Abstract
Cytosolic free Ca2+ mobilization induced by microbe/pathogen-asssociated molecular patterns (MAMPs/PAMPs) plays key roles in plant innate immunity. However, components involved in Ca2+ signaling pathways still remain to be identified and possible involvement of the CBL (calcineurin B-like proteins)-CIPK (CBL-interacting protein kinases) system in biotic defense signaling have yet to be clarified. Recently we identified two CIPKs, OsCIPK14 and OsCIPK15, which are rapidly induced by MAMPs, involved in various MAMP-induced immune responses including defense-related gene expression, phytoalexin biosynthesis and hypersensitive cell death. MAMP-induced production of reactive oxygen species as well as cell browning were also suppressed in OsCIPK14/15-RNAi transgenic cell lines. Possible molecular mechanisms and physiological functions of the CIPKs in plant innate immunity are discussed.
Key words: PAMPs/MAMPs, calcium signaling, CBL-CIPK, hypersensitive cell death, reactive oxygen species
Ca2+ plays an essential role as an intracellular second messenger in plants as well as in animals. Several families of Ca2+ sensor proteins have been identified in higher plants, which decode spatiotemporal patterns of intracellular Ca2+ concentration.1,2 Calcineurin B-Like Proteins (CBLs) comprise a family of Ca2+ sensor proteins similar to both the regulatory β-subunit of calcineurin and neuronal Ca2+ sensors of animals.3,4 Unlike calcineurin B that regulates protein phosphatases, CBLs specifically target a family of protein kinases referred to as CIPKs (CBL-Interacting Protein Kinases).5 The CBL-CIPK system has been shown to be involved in a wide range of signaling pathways, including abiotic stress responses such as drought and salt, plant hormone responses and K+ channel regulation.6,7
Following the recognition of pathogenic signals, plant cells initiate the activation of a widespread signal transduction network that trigger inducible defense responses, including the production of reactive oxygen species (ROS), biosynthesis of phytoalexins, expression of pathogenesis-related (PR) genes and reorganization of cytoskeletons and the vacuole,8 followed by a form of programmed cell death known as hypersensitive response (HR).9,10 Because complexed spatiotemporal patterns of cytosolic free Ca2+ concentration ([Ca2+]cyt) have been suggested to play pivotal roles in defense signaling,1,9 multiple Ca2+ sensor proteins and their effectors should function in defense signaling pathways. Although possible involvement of some calmodulin isoforms11–13 and the calmodulin-domain/calcium-dependent protein kinases (CDPKs)14–19 has been suggested, other Ca2+-regulated signaling components still remain to be identified. No CBLs or CIPKs had so far been implicated as signaling components in innate immunity.
The Role of OsCIPK14/15 in MAMP-Triggered Immunity
We have been establishing a model system to analyze a variety of defense responses including hypersensitive cell death in rice cultured cells by using TvX/EIX (xylanase from Trichoderma viride/ethylene-inducing xylanase) as an elicitor or a MAMP.20 We surveyed the expression patterns of several CIPK genes in response to several MAMPs, including TvX/EIX and N-acetylchitooligosaccharides, and identified two MAMP-inducible CIPKs, OsCIPK14 and OsCIPK15, which are duplicated genes in the rice genome. OsCIPK14/15 interacted with several OsCBLs through the FISL/NAF-motif in yeast cells and showed the strongest interaction with OsCBL4, whose expression was also induced by the MAMP. The recombinant OsCIPK14/15 proteins showed Mn2+-dependent protein kinase activity, which was enhanced both by deletion of their FISL/NAF-motifs and by combination with OsCBL4.21
Functional characterization of the OsCIPK14/15-RNAi lines, as well as the overexpressing lines, suggested that these CIPKs are involved in the regulation of various TvX/EIX-induced defense responses, including mitochondrial dysfunction, hypersensitive cell death, biosynthesis of phytoalexins and PR gene expression.21 During the induction of TvX/EIX-induced defense responses, cell browning is also triggered,20 which was also suppressed in the OsCIPK14/15-knockdown cell lines (Fig. 1). Cell browning has been shown to be accompanied by accumulation of phenolic compounds and lignification in the cell wall during defense reactions against pathogen infection.22 These results imply that OsCIPK14/15 may play an important role in the TvX/EIX-induced reprogramming of secondary metabolism. These consequences of knockdown/overexpression of the CIPKs were similar at least in part with those of a putative voltage-dependent Ca2+ channel, OsTPC1,20 suggesting that these components may play roles in a common defense signaling pathway.
Figure 1.
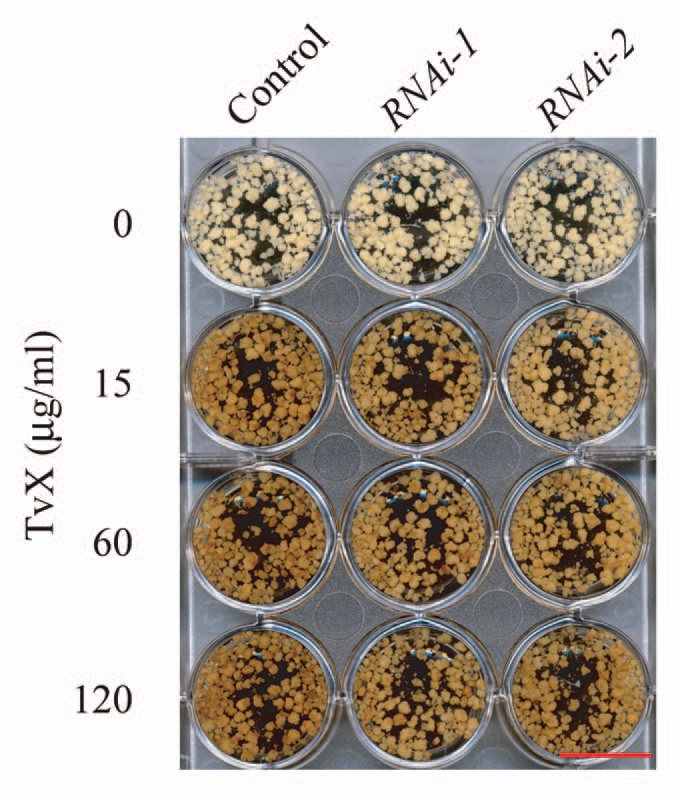
Effects of OsCIPK14/15 suppression on TvX/EI X-induced cell browning. Rice cells of five days after subculture were treated with the TvX/EI X elicitor. Cells 24 h after elicitation are shown as representative of three experiments. Scale bar: 2 cm.
Possible Involvement of OsCIPK14/15 in the Regulation of MAMP-Induced ROS Production
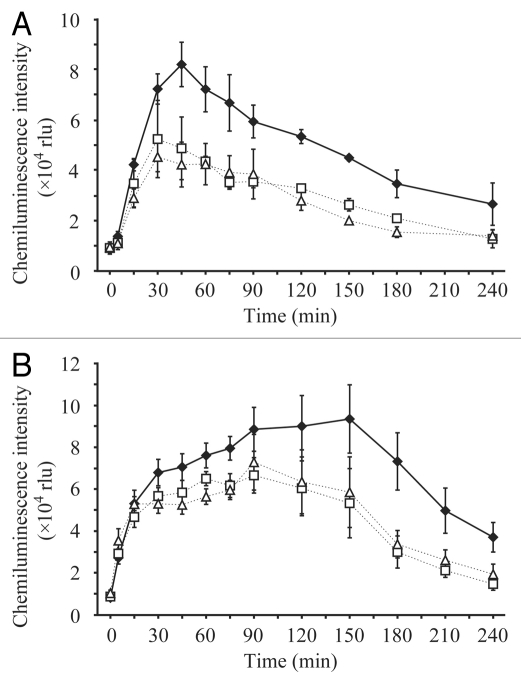
TvX/EIX-induced ROS production was partially impaired (Fig. 2A) in OsCIPK14/15-RNAi-lines. Moreover, ROS production triggered by N-acetylchitooligosaccharides was also substantially reduced in the OsCIPK14/15-RNAi lines (Fig. 2B), suggesting possible involvement of OsCIPK14/15 on MAMPs-induced ROS production in rice cultured cells. External Ca2+ is required not only for hypersensitive cell death but also for NADPH oxidase-mediated ROS generation, which is prerequisite for TvX/EIX-induced hypersensitive cell death in rice.20
Figure 2.
Effects of OsCIPK14/15 suppression on MAMP-induced ROS generation. Time course of ROS (·O2−) generation in the cell suspension after TvX/EI X treatment (120 µg mL−1) (A) and N-acethylchitoheptaose (10 µM) (B). Cells of five days after subculture were washed and resuspended in fresh growth medium 30 min before measurement. A 250 µl aliquot of cells was collected at the indicated time and treated with 2 µM MC LA (Molecular Probes, Eugene, OR). The ·O2−-dependent chemiluminescence was measured with a Lumicounter 2500 (Microtech Nition, Chiba, Japan) with continuous aeration by shaking of the vial. Average values and standard errors of three independent experiments for the control line (black diamond) and two independent RNAi lines (white square for RNAi-1 and white triangle for RNAi-2) are shown.
Rbohs (Respiratory Burst Oxidase Homologues) have been suggested to be involved in oxidative burst and the regulation of hypersensitive cell death in Arabidopsis and rice.23–25 Some rboh proteins have recently been shown to possess ROS-producing NADPH oxidase activity and are synergistically activated by the direct binding of Ca2+ to their EF-hand motifs and protein phosphorylation.26,27 Potato CDPK4 and 5, representative Ca2+ sensor proteins, have been shown to be involved in the regulation of rboh-mediated ROS prodution.17 OsCIPK14/15 may also somehow play roles to activate rbohmediated ROS prodution directly or indirectly to regulate various defense responses including the hypersensitive cell death.
Concluding Remarks
Some OsCBLs, including OsCBL4, or other unidentified factors that interact with OsCIPK15 via the FISL/NAF-motif may regulate the activity and localization of OsCIPK14/15 in the MAMP-triggered signal transduction pathway. Searches for the in vivo substrates of these CIPKs are currently underway to further elucidate the Ca2+ signaling pathways regulating hypersensitive cell death and innate immunity. These findings should shed further light on our understanding of defense signaling pathways.
Acknowledgements
We would like to thank Drs. Daisuke Miki and Ko Shimamoto for the RNAi plasmid (pANDA vector) and Dr. Naoto Shibuya for the gift of N-acethylchitoheptaose.
This work was supported in part by Grants-in-Aid for Scientific Research on Innovative Areas No. 21117516 to K.K. and No. 21200067 to T.K., for Exploratory Research No. 21658118 to K.K. and for Young Scientists (B) No. 21780041 to T.K.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/12407
References
- 1.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang T, Poovaiah BW. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003;8:505–512. doi: 10.1016/j.tplants.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Zhu JK. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- 4.Kudla J, Xu Q, Harter K, Gruissem W, Luan S. Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc Natl Acad Sci USA. 1999;96:4718–4723. doi: 10.1073/pnas.96.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell. 2002;14:S389–S400. doi: 10.1105/tpc.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luan S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14:37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Batistic O, Kudla J. Plant calcineurin-B like proteins and their interacting protein kinases. Biochim Biophys Acta. 2009;1793:985–992. doi: 10.1016/j.bbamcr.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Higaki T, Goh T, Hayashi T, Kutsuna N, Kadota Y, Hasezawa S, et al. Elicitor-induced cytoskeletal rearrangement relates to vacuolar dynamics and execution of cell death: in vivo imaging of hypersensitive cell death in tobacco BY-2 cells. Plant Cell Physiol. 2007;48:1414–1425. doi: 10.1093/pcp/pcm109. [DOI] [PubMed] [Google Scholar]
- 9.Nürnberger T, Scheel D. Signal transmission in the plant immune response. Trends Plant Sci. 2001;6:372–379. doi: 10.1016/s1360-1385(01)02019-2. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg JT, Yao N. The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol. 2004;6:201–211. doi: 10.1111/j.1462-5822.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- 11.Heo WD, Lee SH, Kim MC, Kim JC, Chung WS, Chun HJ, et al. Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses. Proc Natl Acad Sci USA. 1999;96:766–771. doi: 10.1073/pnas.96.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamakawa H, Mitsuhara I, Ito N, Seo S, Kamada H, Ohashi Y. Transcriptionally and post-transcriptionally regulated response of 13 calmodulin genes to tobacco mosaic virus-induced cell death and wounding in tobacco plant. Eur J Biochem. 2001;268:3916–3929. doi: 10.1046/j.1432-1327.2001.02301.x. [DOI] [PubMed] [Google Scholar]
- 13.Takabatake R, Karita E, Seo S, Mitsuhara I, Kuchitsu K, Ohashi Y. Pathogen-induced calmodulin isoforms in basal resistance against bacterial and fungal pathogens in tobacco. Plant Cell Physiol. 2007;48:414–423. doi: 10.1093/pcp/pcm011. [DOI] [PubMed] [Google Scholar]
- 14.Romeis T, Piedras P, Jones JD. Resistance genedependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell. 2000;12:803–816. doi: 10.1105/tpc.12.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romeis T, Ludwig AA, Martin R, Jones JD. Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 2001;20:5556–5567. doi: 10.1093/emboj/20.20.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, Wasternack C, et al. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc Natl Acad Sci USA. 2005;102:10736–10741. doi: 10.1073/pnas.0502954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, et al. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshioka H, Asai S, Yoshioka M, Kobayashi M. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Mol Cells. 2009;28:321–329. doi: 10.1007/s10059-009-0156-2. [DOI] [PubMed] [Google Scholar]
- 19.Boudsocq M, Willman MR, McCormack M, Lee H, Shan L, He P, et al. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature. 2010;464:418–422. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurusu T, Yagala T, Miyao A, Hirochika H, Kuchitsu K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 2005;42:798–809. doi: 10.1111/j.1365-313X.2005.02415.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurusu T, Hamada J, Nokajima H, Kitagawa Y, Kiyoduka M, Takahashi A, et al. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiol. 2010;153:678–692. doi: 10.1104/pp.109.151852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller H, Hohlfeld H, Wray V, Hahlbrock K, Scheel D, Strack D. Changes in the accumulation of soluble and cell wall-bound phenolics in elicitor-treated cell suspension cultures and fungus-infected leaves of Solanum tuberosum. Phytochemistry. 1996;42:389–396. [Google Scholar]
- 23.Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshie Y, Goto K, Takai R, Iwano M, Takayama S, Isogai A, et al. Function of the rice gp91phox homologs OsrbohA and OsrbohE genes in ROS-dependent plant immune responses. Plant Biotech. 2005;22:127–135. [Google Scholar]
- 25.Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 27.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]