Abstract
Reactive oxygen species represent one of the principal factors that cause cell death and scavenging of reactive oxygen species by superoxide dismutase-related pathway is essential for cell survival. The Parkinson disease-related DJ-1 protein (also known as PARK7) has been implicated in resistance against oxidative stress in dopaminergic neurons however, its molecular mechanism has to date been unknown. We have used Arabidopsis thaliana as a model system to demonstrate that DJ-1, in both plant and mammalian cells, directly influence SOD activity in a highly conserved manner thereby preventing cell death. These data not only provides evidence for the molecular mechanisms associated with DJ-1-induced Parkinson disease but also highlight the unprecedented value of plants as a tool in understanding human disease mechanisms.
Key words: DJ-1, stress, cell death, Parkinson disease, Arabidopsis
Reactive oxygen species (ROS) are involved in a myriad of fundamental biological processes including cell signaling and cellular defense pathways in plants and animals.1–3 Despite its role as a signaling molecule, inappropriate and elevated levels of ROS have a major impact on the etiology of neurodegenerative diseases, such as for example Parkinson disease (PD), and in oxidative stress responses in plants. In general ROS can cause damage to DNA, lipids, proteins and various cofactors. During normal physiological conditions, when ROS are continuously generated, antioxidant defense systems are adequately equipped to prevent ROS-induced tissue dysfunction.4,5 However upon elevated ROS generation the cellular antioxidant systems either recruit additional factors to minimize ROS-induced damage or cells suffer the consequences of cell death. Because of this dichotomy, where ROS plays a vital role during growth and development but can also have overwhelming damaging effects, it is clear that strict regulatory mechanisms need to be in place to effectively control ROS levels. In both plant and mammalian cells elevated ROS levels lead to cell death and in various human disease such as PD, Alzheimer disease, amyotrophic lateral sclerosis and Huntington disease proteins involved in stress-related pathways are often mutated.6
In both plants and mammals mitochondria act as an important source of ROS however, plants also produce ROS in chloroplasts as part of photosynthetic activity. Combined with the fact that plants are sessile organisms it suggests that, although similar in nature, plants most probably have more complex antioxidant systems than other organisms.
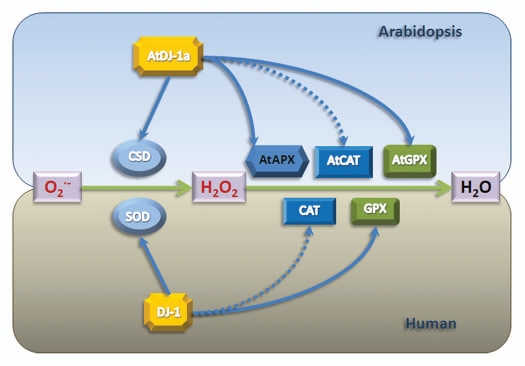
Strategies for removing excess ROS are similar in plants and humans. The principle ROS removal pathway involves superoxide dismutases (SOD) (or copper/zinc superoxide dismutase-CSD in plants), glutathione peroxidases (GPX) and catalases (CAT) localized in the cytosol, mitochondria and chloroplasts (Fig. 1). SOD converts superoxide anion to H2O2, which is then detoxified to H2O by GPX and CAT. In Arabidopsis, besides SOD, GPX and CAT, there are five ascorbate peroxidases (APX) located in the cytosol and chloroplasts, involved in scavenging ROS generated during photosynthesis.7,8 Therefore, SOD, GPX, CAT and APX, together with other auxiliary proteins, form the main line of defense against ROS.
Figure 1.
Involvement of DJ-1-like proteins in ROS scavenging pathways. Produced O2- is converted to H2O2 by SOD in human or CSD (Arabidopsis SOD) in Arabidopsis. H2O2 is then converted to H2O by GPX or CAT (catalase) in human or APX, GPX or CAT in Arabidopsis. DJ-1-like proteins interact with SOD or GPX in humans and CSD, GPX and APX in Arabidopsis. It is assumed here that DJ-1-like proteins may also interact with catalases (broken arrows).
DJ-1 was originally identified as an oncogene and represents a ubiquitous redox-responsive cytoprotective protein with diverse functions where one of its main roles have been attributed to oxidative stress protection.9 Numerous studies have shown that several DJ-1 mutations in humans cause autosomal recessive, early onset PD however, its mode of action has been elusive in terms of having a direct influence on neuronal cell death.10 In an attempt to clarify the mechanism of DJ-1 we established Arabidopsis thaliana as a new and novel model system.11 The Arabidopsis genome contains three DJ-1 homologs compared to the single DJ-1 locus found in humans and we showed that two of these (AtDJ-1b and AtDJ-1c) localizes to chloroplasts whilst one, AtDJ-1a, localizes to the cytosol and nucleus as observed for human DJ-1.11 As mutated DJ-1 in mammals leads to cell death we identified and characterized a DJ-1 loss-of-function mutant which showed increased cell death in aging plants. Using Bimolecular Fluorescence Complementation (BiFC) and isothermal titration calorimetry (ITC) assays we showed that AtDJ-1a interacts with CSD1, the cytosolic SOD in Arabidopsis, and with human SOD1 in plant cells. Further we demonstrated that the human DJ-1 protein interacts with SOD1 in mammalian CHO cells.11 Similar approaches were also employed to show that AtDJ-1a and human DJ-1 had an interaction with GPX2 in plant and mammalian cells.11
Enzyme assays revealed that AtDJ-1a and DJ-1 stimulated SOD/CSD1 activity and that only the copper-loaded forms of AtDJ-1a and DJ-1 had this effect suggesting that AtDJ-1a/DJ-1 may provide copper for SOD/CSD1.11 Although the observed SOD activation provides clues towards the role of DJ-1 in detoxification of ROS, SOD only converts superoxide anion to H2O2 which must further be detoxified to H2O by GPX and CAT. Although we showed that AtDJ-1a and human DJ-1 can interact with AtGPX2 and GPX2, respectively, we observed no changes in GPX2 activity upon DJ-1 interaction. The reason for this may be several-fold. First, cellular GPX2 activity levels may be sufficient to convert SOD-generated H2O2 to H2O. Second, DJ-1 may indeed have no effect on GPX2 activity but simply act as an anchor to dock GPX2 in the vicinity of SOD. To test whether the DJ-1/SOD/GPX2 complex recruits other auxiliary proteins we have also shown that AtDJ-1a interacts with the Arabidopsis cytosolic APX1 protein (Fig. 2, unpublished data). It is also highly possible that DJ-1 interacts with catalase or at least influences its activity (Fig. 1). Although we have no data to date indicating a functional significance of the DJ-1/APX1 interaction we speculate that DJ-1 indeed acts as a scaffold protein bringing together SOD, GPX and possibly APX1 to mediate and control ROS scavenging, ultimately preventing oxidative stress-induced cell death (Fig. 3).
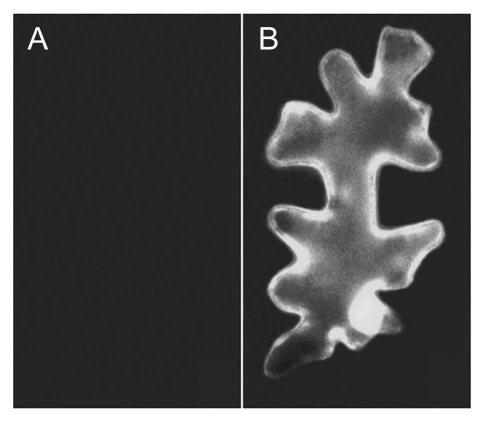
Figure 2.
Interaction of AtDJ-1a with APX1. AtDJ-1a tagged with the N-terminal region of GFP and APX1 tagged with the C-terminal region of GFP gene were co-transformed into tobacco cells. The observed GFP signal in (B) demonstrates an AtDJ-1a/APX1 interaction through reconstitution of functional GFP molecules. (A) Negative control.
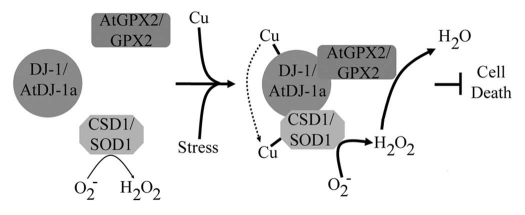
Figure 3.
Working model of AtDJ-1a and DJ-1 mode of action. AtDJ-1a and DJ-1 interacts with SOD and GPX2 leading to SOD activation in a copper-dependent fashion. It is proposed that AtDJ-1a and DJ-1 delivers copper to SOD enhancing its activity whilst GPX2 is anchored by AtDJ-1 and DJ-1 to the protein complex to ensure conversion of the SOD-generated H2O2 to H2O.
The fact that Arabidopsis has three DJ-1 homologs where two of these, AtDJ-1b and AtDJ-1c, are localized to chloroplasts11 underlines the protective role of DJ-1-like proteins during oxidative stress in plants. From our localization studies it appears that AtDJ-1b is localized to the chloroplast stroma whilst AtDJ-1c is localized to both the stroma and the thylakoid membranes (unpublished data). Whether AtDJ-1b and AtDJ-1c act in isolation or in concert and how these two proteins are involved in photosynthesis-induced ROS regulation is unclear but represent exciting future challenges.
The notion that plants can be used as tools to increase our understanding of human disease mechanisms is somewhat obscure to the general scientific community. The fact remains that many discoveries with direct relevance to human health and disease have been elaborated using Arabidopsis, and several processes important to human biology are more easily studied in this versatile model plant.12 The use of Arabidopsis to understand human disease states has several advantages: (1) Arabidopsis represents a well established model organism with a fully annotated genome, (2) The Arabidopsis genome contains homologs of numerous genes involved in human disease, (3) The identification and generation of Arabidopsis mutants is simple and requires little effort, (4) Arabidopsis growth and maintenance requires little infrastructure and running costs and (5) Arabidopsis research has few ethical constraints.
Despite the advantages of Arabidopsis as a model system for elucidating human disease mechanisms it is important to appreciate that Arabidopsis and plant research in general can only reach its full potential in the field of medical research if combined with complementary, and perhaps more conventional, model systems.
Acknowledgements
This works was funded by the Norwegian Research Council.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12298
References
- 1.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 3.Triantaphylides C, Havaux M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 2009;14:219–228. doi: 10.1016/j.tplants.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88:233–240. doi: 10.1139/Y10-016. [DOI] [PubMed] [Google Scholar]
- 5.Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, et al. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot. 2002;53:1305–1319. [PubMed] [Google Scholar]
- 6.Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J, et al. Oxidative stress and aging: Is methylglyoxal the hidden enemy? Can J Physiol Pharmacol. 2010;88:273–284. doi: 10.1139/Y10-001. [DOI] [PubMed] [Google Scholar]
- 7.Kitajima S. Hydrogen peroxide-mediated inactivation of two chloroplastic peroxidases, ascorbate peroxidase and 2-cys peroxiredoxin. Photochem Photobiol. 2008;84:1404–1409. doi: 10.1111/j.1751-1097.2008.00452.x. [DOI] [PubMed] [Google Scholar]
- 8.Mullineaux P, Ball L, Escobar C, Karpinska B, Creissen G, Karpinski S. Are diverse signalling pathways integrated in the regulation of arabidopsis antioxidant defence gene expression in response to excess excitation energy? Philos Trans R Soc Lond B Biol Sci. 2000;355:1531–1540. doi: 10.1098/rstb.2000.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 11.Xu XM, Lin H, Maple J, Bjørkblom B, Alves G, Larsen JP, et al. The Arabidopsis DJ-1a protein confers stress protection through cytosolic SOD activation. J Cell Sci. 2010;123:1644–1651. doi: 10.1242/jcs.063222. [DOI] [PubMed] [Google Scholar]
- 12.Jones AM, Chory J, Dangl JL, Estelle M, Jacobsen SE, Meyerowitz EM, et al. The impact of Arabidopsis on human health: diversifying our portfolio. Cell. 2008;133:939–943. doi: 10.1016/j.cell.2008.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]