. 1B), suggesting that, in our system, at high densities, mycorrhizae may become parasitic and may compete for resources (e.g., carbon) with the induced host plant response to leaf damage.
Figure 1.
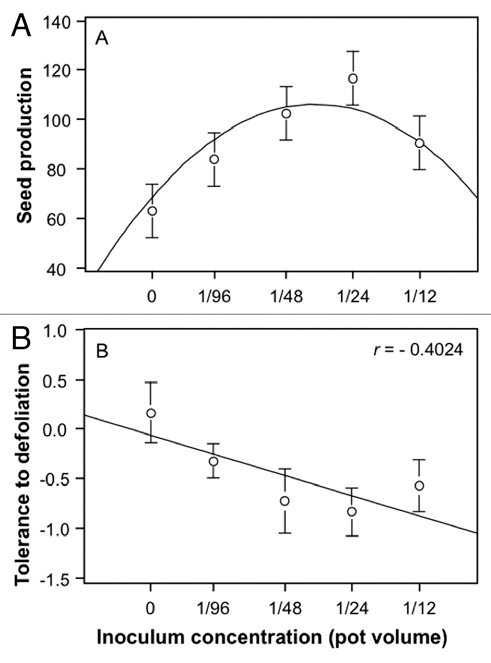
Effect of a gradient in AM fungi inoculum concentration on D. stramonium performance. (A) Non-linear relation between seed production and inoculum concentration. In general, plants achieved their maximal performance at an inoculum concentration of 1/24th total pot volume. (B) Tolerance to defoliation decreased linearly with inoculum concentration. Tolerance was calculated as the difference in standardized seed production between related damaged and undamaged genetically related plants corresponding to six genetic full-sib families.
In order to know whether the effects we found in the greenhouse using commercial inoculum could be expected in the field, we addressed whether or not D. stramonium performance and tolerance were similarly influenced by whole soil field communities; including AM fungi, pathogens, root herbivores, etc. Unfortunately, D. stramonium is not native to the area where this research was undertaken, so we collected soil immediately below plants of a native congener Datura wrightii, a perennial herb that grows at the Putah Creek Reserve (UC, Davis). Pots were inoculated at a 1/12th total pot volume with this live soil and plants were grown concurrently with those in the previous experiment. We compared plant performance and tolerance under the live soil treatment and the last level of the commercial AMF inoculum gradient (both inoculated at a 1/12th total pot volume). Results indicated no differences in foliar area (F1,94 = 1.18; p = 0.2782), root mass (F1,94 = 0.99; p = 0.3222), flowering day (χ2 = 0.31; p = 0.5804) and fitness (χ2 = 0.03; p = 0.8691). Moreover, root colonization levels were (F1,94 = 0.75; p = 0.3877) in both 1/12th volume vs. live soil, as well as in the 0 AMF and sterilized soil (F1,94 = 2.56; p = 0.1130). Despite these similarities, plant tolerance did differ significantly between AMF and live soil treatments (F1,94 = 5.49; p = 0.0411), tolerance being greater under the live soil treatment (0.3755 ± 0.0311 tolerance) relative to the 1/12th AM fungal treatment (−0.5744 ± 0.2714 tolerance). This result suggests that the expression of plant tolerance may also depend on the identity of AMF colonizing roots or the number and identities of soil bacteria. We did not know which microbial species were in the soils we collected.
We show that, when inoculated over a gradient of abundance, Glomus AM fungal colonization consistently decreased tolerance to herbivory. The presence of mycorrhizae could therefore decrease the adaptive value of traits increasing tolerance. We also show here that though live soil inoculum had similar effects in magnitude and direction to those of commercial AMF incoculum on growth and fitness, live soil biota collected under a congener of D. stramonium increased tolerance to herbivory at the same levels of root colonization. Overall, the results of this study indicate that the interaction between soil biotic components and the response of D. stramonium to leaf damage is highly conditional; and can depend on amounts of root colonization, as well as perhaps identities of AM fungi and bacteria. In both cases, soil biota affected the impact of damage to leaves aboveground. AM fungi may mediate the efficacy of tolerance as a defense, and this effect may be especially important in light of herbivore adaptation, when tolerance may be favored over resistance as a plant defense strategy.10
Acknowledgements
This study was financed by PAPIIT IN 200807 to J.F. and the California Agricultural Experiment Station and the College of Biological Sciences at UC Davis to S.Y.S. E.G. is grateful to the Programa de Movilidad Internacional de Estudiantes (DGEP, UNAM), Posgrado en Ciencias Biológicas (UNAM) and CONACyT.
Abbreviations
- AM
arbuscular mycorrhizal
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12292
References
- 1.Hodge A. Microbial ecology of the arbuscular mycorrhiza. FEMS Microbiol Ecol. 2000;32:91–96. doi: 10.1111/j.1574-6941.2000.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith SE, Read DJ. Mycorrhizal symbiosis. 2nd edn. London UK: Academic Press; 1997. [Google Scholar]
- 3.Francis R, Read DJ. Mutualism and antagonism in the mycorrhizal symbiosis, with special reference to impacts on plant community structure. Can J Bot. 1995;73:1301–1309. [Google Scholar]
- 4.Johnson NC, Graham JH, Smith FA. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997;135:575–585. [Google Scholar]
- 5.Jones MD, Smith SE. Exploring functional definitions of mycorrhizas: are mycorrhizas always mutualisms? Can J Bot. 2004;82:1089–1109. [Google Scholar]
- 6.Ramos-Zapata JA, Campos-Navarrete MJ, Parra-Tabla V, Abdala-Roberts L, Navarro-Alberto J. Genetic variation in the response of the weed Ruellia nudiflora (Acanthaceae) to arbuscular mycorrhizal fungi. Mycorrhiza. 2010;20:275–280. doi: 10.1007/s00572-009-0282-x. [DOI] [PubMed] [Google Scholar]
- 7.Gange AC, Ayres RL. On the relation between arbuscular mycorrhizal colonization and plant “benefit”. Oikos. 1999;87:615–621. [Google Scholar]
- 8.Garrido E, Bennett AE, Fornoni J, Strauss SY. Variation in arbuscular mycorrhizal fungi colonization modifies the expression of tolerance to above-ground defoliation. J Ecol. 2010;98:43–49. [Google Scholar]
- 9.Bever JD. Feedback between plants and their soil communities in an old field community. Ecology. 1994;75:1965–1977. [Google Scholar]
- 10.Jokela J, Schmid-Hempel P, Rigby MC. Dr. Pangloss restrained by the Red Queen—steps towards a unified defence theory. Oikos. 2000;89:267–274. [Google Scholar]


