Abstract
Background:
Diffuse cerebral vasospasm following aneurysmal subarachnoid hemorrhage (SAH) refractory to medical management can be treated with intra-arterial administration of vasodilators, but valid bedside monitoring for the diagnosis and therapeutic assessment is poorly available. We demonstrate the successful application of regional cerebral oxygen saturation (rSO2) monitoring with multichannel near-infrared spectroscopy (NIRS) in assisting intra-arterial infusions of fasudil hydrochloride to a patient suffering from post-SAH vasospasm in the distal vascular territories.
Case Description:
A 63-year-old man presented with SAH and intracerebral hematoma due to ruptured right middle cerebral artery aneurysm developed aphasia and right-sided weakness on day 9 after SAH onset. Delayed cerebral ischemia attributable to diffuse vasospasm in the distal territories of the left anterior and middle cerebral arteries was suspected. Since the symptoms persisted despite maximal hyperdynamic therapy with dobutamine, intra-arterial fasudil treatment in the setting of rSO2 monitoring including the spasm-affected vascular territory with four-channel flexible NIRS sensors was subsequently performed. Decreased and fluctuating rSO2 in angiographically documented vasospastic territories increased immediately after intra-arterial fasudil infusion in accordance with relief of vasospasm that correlated with neurological improvement. The procedure was repeated on day 11 since the effect was transient and neurological deterioration and reduction of rSO2 recurred. The deficits resolved accompanied by uptake and maintenance of rSO 2 following the intra-arterial fasudil, resulting in favorable functional outcome.
Conclusion:
Continuous rSO2 monitoring with multichannel NIRS is a feasible strategy to assist intraarterial fasudil therapy for detecting and treating the focal ischemic area exposed to diffuse vasospasm.
Keywords: Fasudil hydrochloride, intra-arterial infusion, near-infrared spectroscopy, subarachnoid hemorrhage, vasospasm
INTRODUCTION
Cerebral vasospasm is the most serious complication of aneurysmal subarachnoid hemorrhage (SAH), in which 40% of patients experience-delayed cerebral ischemia (DCI) attributable to vasospasm, leading to infarction and thus long-term morbidity and mortality.[1,28] The current strategy for the treatment of clinical deterioration caused by DCI includes hypervolemia, hypertension, and hemodilution (triple H),[23] or hyperdynamic therapy.[8,19,20] However, if symptoms are refractory to maximal medical management and vasospasm distributed diffusely to distal vasculatures, endovascular treatment such as intra-arterial vasodilator infusions provides a useful invasive therapeutic option.[9]
Fasudil hydrochloride is a potent vasodilator that inhibits Rho-kinase, which is involved in the development of cerebral vasospasm. Numerous findings in Japan have demonstrated the clinical effectiveness of intra-arterial fasudil infusions in inducing angiographic improvement of vasospasm and resolution of DCI with fewer adverse effects on intracranial pressure and less induction of convulsions.[5,26] However, the real-time effectiveness of intra-arterial fasudil in reversing reduced regional cerebral blood flow (rCBF) affected by vasospasm, which may be a key to assess successful outcome of endovascular therapy, remains unclear.
We have recently introduced continuous regional cerebral hemoglobin oxygen saturation (rSO 2 ; venous-weighted % saturation of hemoglobin derived from the INVOS NIRS device) monitoring to assess the efficacy of hyperdynamic therapy in improving rCBF in the territory affected by vasospasm after SAH.[17] Here, we demonstrate the successful application of rSO2 monitoring with multichannel near-infrared spectroscopy (NIRS) in assisting intra-arterial infusions of fasudil hydrochloride to a patient suffering from post-SAH vasospasm in the distal vascular territories. We used four-channel flexible NIRS sensors to detect rSO2 in the vascular territories affected by vasospasm more accurately, in conjunction with systemic hemodynamic monitoring derived from a radial artery waveform-based pulse contour cardiac output device throughout the treatment period.
CASE REPORT
A 63-year-old man admitted to the stroke service at our center presented with the sudden onset of headache, vomiting, and right-sided hemiparesis followed by loss of consciousness (World Federation of Neurological Surgery grade IV). A computed tomography (CT) scan revealed diffuse thick SAH combined with a large right temporal intracerebral hematoma [Figure 1a], and a ruptured right middle cerebral artery aneurysm [Figure 1b] was successfully clipped following extensive hematoma evacuation[14] by emergent surgery performed at 6 hours after SAH onset. The early postoperative course was uneventful, and the neurological deficits disappeared with the exception of mild left hemispatial neglect. He received standard post-SAH fluid and drug management at our institution.[19,20] On the ninth day after SAH onset, however, he became restless and then developed aphasia and right-sided hemiparesis. Transcranial Doppler (TCD) measurements were compatible with mild left middle cerebral artery (MCA) vasospasm (left MCA peak velocity, 149 cm/s; mean velocity 120 cm/s). Diffusion-weighted magnetic resonance (MR) imaging performed immediately after the onset of symptoms revealed no apparent ischemic findings [Figure 1c], but subsequent technetium 99 m hexamethylpropyleneamine oxime single photon emission CT (Tc-99 m HMPAO SPECT) revealed a mild reduction in rCBF in the left anterior cerebral artery (ACA) and MCA territories Figure 1d] indicative of clinical deterioration attributable to vasospasm.[28] Hyperdynamic therapy with dobutamine (initial dose: 3 μg/kg/min; increased in 3 μg/kg/min increments every 4 hours to a level at which the deterioration resolved)[6,8,17,18] combined with mild hypervolemia with supplemental low-molecular-weight dextran (500 mL/day)[13] was initiated.
Figure 1.
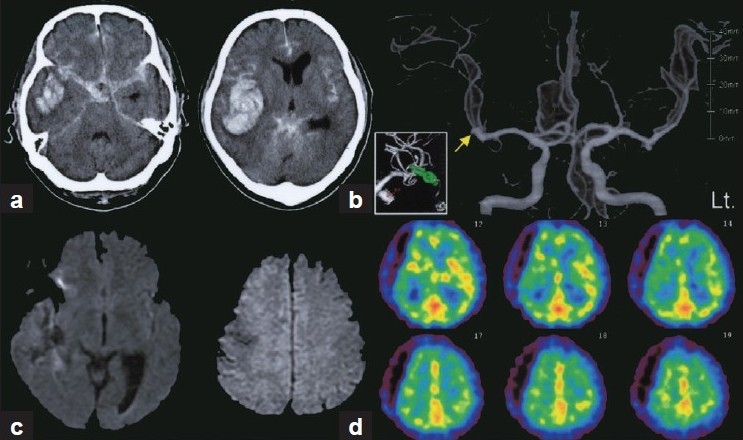
(a) CT scans showing SAH combined with a massive right temporal intracerebral hematoma. (b) Preoperative 3-dimensional CT angiography showing bilateral MCA aneurysms. Ruptured aneurysm on the right side (arrow) was successfully clipped (inset). Clinical deterioration attributable to vasospasm was suspected based on findings of no apparent ischemic lesion on diffusion-weighted MR images (c) and relatively decreased rCBF in the left ACA and MCA territories on Tc-99 m HMPAO SPECT (d)
Cerebral digital subtraction angiography (DSA) was performed under local anesthesia via a transfemoral approach since there was no clinical improvement after 2 hours of maximal hemodynamic augmentation with dobutamine (12 μg/kg/min) to raise cardiac output to supranormal plateau level (cardiac index >5.0 L/min/m 2 ), assisted by radial artery waveform-based pulse contour cardiac output monitoring (FloTrac system version 3.02, Edwards Lifesciences, Irvine, CA, USA).[15,16] Prior to DSA, transcranial rSO2 was measured by a NIRS cerebral oximetry device (INVOS 5100C, Somanetics, Troy, MI, USA), which was composed of a light-emitting diode and two detectors located 30 and 40 mm from the diode, allowing compensation for NIR absorption from the scalp and the skull to determine rSO2 in the underlying area of the brain. Each of the four-channel sensors (SAFB-SM, Somanetics, Troy, MI, USA) was placed symmetrically on the scalp of the patients at the approximate location overlying the spasm-affected vascular territory and on the mirror--image location in the opposite hemisphere.[17]
DSA revealed severe vasospasm of the distal portion of the A1 segment of the left ACA and of the proximal left A2 and M2 segments and diffuse spasm in their branches [Figure 2a]. After systemic heparinization, a 5-Fr. guiding catheter was advanced to the cervical portion of the internal carotid artery and a microcatheter was placed at the top of the internal carotid artery[Figure 2b], Arrow] and fasudil hydrochloride (25 mg) was infused at a rate of 1.0 mg/mL (consisting of 60 mg of fasudil hydrochloride mixed in 60 mL of normal saline) for distribution into both the ACA and MCA territories, resulting in significant reversal of vasospasm in distal A1 and M1 segments [Figure 2c] and improvement of cerebral circulation time (as measured by the interval between the first image in which contrast is visible above the supraclinoid internal carotid artery and the peak filling of the cortical parietal veins)[11] from 6.6 to 5.3 seconds. Since focal severe vasospasm still existed in the distal potion of M3 (superior division), superselective infusion of fasudil (25 mg) into the MCA distribution was performed by directing the microcatheter into the distal portion of M1 [Figure 2c, arrow]. Although further dilation could not be obtained in the M3 segment, good angiographic results were obtained in the remaining spastic vessels [Figure 2d]. Within an hour of completion of the procedure, his symptoms improved.
Figure 2.
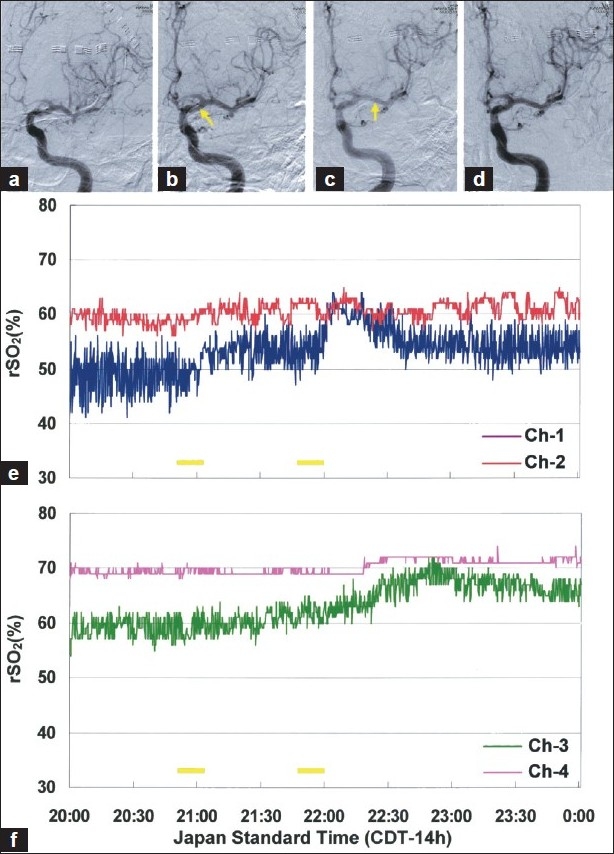
(a) Left internal carotid artery angiogram demonstrates diffuse angiographic vasospasm. (b) Intra-arterial fasudil infusion from the top of internal carotid artery (arrow) and subsequent selective infusion from distal portion of M1 (c, arrow), resulting in significant reversal of vasospasm (d). Low and fluctuating rSO2 detected in the left ACA territory (E, Ch-1) increased immediately after each fasudil infusion, while rSO2 in the MCA territory (F, Ch-3) gradually elevated following infusion from the distal M1 segment. Each yellow bar represents intra-arterial fasudil infusion
The intra-arterial fasudil likewise produced improvement of low and fluctuating rSO2 in the left ACA--MCA territory immediately after onset of each infusion[Figure 2e, Ch-1], and the left MCA territory flow then gradually increased following superselective infusion in the distal M1 segment[Figure 2f, Ch-3]. TCD velocities normalized after the procedure (left MCA peak velocity, 132 cm/s; mean velocity 98 cm/s), compatible with angiographic improvement of the M1 vasospasm.
The postprocedural course was uneventful, and the symptoms were managed with hyperdynamic therapy under stable but slightly reduced (< 10%) rSO2 on the left side. However, he developed aphasia and weakness of the right hand with decreased voluntary movements over the course of the next 24-36 hours. TCD velocities consistently remained within normal range, but decreased and unstable rSO2 in the left ACA--MCA territory were detected. Diffusion-weighted MR imaging revealed small hyperintense signals in the left insular cortex and medial frontal cortex [Figure 3a]. Tc-99 m HMPAO SPECT detected decreased rCBF in the left ACA and MCA territories, in contrast to hyperperfusion observed in the superior trunk of the left MCA territory [Figure 3b. Recurrent vasospasm in the distal vascular territories, particularly in the left ACA and MCA territories, was strongly suspected and DSA was performed immediately as an additional.
Figure 3.
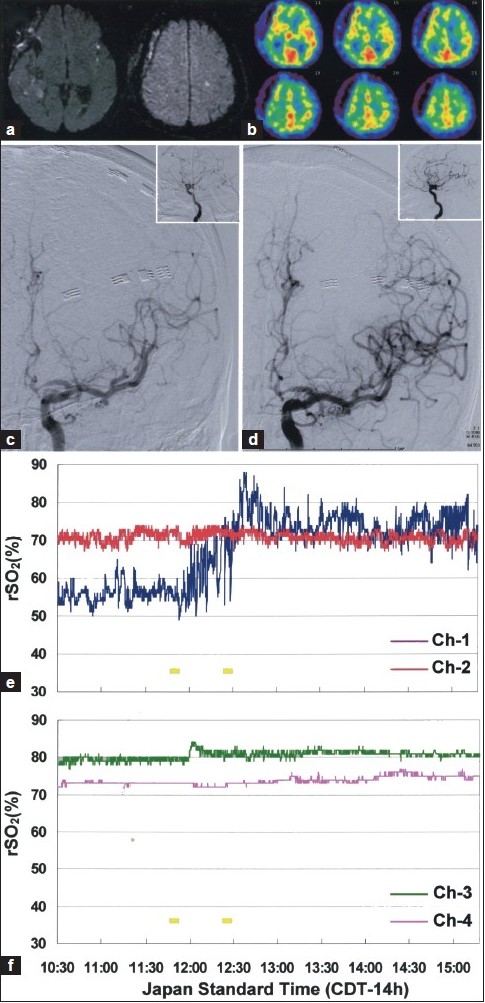
Diffusion-weighted MR images (a) and SPECT (b) showing new small infarctions and decreased rCBF in the left ACA--MCA territory. (c) DSA showing moderate vasospasm of distal A1 and diffuse vasospasm in the distal ACA and MCA branches. (d) Intra-arterial fasudil infusions resulted in an improvement of the distal vasospasm and cerebral circulation time (inset). Low and fluctuating rSO2 detected in the left ACA--MCA territories increased immediately after each infusion and recovered close to the contralateral recordings (e, Ch-1). Each yellow bar represents intra-arterial fasudil infusion
Repeated DSA demonstrated moderate vasospasm of the distal A1 and diffuse vasospasm in the distal ACA and MCA branches as well as persistent focal spasm in the distal potion of the M3 segment [Figure 3c]. Then intra-arterial fasudil infusions (25 mg/each) were performed again, resulting in improvement of the distal vasospasm and cerebral circulation time[Figure 3c d, inset] from 5.8 to 4.9 seconds. In response to intra-arterial fasudil infusions, rSO2 in the left ACA--MCA territory rapidly increased to a level close to that on the contralateral side [Figure 3e]. The patient's symptoms gradually resolved over the next 72 hours accompanied by maintenance of stable rSO2 values. Cardiac output (> 5.0 L/min/m 2 ), systolic blood pressure (< 180 mmHg), and heart rate (< 130 bpm) were also maintained within each target for hyperdynamic therapy by titration of dobutamine dose and intravenous calcium antagonists.[19,20] No apparent periprocedural clinical/vital signs or radiological findings of increased intracranial pressure or hemorrhage associated with the intra-arterial fasudil therapy were observed. Follow-up MR angiography on day 14 (corresponding to 3 days after the second endovascular therapy) confirmed improvement of vasospasm [Figure 4a]. Diffusion-weighted MR imaging revealed no additional ischemic findings after repeated endovascular therapy [Figure 4b]. Abnormal rCBF distributions in the left hemisphere were also normalized [Figure 4c]. The patient underwent ventriculoperitoneal shunt placement on day 30 for the treatment of post-SAH normal pressure hydrocephalus [Figure 4d], resulting in favorable functional outcome with a modified Rankin Scale score of 1 at 2 months.
Figure 4.
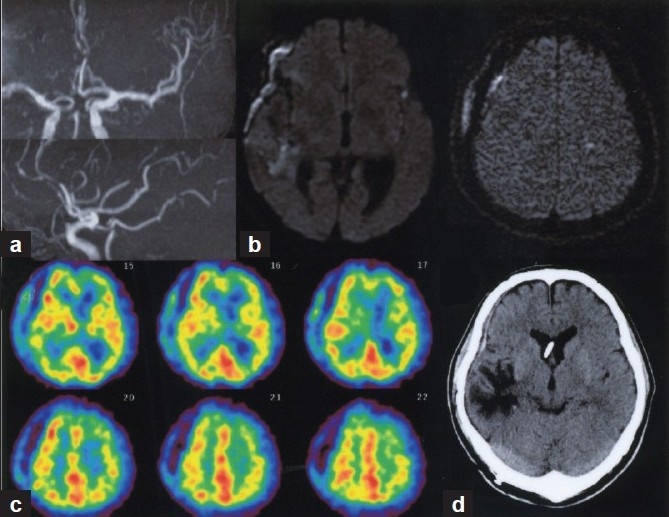
(a) Follow-up MR angiography on day 14 showing improvement of vasospasm. (b) Diffusion-weighted MR images demonstrated no additional ischemic findings after repeated endovascular therapy. (c) Tc-99 m HMPAO SPECT confirmed normalized rCBF distribution in the left hemisphere. (d) CT scan at 2 months after SAH showing no apparent ischemia in the left hemisphere
DISCUSSION
Monitoring of cerebral autoregulation from a focal brain region using direct brain tissue oxygenation measurement has been shown to be of prognostic value in patients with SAH.[7] As observed in this case, NIRS but not TCD successfully tracked real-time hemodynamic changes in a specific brain region coinciding with neurological deterioration and findings from imaging studies (MRI and SPECT) following intra-arterial fasudil therapy. Currently, TCD is a widely available and highly affordable noninvasive technique for screening of vasospasm; however, it may not necessarily assist in the identification of focal ischemia or monitoring treatment effects because of its poor sensitivity in detecting distal M1 and M2 vessel vasospasm.[24,25] One important factor responsible for the discrepancy between NIRS and TCD measurements may be the area of interest for assessment of CBF changes. With TCD, only the CBF of the brain supplied by the blood flow of MCA is usually monitored, while the NIRS rSO2 reflects changes in cerebral blood oxygenation where the flexible sensor can be placed. Since continuous rCBF monitoring using thermal diffusion flowmetry implanted into the brain tissue is more effective for intra-arterial papaverine therapy than TCD in reversing vasospasm,[27] noninvasive NIRS may also be an alternative CBF-based neuromonitoring strategy.
Our findings indicate the importance of appropriate NIRS sensor location determined by exploring the vascular territory affected by vasospasm for accurate determination of rSO2 changes over time. In a recent study of rSO2 readings using the INVOS device, however, no clear trends in rSO2 change associated with intra-arterial vasodilator therapy for angiographic vasospasm were detected.[21] On the other hand, there are also reports suggesting that rSO2 may enhance reliability in detecting focal cerebral ischemia[4,22] or be useful for assessing the therapeutic effectiveness of intra-arterial papaverine therapy for severe symptomatic vasospasm after SAH.[12] We believe that such discrepancies may simply be due to recording only from the forehead space over the ACA/MCA watershed area with two-channel sensors, which may thus have lost the specific areas of interest where vasospasm impaired microcirculatory autoregulation.
Intra-arterial fasudil infusion is an effective modality for treating vasospasm after SAH. It is important to note that the vasodilative effect of fasudil hydrochloride is transient and temporary and that approximately 40% of patients required multiple treatments for recurrent vasospasm, despite angiographic improvement in 86-100% and clinical improvement in 44-82% of cases.[26] A decrease in focal rSO2 readings in the treated territories preceded by clinical deterioration may, as shown in this case, be a warning sign necessitating further investigation. Systemic blood pressure depression (> 20 mmHg)[26] and convulsions (12.9%)[5] have been reported as adverse effects of intra-arterial fasudil therapy. We tried to avoid such serious risks by application of continuous hemodynamic monitoring with a less-invasive device and use of the recommended constant rate of fasudil delivery (< 3 mg/min) with an infusion pump.
Application of NIRS to detecting and managing vasospasm has several pitfalls and limitations. The quality of NIRS measurements can potentially be restricted by polycythemia, and the increased area of the cerebrospinal fluid layer.[10,30] The four-channel flexible and thin NIRS sensors in the current INVOS device enables close skin contact for longer periods of time and more stable monitoring than with any other available NIRS probes; however, it should be kept in mind that the method of analysis using continuous-wave light NIRS reflects O2 delivery by providing an accurate, venous-weighted measure of hemoglobin saturation in the tissues beneath the sensors[2] but does not provide quantitative measurement of hemoglobin concentrations as demonstrated by time-resolved or frequency-domain NIRS.[3,29] It is also important to note that the near-infrared light penetrates 2.0-2.5 cm into the head and hence interrogates only the cortical regions and not deeper brain structures. Further studies to determine criteria for selecting patients are warranted when this bedside monitoring is more effective in monitoring treatment of vasospasm with intra-arterial fasudil infusions.
In conclusion, this case illustrates the value of multichannel NIRS to assist intra-arterial fasudil therapy in detecting and treating diffuse/distal vasospasm in selected situations such as when serial TCD monitoring is ineffective. Further studies in a larger study population are needed to reflect better clinical outcome with this monitoring strategy.
Acknowledgments
The authors thank Drs. Kentaro Hikichi and Shotaro Yoshioka for their excellent endovascular procedures. This work was supported by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (C22592026) and Project Research Grant from Akita Prefecture (H221001, H231105).
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/68/81728
Contributor Information
Tatsushi Mutoh, Email: tmutoh@tiara.ocn.ne.jp.
Shinya Kobayashi, Email: kobayashi@akita-noken.jp.
Noriyuki Tamakawa, Email: tamakawa@akita-noken.jp.
Tatsuya Ishikawa, Email: teddyish@akita-noken.jp.
REFERENCES
- 1.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 2.Booth EA, Dukatz C, Ausman J, Wider M. Cerebral and somatic venous oximetry in adults and infants. Surg Neurol Int. 2010;1:75. doi: 10.4103/2152-7806.73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderon-Arnulphi M, Alaraj A, Slavin KV. Near infrared technology in neuroscience: Past, present and future. Neurol Res. 2009;31:605–14. doi: 10.1179/174313209X383286. [DOI] [PubMed] [Google Scholar]
- 4.Ekelund A, Kongstad P, Saveland H, Romner B, Reinstrup P, Kristiansson KA, et al. Transcranial cerebral oximetry related to transcranial Doppler after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1998;140:1029–35. doi: 10.1007/s007010050211. discussion 1035-6. [DOI] [PubMed] [Google Scholar]
- 5.Enomoto Y, Yoshimura S, Yamada K, Iwama T. Convulsion during intra-arterial infusion of fasudil hydrochloride for the treatment of cerebral vasospasm following subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2010;50:7–11. doi: 10.2176/nmc.50.7. discussion 11-2. [DOI] [PubMed] [Google Scholar]
- 6.Hadeishi H, Mizuno M, Suzuki A, Yasui N. Hyperdynamic therapy for cerebral vasospasm. Neurol Med Chir (Tokyo) 1990;30:317–23. doi: 10.2176/nmc.30.317. [DOI] [PubMed] [Google Scholar]
- 7.Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38:981–6. doi: 10.1161/01.STR.0000257964.65743.99. [DOI] [PubMed] [Google Scholar]
- 8.Joseph M, Ziadi S, Nates J, Dannenbaum M, Malkoff M. Increases in cardiac output can reverse flow deficits from vasospasm independent of blood pressure: A study using xenon computed tomographic measurement of cerebral blood flow. Neurosurgery. 2003;53:1044–51. doi: 10.1227/01.neu.0000088567.59324.78. discussion 1051-2. [DOI] [PubMed] [Google Scholar]
- 9.Jun P, Ko NU, English JD, Dowd CF, Halbach VV, Higashida RT, et al. Endovascular treatment of medically refractory cerebral vasospasm following aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2010;31:1911–6. doi: 10.3174/ajnr.A2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishi K, Kawaguchi M, Yoshitani K, Nagahata T, Furuya H. Influence of patient variables and sensor location on regional cerebral oxygen saturation measured by INVOS 4100 near-infrared spectrophotometers. J Neurosurg Anesthesiol. 2003;15:302–6. doi: 10.1097/00008506-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Liu JK, Couldwell WT. Intra-arterial papaverine infusions for the treatment of cerebral vasospasm induced by aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2005;2:124–32. doi: 10.1385/NCC:2:2:124. [DOI] [PubMed] [Google Scholar]
- 12.Luer MS, Dujovny M, Slavin KV, Hernandez-Avila G, Ausman JI. Regional cerebral oxygen saturation during intra-arterial papaverine therapy for vasospasm: Case report. Neurosurgery. 1995;36:1033–6. doi: 10.1227/00006123-199505000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Arai H, Nakajima K, Tajima A, Maeda M. Hemorheological and hemodynamic analysis of hypervolemic hemodilution therapy for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 1995;26:1620–6. doi: 10.1161/01.str.26.9.1620. [DOI] [PubMed] [Google Scholar]
- 14.Mutoh T, Ishikawa T, Moroi J, Suzuki A, Yasui N. Impact of early surgical evacuation of sylvian hematoma on clinical course and outcome after subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2010;50:200–8. doi: 10.2176/nmc.50.200. [DOI] [PubMed] [Google Scholar]
- 15.Mutoh T, Ishikawa T, Nakase T, Yasui N. Performance of the refined FloTrac system (3rd generation device) for uncalibrated continuous cardiac output monitoring during hyperdynamic therapy of cerebral vasospasm after subarachnoid hemorrhage. Crit Care Med. 2009;37(Suppl 12):A1288. [Google Scholar]
- 16.Mutoh T, Ishikawa T, Nishino K, Yasui N. Evaluation of the FloTrac uncalibrated continuous cardiac output system for perioperative hemodynamic monitoring after subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2009;21:218–25. doi: 10.1097/ANA.0b013e3181a4cd8b. [DOI] [PubMed] [Google Scholar]
- 17.Mutoh T, Ishikawa T, Suzuki A, Yasui N. Continuous cardiac output and near-infrared spectroscopy monitoring to assist in management of symptomatic cerebral vasospasm after subarachnoid hemorrhage. Neurocrit Care. 2010;13:331–8. doi: 10.1007/s12028-010-9383-9. [DOI] [PubMed] [Google Scholar]
- 18.Mutoh T, Ishikawa T, Yasui N. Application of the FloTracTM arterial pressure-based continuous cardiac output monitor to dobutamine-induced hyperdynamic therapy for cerebral vasospasm after subarachnoid hemorrhage. No Shinkei Geka. 2009;37:1085–93. [PubMed] [Google Scholar]
- 19.Mutoh T, Kazumata K, Ajiki M, Ushikoshi S, Terasaka S. Goal-directed fluid management by bedside transpulmonary hemodynamic monitoring after subarachnoid hemorrhage. Stroke. 2007;38:3218–24. doi: 10.1161/STROKEAHA.107.484634. [DOI] [PubMed] [Google Scholar]
- 20.Mutoh T, Kazumata K, Ishikawa T, Terasaka S. Performance of bedside transpulmonary thermodilution monitoring for goal-directed hemodynamic management after subarachnoid hemorrhage. Stroke. 2009;40:2368–74. doi: 10.1161/STROKEAHA.109.547463. [DOI] [PubMed] [Google Scholar]
- 21.Naidech AM, Bendok BR, Ault ML, Bleck TP. Monitoring with the Somanetics INVOS 5100C after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2008;9:326–31. doi: 10.1007/s12028-008-9077-8. [DOI] [PubMed] [Google Scholar]
- 22.Rothoerl RD, Faltermeier R, Burger R, Woertgen C, Brawanski A. Dynamic correlation between tissue PO2 and near infrared spectroscopy. Acta Neurochir Suppl. 2002;81:311–3. doi: 10.1007/978-3-7091-6738-0_79. [DOI] [PubMed] [Google Scholar]
- 23.Sen J, Belli A, Albon H, Morgan L, Petzold A, Kitchen N. Triple-H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2003;2:614–21. doi: 10.1016/s1474-4422(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 24.Suarez JI, Qureshi AI, Yahia AB, Parekh PD, Tamargo RJ, Williams MA, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: Evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30:1348–55. doi: 10.1097/00003246-200206000-00035. [DOI] [PubMed] [Google Scholar]
- 25.Suwanwela NC, Phanthumchinda K, Suwanwela N. Transcranial doppler sonography and CT angiography in patients with atherothrombotic middle cerebral artery stroke. AJNR Am J Neuroradiol. 2002;23:1352–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka K, Minami H, Kota M, Kuwamura K, Kohmura E. Treatment of cerebral vasospasm with intra-arterial fasudil hydrochloride. Neurosurgery. 2005;56:214–23. doi: 10.1227/01.neu.0000147975.24556.bc. [DOI] [PubMed] [Google Scholar]
- 27.Vajkoczy P, Horn P, Bauhuf C, Munch E, Hubner U, Ing D, et al. Effect of intra-arterial papaverine on regional cerebral blood flow in hemodynamically relevant cerebral vasospasm. Stroke. 2001;32:498–505. doi: 10.1161/01.str.32.2.498. [DOI] [PubMed] [Google Scholar]
- 28.Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- 29.Yokose N, Sakatani K, Murata Y, Awano T, Igarashi T, Nakamura S, et al. Bedside monitoring of cerebral blood oxygenation and hemodynamics after aneurysmal subarachnoid hemorrhage by quantitative time-resolved near-infrared spectroscopy. World Neurosurg. 2010;73:508–13. doi: 10.1016/j.wneu.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 30.Yoshitani K, Kawaguchi M, Miura N, Okuno T, Kanoda T, Ohnishi Y, et al. Effects of hemoglobin concentration, skull thickness, and the area of the cerebrospinal fluid layer on near-infrared spectroscopy measurements. Anesthesiology. 2007;106:458–62. doi: 10.1097/00000542-200703000-00009. [DOI] [PubMed] [Google Scholar]


