Abstract
Background:
Covered stents are used endovascularly to seal arterial wall defects while preserving vessel patency. This report describes our experience with the use of covered stents to treat cervical pathology, and a review of the literature in regards to this topic is presented.
Case Description:
Two patients presenting with the carotid blowout syndrome and one patient with a vertebrojugular fistula were treated with covered stents. This allowed for preservation of the vessel and was a treatment alternative to cerebral bypass.
Conclusion:
Covered stents provide a viable means of preserving the cervical vessels in selected patients; however, long-term follow-up is necessary to determine stent patency and permanency of hemostasis.
Keywords: Covered stent, carotid blow-out, endovascular, vertebrojugular fistula
INTRODUCTION
Covered stents consist of a synthetic material that either covers or is attached to a metallic stent to create a graft endoprosthesis. The covering excludes breaches to the integrity of the arterial wall, while preserving vessel patency. Covered stents have been used to treat aneurysms,[43,53,58] traumatic arterial injuries,[79,102,108] and arteriovenous fistulas (AVF)[43,79,102,108] of the axillary, subclavian, iliac, femoral, and popliteal arteries.
The emergence of neuroendovascular techniques offers an alternative treatment for patients in whom surgery is contraindicated. Advantages of an endovascular approach include: an easier access, less invasiveness, performance under local anesthesia, less post-procedural pain and disability, and less expense.[79] The Food and Drug Administration (FDA) has not approved the use of covered stents for neuroendovascular interventions; however, these devices offer a potentially less morbid alternative in the treatment of complex, extracranial disease where surgical options are limited. We describe our experience with the placement of covered stents in the treatment of the carotid blowout syndrome (CBS) and a vertebrojugular fistula (VJF). We also review the present literature regarding the use of covered stents in extracranial cerebral circulation.
CASE REPORTS
The Institutional Review Board approval was obtained for the retrospective review of all extracranial neuroendovascular interventions between January 2006 and June 2009. Three patients treated with a covered stent endoprosthesis were identified and their charts were reviewed. One patient was diagnosed with VJF. Two patients were diagnosed with CBS, one of whom was with bilateral CBS. The off-label use of the covered stent was disclosed in all cases, and informed consent was obtained for all procedures.
Case 1
A 51-year-old woman presented with a ten-week history of persistent left-sided neck pain and pulsatile tinnitus. Cerebral angiography demonstrated a large, high-flow AVF originating from the left vertebral artery (VA) and draining into the internal jugular veins bilaterally [Figure 1a]. Retrograde flow from the right VA also supplied the fistula [Figure 1b]. Diffuse, advanced fibromuscular dysplasia was observed in the cervical segments of both the vertebral arteries and was the presumed underlying etiology. Flow measurements with quantitative magnetic resonance angiography (Q-MRA) using the Non-invasive Optimal Vessel Analysis (NOVA) software (VasSol Inc., Chicago, IL) demonstrated that the fistula was supplied by 744 ml / minute of antegrade flow through the left VA and 55 ml / minute of retrograde flow through the right VA.
Figure 1.
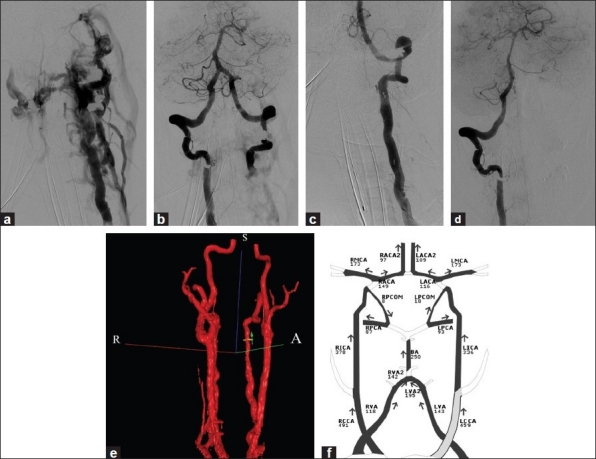
(a) Left VA angiogram showing an AVF draining into bilateral internal jugular veins and diffuse fibromuscular dyspastic change; (b) Right VA injection showing retrograde filling of the distal left VA into the right VA-jugular vein fistula, and diffuse fibromuscular dysplastic change; (c) after deployment of the stent-graft showing complete occlusion of the AVF with normalization of the antegrade flow within the intracranial portion of the left VA; (d) Right VA injection showing no cross flow of contrast into the left intracranial VA; (e) 3 D-imaging from a Q-MRA, showing reconstitution of the left VA with no opacification of the fistula; (f) Q-MRA flow maps indicating 151 ml / minute of antegrade blood flow within the left VA compared to 744 ml / minute at the baseline
Due to the complexity of the fistula, surgery and endovascular coiling were not feasible. Additionally, preservation of the VA was preferred given the presence of fibromuscular dysplasia. The procedure was performed under general anesthesia. Loading doses of intravenous Heparin (5000 U) and eptifibatide (180 μg / kg) were administered initially and eptifibatide (2 μg / kg / minute) was continued throughout the procedure. A Fluency Plus covered stent (Bard Inc.; Karlsruhe, Germany) was deployed successfully across the fistula. Control angiography of the left VA demonstrated complete closure of the fistula with preservation of flow to the intracranial circulation [Figure 1c], and reversal of the contralateral flow from the right VA [Figure 1d]. Post procedural Q-MRA demonstrated normalization of the antegrade flow through the left VA distal to the fistula at 143 ml / minute, with a combined total of 261 ml / minute within both vertebral arteries, (the normal range of combined vertebral flow was 99 – 281 mg / minute) [Figures 1e and f].
Postoperatively the patient remained neurologically intact and was started on dual anti-platelet therapy with daily aspirin (325 mg) and clopidogrel (75 mg). Eptifibatide was discontinued the morning after the procedure. On follow-up, the patient's tinnitus had resolved, and angiography at four months demonstrated complete exclusion of the VJF and patency of the covered stent.
Case 2
A 62-year-old male with a history of stage II laryngeal squamous cell carcinoma was treated with total laryngectomy, bilateral neck dissection, tracheostomy, and adjuvant radiation therapy. The patient suffered tumor recurrence in the pharynx, and was being treated with palliative chemotherapy. He presented with severe, acute bleeding from the mouth and bilateral nares, which required emergent intubation and extensive packing of the nasal and oral cavities. Angiography demonstrated left common carotid artery (CCA) blowout with a pseudoaneurysm at the carotid bifurcation [Figure 2a]. Circumferential ulceration suggestive of CBS was also identified at the bifurcation of the right internal carotid artery (ICA).
Figure 2.
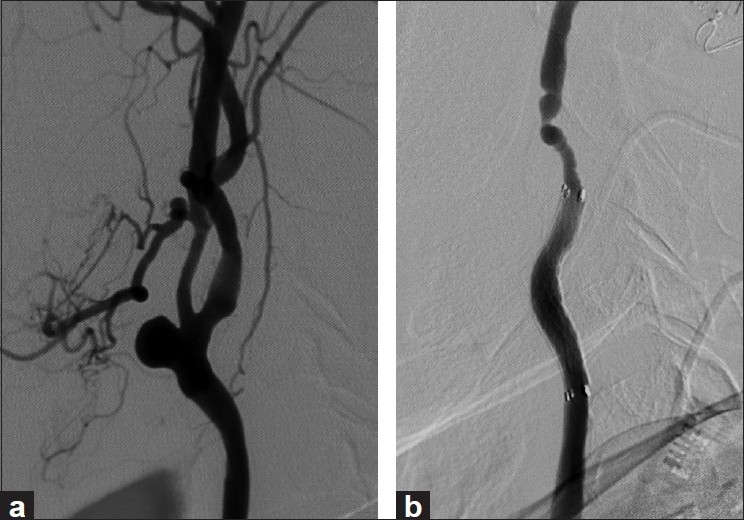
(a) Left CCA angiogram showing a pseudoaneurysm at the carotid bifurcation; (b) exclusion of the pseudoaneurysm after deployment of the covered stent
Due to surgical scarring, radiation, and tumor recurrence, the surgery carried a high risk of morbidity and mortality. It was elected to endovascularly reconstruct the carotid arteries with covered stents. A Fluency Plus stent-graft was successfully deployed effectively covering the common carotid bifurcation and the proximal aspect of the cervical segment of the left ICA. Control angiography showed complete exclusion of the pseudoaneurysm as well as the external carotid artery [Figure 2b].
A Fluency Plus stent-graft was also deployed across the right carotid bifurcation. During advancement of the Stent, dissection of the proximal CCA was noted. Subsequently, two Precise Nitinol stents (Cordis Corporation; Miami Lakes, FL) were successfully deployed across the iatrogenic dissection. Control angiography demonstrated the near-normal caliber of the right common carotid artery with the three stents in tandem. Systemic heparinization was continued for 24 hours, after which the patient was maintained on dual anti-platelet therapy. Post-procedurally no new neurological deficits were identified. He was ultimately discharged to a nursing home and was lost to follow-up.
Case 3
A 53-year-old female with a history of stage II squamous cell carcinoma of the larynx was treated with radical laryngectomy with bilateral neck dissection, tracheostomy, and radiation therapy. The patient suffered tumor recurrence in the pharynx, and presented with severe, acute bleeding from the mouth. She was emergently intubated and hemostasis was achieved by packing the nasal and oral cavities. Angiography demonstrated a left CCA blowout with a pseudoaneurysm at the carotid bifurcation. This was treated with endovascular deployment of a Fluency covered stent graft with no immediate complications and was maintained on dual anti-platelet therapy. The patient died six months later from cancer recurrence.
REVIEW OF THE LITERATURE
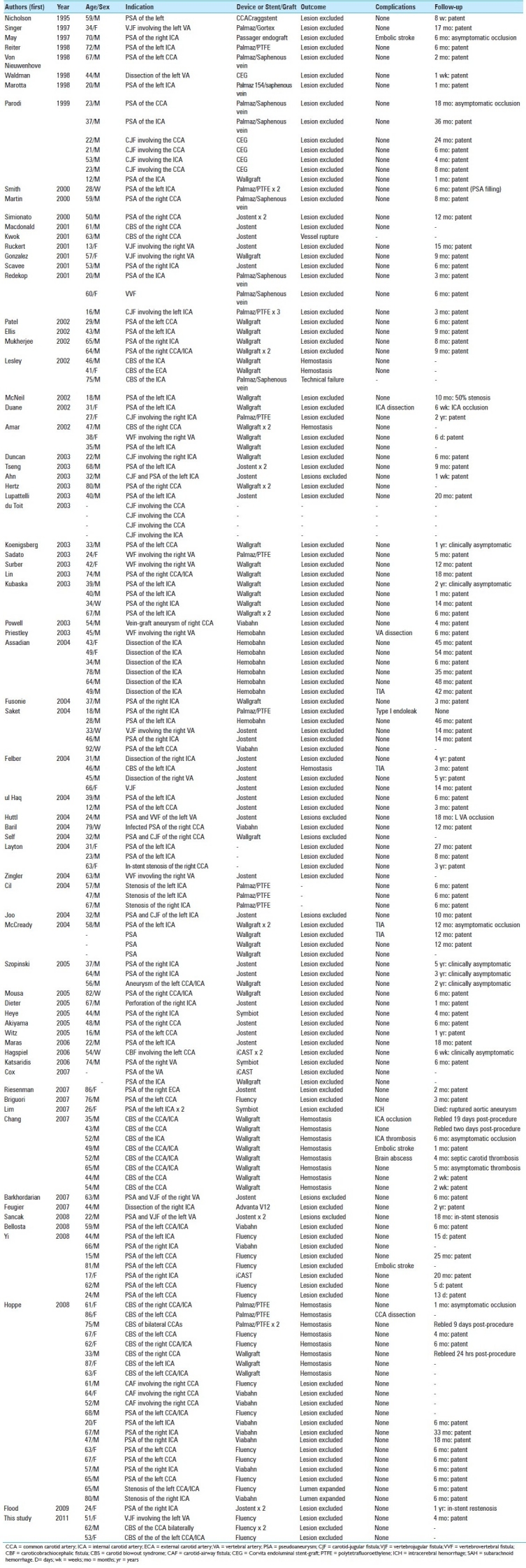
We conducted a systematic review of the English-speaking medical literature using the PubMed service of the National Library of Medicine / National Institutes of Health and OVID Medline databases to identify all publications documenting the use of covered stents in the extracranial cerebral circulation. The search included the keywords ‘Stents’[Mesh] AND (‘Vertebral Artery’[Mesh] OR ‘Carotid Artery Injuries’[Mesh] NOT ‘Intracranial Arterial Diseases’[Mesh] NOT ‘Subclavian artery’[MESH] NOT ‘Carotid-Cavernous Sinus Fistula’[Mesh]. Additionally, the reference lists of the relevant articles were checked until no further publications were found. These publications are summarized in Table 1.[1,2,4,5,7,8,11,13,15,16,19,25,27–32,34,35,37,38,41,42,45,49,51,52,54,57,59–63,65,66,68–73,75–77,79,80,82–85,87,89–92,94–98,100,101,103,104,106,109,112–114]
Table 1.
Extracranial neurovascular interventions with covered stents: bibliography review
DISCUSSION
Indications for covered stents
A total of 150 patients, including the present cases, were endovascularly implanted with 164 covered stents for the treatment of extracranial disease of the carotid or vertebral arteries [Table 1]. Publication bias may have resulted in the underestimation of these figures, as failed procedures are occasionally not publicized. The most commonly reported indications included 81 pseudoaneurysms, 27 cases of CBS, and 23 AVFs. Additional reported indications included dissections, carotid-airway fistulas, in-stent stenosis, and atherosclerosis [Table 1].
A pseudoaneurysm forms secondary to vessel wall trauma that results in a periarterial hematoma contained in the ingrowth of the fibrotic tissue. As the center of the hematoma dissolves, a potential space for blood flow is created, which under arterial pressure gradually enlarges to form an aneurysmal sac.[9] Blood may also dissect through the subintimal or subadventitial space narrowing the true lumen of the vessel.[22] Pseudoaneurysms may be caused by blunt or penetrating craniocervical trauma, spontaneous dissection, and as a rare complication of carotid endarterectomy.[20] The most common clinical presentations include thromboembolic symptoms[78] or a pulsatile cervical mass or bruit on physical examination.[74] Treatment is recommended to reduce the risk of stroke and rupture.
Arteriovenous fistulas involving the extracranial carotid and vertebral arteries are rare.[27,107] Like pseudoaneurysms, these lesions most commonly occur secondary to blunt or penetrating neck trauma.[50] Iatrogenic lesions are caused by an arterial puncture with concomitant damage to an adjacent vein.[102] Other causes include systemic diseases such as neurofibromatosis[98] and fibromuscular dysplasia,[39] which is the presumed etiology in our patient. Treatment is necessary, because shunting through the high-flow AVF may result in cardiac overload.[44] Additionally, as seen in our patient, vertebral AVFs may produce a continuous or pulsatile tinnitus also necessitating treatment.[10]
Carotid blowout syndrome is a term used to describe a rupture of the extracranial carotid artery or its branches. Patients commonly present with acute transoral or transcervical hemorrhage.[14] CBS is a rare, but life-threatening complication of head and neck cancer occurring in 4.3% of these patients.[67] The etiological factors related to previous surgery and adjuvant radiation therapy have been implicated.[67,93] Our patients with CBS presented with life-threatening bleeding that necessitated packing and acute parent vessel reconstruction with covered stents.
Conventional surgical treatment
Although standard surgical procedures on the extracranial carotid and vertebral arteries are generally straightforward, complex lesions such as those previously discussed pose unique challenges. Historically, surgical options for the treatment of extracranial pseudoaneurysms included vessel ligation, extracranial–intracranial bypass, and direct vessel repair; however, these surgeries are technically challenging. In particular, ICA pseudoaneurysms near the skull base necessitate extensive exposures to achieve proximal and distal control, which may result in significant morbidity and mortality.[21,33] Direct repair of VA pseudoaneurysms is also associated with high morbidity and mortality due to the anatomic depth of the vessel, extensive periarterial plexus, prevalence of collateral blood flow, and risk of vertebrobasilar ischemia.[12,24,40]
Surgical treatment of AVFs is similarly difficult. Ideally, the procedure entails interruption of the fistula with arterial and venous reconstruction.[86,88] However, the technical challenge and consequent morbidity related to surgical exposure are similar to those of pseudoaneurysm repair.[29] Furthermore, the large caliber of the extracranial carotid and vertebral arteries produces large shunts and pressure differences. This results in rich arterial and venous collateral formation and arterialization of the thin-walled veins, which makes surgery even more delicate. If the fistula cannot be fully trapped, ligation of the affected vessel is the only other surgical option.
Surgical management of CBS involves emergent ligation of the affected common or proximal internal carotid artery; however, vessel sacrifice increases the risk of stroke.[46] Additionally, these patients are generally high risk candidates for general anesthesia. Furthermore, identification of the source of bleeding can be extremely difficult due to the tumor bulk, coexisting infection or dense scarring secondary to the previous treatment. Consequently, these procedures have been associated with a 60% incidence of neurological complications and 40% overall mortality.[14,17]
Advantages of covered stents in the extracranial vessels
Preserving vessel patency is ideal when treating vascular lesions of the extracranial cerebral circulation. Sacrificing extracranial cerebral vessels increases morbidity and mortality, especially in patients with poor collateral circulation.[23,56] Indeed, iatrogenic vertebrobasilar insufficiency has been reported after deliberate sacrifice of one vertebral artery, even after an antecedent balloon test occlusion suggested its safety.[3] Additionally, preservation of the complete extracranial cerebral vasculature is desirable in young patients[49,90] and patients with systemic vasculatides or connective tissue disorders, where future neurovascular lesions must be anticipated[62,91,98]
Because of the risk of surgical complications, endovascular approaches are useful for extracranial cerebral vasculature. Balloon occlusion and coil embolization are commonly used endovascular techniques; however, these procedures sacrifice the parent vessel. Another technique is overlapping bare metal stents, which has been used to trigger hemodynamic changes that accelerate the thrombosis of dissecting pseudoaneurysms; however, this technique has limited utility in cases of an expanding pseudoaneurysm or active bleeding, where an immediate, blood-tight seal is required. Covered stents circumvent these limitations by immediately excluding breaches of the vessel wall while maintaining parent vessel patency.
History of covered stents in the extracranial vessels
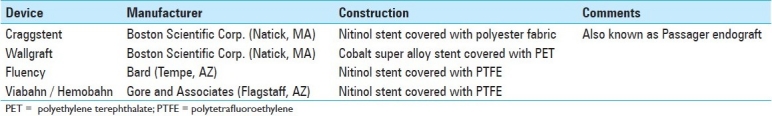
The earliest covered stent used for neurovascular intervention was the Craggstent (Boston Scientific Corp., Natick, MA), later renamed the Passager endograft, which was used in two cases to treat carotid artery pseudoaneurysms.[71,77] This device consisted of a self-expanding Nitinol stent covered by a polyester fabric designed and marketed for bypass grafting in the iliac and superficial femoral arteries. The primary disadvantage of this device was that the delivery catheter was too short to allow a percutaneous approach from the groin, thus necessitating direct puncture of the CCA. Early neurovascular interventions were also performed with homemade devices made of Gortex,[97] PTFE[45,85] or autologous-vein[69] grafts sutured to balloon-expandable Palmaz stents (Johnson and Johnson, New Brunswick, NJ).
The Wallgraft (Boston Scientific, Natick, MA), which consists of a PET (Dacron; E.I. duPont de Nemours and Co., Wilmington, DE) covered self-expanding cobalt super alloy stent, is more widely used. The longitudinal flexibility of this device allows for better conformability to the tortuous arterial walls than the homemade devices;[45] however, the Wallgraft has several important disadvantages. First, PET is highly immunogenic,[26,48,55] which animal studies suggest, increases the rate of vessel thrombosis.[64] Second, the Wallgraft delivery system requires a 9-French arterial sheath in the carotid and vertebral arteries, which may increase the risk of pseudoaneurysms or groin hematomas at the femoral puncture site. Finally, the PET covering is initially porous until a clot forms to seal the fabric. In acute bleeding situations, such as CBS, the fabric does not seal rapidly enough to prevent exsanguination.[99]
Newer self-expanding devices include the Fluency and Viabahn, which were FDA approved for the treatment of tracheobronchial strictures in 2003 and 2005, respectively. Both are composed of a Nitinol stent covered with PTFE. These devices are more flexible, conform more easily to the vessel walls, and the PTFE covering is less thrombogenic. In recent times, these devices have become available in long delivery sheaths necessary for placement in the extracranial cerebral circulation; however, large delivery sheaths are still required. Other self-expanding devices have been used including the Symbiot,[52,62] which is only available in Europe, and the Corvita Endoluminal Stent-Graft,[109] which did not come into the market. The primary advantage of self-expanding stents is that they re-expand after external compression. Therefore, self-expanding stents are preferred for the carotid artery[81] and at the carotid bifurcation, to accommodate the difference in diameters between the common and internal carotid arteries.[11] In all our cases, four Fluency stents were used, which were all self-expanding and configured appropriately to match the size of the CCA, ICA, and VA, with immediate angiographic reconstruction of the parent vessel and isolation of the pseudoaneurysm / fistula connection.
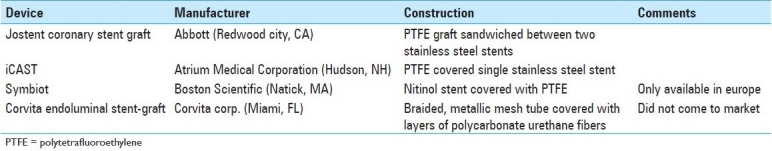
Commercially available balloon-expandable covered stents are also available [Table 2]. The most common of these devices is the Jostent Coronary Stent Graft (Abbott, Redwood City, CA), which was FDA approved in 2001. This device consists of a PTFE graft sandwiched between two stainless steel stents. Also available is the iCAST (Atrium Medical Corporation, Hudson, NH), a PTFE-covered, single stainless steel stent, which was FDA approved in 2005, for the treatment of tracheobronchial strictures. An advantage of a balloon-expandable deployment system in treating AVFs is the ability to over-dilate the vessel.[90] This firmly fixes the stent, thus avoiding an endoleak between the graft and vessel wall. However, the several drawbacks to these devices are that they are expensive, lack flexibility, are susceptible to mechanical distortion, and require high deployment pressure.[31] The latter is particularly important when treating fragile vessels.[45]
Table 2.
Balloon-expandable covered stents used in extracranial neurovasculature
Technical success
Table 3 summarizes the outcomes of previously reported cases. In our literature review, three technical failures were reported,[15,59,61,62] resulting in a technical success rate of 98.2%. Kwok et al.,[52] reported carotid artery rupture during deployment of a balloon-expandable covered stent for the treatment of CBS. The authors postulated that the cause of the rupture was due to the high pressure required to inflate the stent in an artery already torn by normal blood pressure. In another patient treated for CBS, Lesley et al.,[61] reported a vein-covered Palmaz stent that failed to deploy. Finally, surgeons treating a patient with Ehlers-Danlos Syndrome type IV were unable to treat an ICA aneurysm just below the skull base, because the Symbiot covered stent would not negotiate the vessel tortuosity.[62]
Table 3.
Self-expanding covered stents used in extracranial neurovasculature
Complications associated with covered stents
Immediate complications occurred in 15 of 164 procedures (9.1%). Embolic complications during covered stent placement are due to dissection or rupture and embolization of the atheromatous plaque. Two patients (1.2%) described by May et al.,[71] and Chang et al.,[15] suffered embolic strokes, the former during treatment of a pseudoaneurysm post carotid endarterectomy, and the latter for CBS. Six additional patients experienced post-procedural transient ischemic attacks.[5,31]
Dissections were encountered in three cases (1.8%).[28,45,83] Duane et al.,[8] reported an ICA dissection during covered stent placement for the treatment of a traumatic pseudoaneurysm. The patient was maintained on anticoagulation therapy, because endovascular treatment was delayed in order to allow healing of the operative site; however, at follow-up the stent was thrombosed. Priestley et al.,[83] reported a VA dissection during treatment for an AVF that resolved spontaneously by six months. Hoppe et al.,[45] reported a dissection of the CCA in a patient being treated for CBS. The dissection was treated with two bare self-expanding Nitinol stents and a follow-up angiography demonstrated normal luminal integrity.
Additional immediate complications were reported by Chang et al.,[15] who described two cases (1.8%) of acute, asymptomatic thrombosis of the CCA after covered stent deployment for the treatment of CBS. In one case the thrombosis was successfully lysed by intravenous glycoprotein IIb / IIIa inhibitor infusion. Also, Lim et al.,[62] reported a patient with Ehlers-Danlos Syndrome type IV, treated for a carotid dissecting pseudoaneurysm, who died five hours after the procedure from a previously undiagnosed ruptured abdominal aortic aneurysm. Such remote vascular complications have been reported during neurointerventional procedures in these patients;[47,105] however, the authors did not encounter any difficulties traversing the abdominal aorta. Finally, Kwok et al.,[59] reported rupture of the target vessel during covered stent deployment as discussed a little earlier in the text.
Seven of the 25 patients (28%) treated for CBS suffered re-hemorrhage after initial hemostasis was achieved with covered stent implantation.[15,45] Four patients suffered re-hemorrhage secondary to disease progression,[40,54] between 19 days and two months after the initial treatment. One patient suffered a fatal re-bleed two days after treatment, possibly from inadequate covered stent placement due to the patient's critical clinical status. Another patient suffered a re-bleed nine days after covered stent placement, due to a residual flow around the device. Attempted balloon angioplasty expansion of the stent caused it to rupture, resulting in massive extravasation. The re-bleed in one patient was attributed to disseminated intravascular coagulation, as there were no complications associated with the placement of a covered stent. Other reported delayed complications included one patient who developed multiple brain abscesses secondary to septic thrombosis of the carotid artery[40] and one patient treated with a PTFE covered Palmaz stent, who developed a small type-1 endoleak, three days after the procedure.[91]
Subacute thrombosis and intimal hyperplasia leading to in-stent stenosis or vessel occlusion are the primary complications associated with covered stents. The graft material may delay endothelialization[26] resulting in thrombotic occlusion of the stented vessel.[6] Additionally, traumatized vessels are hypercoagulable. Placement of a covered stent further retards the flow, which may compound the risk of occlusion.[18] Other factors contributing to stent occlusion include graft material,[26,48,55] small vessel size, dissection, and under-dilation of the stent.[84] In contrast, the synthetic layers of the covered stents theoretically act as mechanical barriers that allow only minimal intimal hyperplasia. However, end-stent stenosis is more common with covered stents compared to conventional stents,[36] and it has been suggested that stenosis primarily occurs adjacent to a bend or kink.[111] There is no evidence to suggest an appropriate time to stop dual anti-platelet therapy. There is no consensus in the reviewed published literature regarding the type and duration of anti-platelets and / or anti-coagulation therapy. In some of the published data, patients were treated with aspirin alone; other reports indicate treatment with aspirin and plavix, others with aspirin and warfarin. The duration of anti-coagulation and / or anti-platelets vary from one month to lifetime treatment. Keeping in mind the large area of synthetic graft material exposure to the circulation, and based on the best evidence from conventional carotid artery stenting literature for atherosclerotic carotid artery disease, all our patients were maintained on dual anti-platelet therapy for six months, and thereafter continued on aspirin alone.
The long-term patency of covered stents in extracranial cerebral circulation is unknown. Nine of the 109 patients who underwent angiographic follow-up developed total occlusion of the stented vessel,[15,28,45,49,71,72,79] which gave an overall occlusion rate of 8.3%. These cases included four ICA pseudoaneurysms, one VA pseudoaneurysm, and four patients with CBS. Occlusion was asymptomatic in all nine patients. Follow-up in the patients with pseudoaneurysms ranged from two to twenty-three months. Duane et al.,[28] cited persistent narrowing at the end of the stent due to an intimal flap as the possible cause of the occlusion. Huttl et al.,[49] described total occlusion of a Jostent in the VA at two months, which was attributed to mechanical distortion caused by external mechanical compression. Four cases of subacute carotid thrombosis were reported in patients treated for CBS;[15,45] nevertheless, the use of covered stents for immediate hemostatic control in CBS is a reasonable alternative to surgical ligation or permanent balloon occlusion.
In-stent stenosis due to intimal hyperplasia was described in an additional three cases of traumatic ICA pseudoaneurysms,[34,73,79] as also one case of a VA pseudoaneurysm with multiple AVFs.[92] McNeil et al.,[73] and Flood et al.,[34] each reported 50% ICA in-stent stenosis following placement of a Wallgraft and Jostent at 10 months and one year, respectively. Parodi et al.,[79] reported 90% ICA in-stent stenosis following placement of a vein-covered Palmaz stent, which progressed to complete occlusion 39 months after initial covered stent placement. Sancak et al.,[92] reported 50% VA re-stenosis at 18 months successfully treated by balloon angioplasty.
Additional neurovascular applications
With newer generations of covered stents, intracranial applications might be expanded. Wang et al.,[110] recently reported 10 patients with direct carotid cavernous fistulas treated with covered stent implantation. Technical failure occurred in one patient due to the rigidity of the covered stent and tortuosity of the ICA. A second patient had recurrence of symptoms the morning after the procedure necessitating ipsilateral ICA occlusion with detachable coils. Of the remaining eight patients, follow-up angiography ranging from five to forty-eight months showed complete exclusion of all direct carotid cavernous fistulas and stent patency without in-stent stenosis.
CONCLUSION
Covered stents are useful for extracranial neuroendovascular interventions in selected patients, for the treatment of a variety of lesions, especially pseudoaneurysms, AVFs, and CBS. However, larger studies are required to determine the true incidence of periprocedural complications. The three cases described in this article, and a review of the present literature, suggest that embolic events and dissections are the most frequent immediate complications. Studies evaluating the long-term safety, stent patency, and permanency of hemostasis are also needed. The widespread use of covered stents requires the development of more flexible devices with longer delivery systems, specifically designed for neuroendovascular intervention.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/67/81725
Contributor Information
Ali Alaraj, Email: alaraj@uic.edu.
Adam Wallace, Email: adam.n.wallace@gmail.com.
Sepideh Amin-Hanjani, Email: hanjani@uic.edu.
Fady T Charbel, Email: fcharbel@uic.edu.
Victor Aletich, Email: valetich@uic.edu.
REFERENCES
- 1.Ahn JY, Chung YS, Lee BH, Choi SW, Kim OJ. Stent-graft placement in a traumatic internal carotid-internal jugular fistula and pseudoaneurysm. J Clin Neurosci. 2004;11:636–9. doi: 10.1016/j.jocn.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama Y, Nakahara I, Tanaka M, Iwamuro Y, Hayashi J, Harada K, et al. Urgent endovascular stent-graft placement for a ruptured traumatic pseudoaneurysm of the extracranial carotid artery. J Trauma. 2005;58:624–7. doi: 10.1097/01.ta.0000096662.79685.ea. [DOI] [PubMed] [Google Scholar]
- 3.Amar AP, Levy ML, Giannotta SL. Iatrogenic vertebrobasilar insufficiency after surgery of the subclavian or brachial artery: Review of three cases. Neurosurgery. 1998;43:1450–7. [PubMed] [Google Scholar]
- 4.Amar AP, Teitelbaum GP, Giannotta SL, Larsen DW. Covered stent-graft repair of the brachiocephalic arteries: technical note. Neurosurgery. 2002;51:247–52. doi: 10.1097/00006123-200207000-00040. [DOI] [PubMed] [Google Scholar]
- 5.Assadian A, Senekowitsch C, Rotter R, Zolss C, Strassegger J, Hagmuller GW. Long-term results of covered stent repair of internal carotid artery dissections. J Vasc Surg. 2004;40:484–7. doi: 10.1016/j.jvs.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Baldus S, Koster R, Reimers J, Hamm CW. Membrane-covered stents for the treatment of aortocoronary vein graft disease. Catheter Cardiovasc Interv. 2000;50:83–8. doi: 10.1002/(sici)1522-726x(200005)50:1<83::aid-ccd18>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Baril DT, Ellozy SH, Carroccio A, Patel AB, Lookstein RA, Marin ML. Endovascular repair of an infected carotid artery pseudoaneurysm. J Vasc Surg. 2004;40:1024–7. doi: 10.1016/j.jvs.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Barkhordarian S. Stent graft repair of traumatic vertebral pseudoaneurysm with arteriovenous fistula. Vasc Endovascular Surg. 2007;41:153–7. doi: 10.1177/1538574406298085. [DOI] [PubMed] [Google Scholar]
- 9.Beale PJ. Late development of a false aneurysm of the common carotid artery. Br J Surg. 1971;58:76–8. doi: 10.1002/bjs.1800580117. [DOI] [PubMed] [Google Scholar]
- 10.Beaujeux RL, Reizine DC, Casasco A, Aymard A, Rufenacht D, Khayata MH, et al. Endovascular treatment of vertebral arteriovenous fistula. Radiology. 1992;183:361–7. doi: 10.1148/radiology.183.2.1561336. [DOI] [PubMed] [Google Scholar]
- 11.Bellosta R, Sesana M, Baglini R, Luzzani L, Talarico M, Sarcina A. Endovascular treatment of a symptomatic carotid artery aneurysm with a stent graft. Vasc Endovascular Surg. 2008;42:276–8. doi: 10.1177/1538574407312650. [DOI] [PubMed] [Google Scholar]
- 12.Blickenstaff KL, Weaver FA, Yellin AE, Stain SC, Finck E. Trends in the management of traumatic vertebral artery injuries. Am J Surg. 1989;158:101–5. doi: 10.1016/0002-9610(89)90355-3. [DOI] [PubMed] [Google Scholar]
- 13.Briguori C, Selvetella L, Baldassarre MP. Endovascular repair of a carotid pseudoaneurysm with Fluency Plus stent graft implantation. J Invasive Cardiol. 2007;19:E254–7. [PubMed] [Google Scholar]
- 14.Chaloupka JC, Putman CM, Citardi MJ, Ross DA, Sasaki CT. Endovascular therapy for the carotid blowout syndrome in head and neck surgical patients: Diagnostic and managerial considerations. AJNR Am J Neuroradiol. 1996;17:843–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Chang FC, Lirng JF, Luo CB, Guo WY, Teng MM, Tai SK, et al. Carotid blowout syndrome in patients with head-and-neck cancers: Reconstructive management by self-expandable stent-grafts. AJNR Am J Neuroradiol. 2007;28:181–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Cil BE, Akpinar E, Peynircioglu B, Cekirge S. Utility of covered stents for extracranial internal carotid artery stenosis. AJNR Am J Neuroradiol. 2004;25:1168–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Citardi MJ, Chaloupka JC, Son YH, Ariyan S, Sasaki CT. Management of carotid artery rupture by monitored endovascular therapeutic occlusion (1988-1994) Laryngoscope. 1995;105:1086–92. doi: 10.1288/00005537-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Cothren CC, Moore EE, Ray CE, Jr, Ciesla DJ, Johnson JL, Moore JB, et al. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. Arch Surg. 2005;140:480–5. doi: 10.1001/archsurg.140.5.480. [DOI] [PubMed] [Google Scholar]
- 19.Cox MW, Whittaker DR, Martinez C, Fox CJ, Feuerstein IM, Gillespie DL. Traumatic pseudoaneurysms of the head and neck: Early endovascular intervention. J Vasc Surg. 2007;46:1227–33. doi: 10.1016/j.jvs.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 20.De Filippo CM, Modugno P, Nasso G, Canosa C, Spatuzza P, Testa N, et al. Pseudoaneurysm after patch-free carotid bifurcation endarterectomy: A case report. Vasc Endovascular Surg. 2007;41:448–51. doi: 10.1177/1538574407301685. [DOI] [PubMed] [Google Scholar]
- 21.de los Reyes RA, Moser FG, Sachs DP, Boehm FH. Direct repair of an extracranial vertebral artery pseudoaneurysm: case report and review of the literature. Neurosurgery. 1990;26:528–33. doi: 10.1097/00006123-199003000-00025. [DOI] [PubMed] [Google Scholar]
- 22.DeFatta RJ, Verret DJ, Bauer P. Extracranial internal carotid artery pseudoaneurysm. Int J Pediatr Otorhinolaryngol. 2005;69:1135–9. doi: 10.1016/j.ijporl.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Demetriades D, Theodorou D, Asensio J, Golshani S, Belzberg H, Yellin A, et al. Management options in vertebral artery injuries. Br J Surg. 1996;83:83–6. doi: 10.1002/bjs.1800830126. [DOI] [PubMed] [Google Scholar]
- 24.Detwiler K, Godersky JC, Gentry L. Pseudoaneurysm of the extracranial vertebral artery.Case report. J Neurosurg. 1987;67:935–9. doi: 10.3171/jns.1987.67.6.0935. [DOI] [PubMed] [Google Scholar]
- 25.Dieter RS, Ikram S, Satler LF, Babrowicz JC, Reddy B, Laird JR. Perforation complicating carotid artery stenting: the use of a covered stent. Catheter Cardiovasc Interv. 2006;67:972–5. doi: 10.1002/ccd.20744. [DOI] [PubMed] [Google Scholar]
- 26.Dolmatch BL, Tio FO, Li XD, Dong YH. Patency and tissue response related to two types of polytetrafluoroethylene-covered stents in the dog. J Vasc Interv Radiol. 1996;7:641–9. doi: 10.1016/s1051-0443(96)70822-9. [DOI] [PubMed] [Google Scholar]
- 27.du Toit DF, Leith JG, Strauss DC, Blaszczyk M, Odendaal Jde V, Warren BL. Endovascular management of traumatic cervicothoracic arteriovenous fistula. Br J Surg. 2003;90:1516–1521. doi: 10.1002/bjs.4343. [DOI] [PubMed] [Google Scholar]
- 28.Duane TM, Parker F, Stokes GK, Parent FN, Britt LD. Endovascular carotid stenting after trauma. J Trauma. 2002;52:149–53. doi: 10.1097/00005373-200201000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Duncan IC, Fourie PA. Percutaneous management of concomitant post-traumatic high vertebrovertebral and caroticojugular fistulas using balloons, coils, and a covered stent. J Endovasc Ther. 2003;10:882–6. doi: 10.1177/152660280301000506. [DOI] [PubMed] [Google Scholar]
- 30.Ellis PK, Kennedy PT, Barros D'sa AA. Successful exclusion of a high internal carotid pseudoaneurysm using the Wallgraft endoprosthesis. Cardiovasc Intervent Radiol. 2002;25:68–9. doi: 10.1007/s00270-001-0058-y. [DOI] [PubMed] [Google Scholar]
- 31.Felber S, Henkes H, Weber W, Miloslavski E, Brew S, Kuhne D. Treatment of extracranial and intracranial aneurysms and arteriovenous fistulae using stent grafts. Neurosurgery. 2004;55:631–8. doi: 10.1227/01.neu.0000134455.02947.1f. [DOI] [PubMed] [Google Scholar]
- 32.Feugier P, Vulliez A, Bina N, Floccard B, Allaouchiche B. Urgent endovascular covered-stent treatment of internal carotid artery injury caused by a gunshot. Eur J Vasc Endovasc Surg. 2007;34:663–5. doi: 10.1016/j.ejvs.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Fleischer AS, Guthkelch AN. Management of high cervical-intracranial internal carotid artery traumatic aneurysms. J Trauma. 1987;27:330–2. doi: 10.1097/00005373-198703000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Flood J, Bussiere M, Teefy P, Gulka I, Lownie S, Pelz D. In-stent stenosis following covered stent-graft placement. Can J Neurol Sci. 2009;36:248–51. doi: 10.1017/s031716710012030x. [DOI] [PubMed] [Google Scholar]
- 35.Fusonie GE, Edwards JD, Reed AB. Covered stent exclusion of blunt traumatic carotid artery pseudoaneurysm: Case report and review of the literature. Ann Vasc Surg. 2004;18:376–9. doi: 10.1007/s10016-004-0037-2. [DOI] [PubMed] [Google Scholar]
- 36.Gercken U, Lansky AJ, Buellesfeld L, Desai K, Badereldin M, Mueller R, et al. Results of the Jostent coronary stent graft implantation in various clinical settings: Procedural and follow-up results. Catheter Cardiovasc Interv. 2002;56:353–60. doi: 10.1002/ccd.10223. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez A, Mayol A, Gil-Peralta A, Gonzalez-Marcos JR. Endovascular stent-graft treatment of an iatrogenic vertebral arteriovenous fistula. Neuroradiology. 2001;43:784–6. doi: 10.1007/s002340100586. [DOI] [PubMed] [Google Scholar]
- 38.Hagspiel KD, Komorowski DJ, Shih MC, Peeler BB, Jensen ME. Treatment of carotid arteriovenous fistula with balloon-expandable tracheobronchial covered stent. J Vasc Interv Radiol. 2006;17:585–6. doi: 10.1097/01.RVI.0000204855.05387.7A. [DOI] [PubMed] [Google Scholar]
- 39.Halbach VV, Higashida RT, Hieshima GB. Treatment of vertebral arteriovenous fistulas. AJR Am J Roentgenol. 1988;150:405–12. doi: 10.2214/ajr.150.2.405. [DOI] [PubMed] [Google Scholar]
- 40.Hanakita J, Suwa H, Nishihara K, Iihara K, Sakaida H. Giant pseudoaneurysm of the extracranial vertebral artery successfully treated using intraoperative balloon catheters. Neurosurgery. 1991;28:738–41. doi: 10.1097/00006123-199105000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Hertz JA, Minion DJ, Quick RC, Moore EM, Schwartz TH, Endean ED. Endovascular exclusion of a postendarterectomy carotid pseudoaneurysm. Ann Vasc Surg. 2003;17:558–61. doi: 10.1007/s10016-003-0034-x. [DOI] [PubMed] [Google Scholar]
- 42.Heye S, Maleux G, Vandenberghe R, Wilms G. Symptomatic internal carotid artery dissecting pseudoaneurysm: Endovascular treatment by stent-graft. Cardiovasc Intervent Radiol. 2005;28:499–501. doi: 10.1007/s00270-004-0269-0. [DOI] [PubMed] [Google Scholar]
- 43.Hilfiker PR, Razavi MK, Kee ST, Sze DY, Semba CP, Dake MD. Stent-graft therapy for subclavian artery aneurysms and fistulas: Single-center mid-term results. J Vasc Interv Radiol. 2000;11:578–84. doi: 10.1016/s1051-0443(07)61609-1. [DOI] [PubMed] [Google Scholar]
- 44.Holman E. Abnormal arteriovenous communications. Great variability of effects with particular reference to delayed development of cardiac failure. Circulation. 1965;32:1001–9. doi: 10.1161/01.cir.32.6.1001. [DOI] [PubMed] [Google Scholar]
- 45.Hoppe H, Barnwell SL, Nesbit GM, Petersen BD. Stent-grafts in the treatment of emergent or urgent carotid artery disease: review of 25 cases. J Vasc Interv Radiol. 2008;19:31–41. doi: 10.1016/j.jvir.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 46.Hoppe H, Wang SL, Petersen BD. Intravascular US-guided direct intrahepatic portocaval shunt with an expanded polytetrafluoroethylene-covered stent-graft. Radiology. 2008;246:306–14. doi: 10.1148/radiol.2461062191. [DOI] [PubMed] [Google Scholar]
- 47.Horowitz MB, Purdy PD, Valentine RJ, Morrill K. Remote vascular catastrophes after neurovascular interventional therapy for type 4 Ehlers-Danlos Syndrome. AJNR Am J Neuroradiol. 2000;21:974–6. [PMC free article] [PubMed] [Google Scholar]
- 48.Hussain FM, Kopchok G, Heilbron M, Daskalakis T, Donayre C, White RA. Wallgraft endoprosthesis: Initial canine evaluation. Am Surg. 1998;64:1002–6. [PubMed] [Google Scholar]
- 49.Huttl K, Sebestyen M, Entz L, Molnar AA, Nemes B, Berczi V. Covered stent placement in a traumatically injured vertebral artery. J Vasc Interv Radiol. 2004;15:201–2. doi: 10.1097/01.rvi.0000109406.52762.98. [DOI] [PubMed] [Google Scholar]
- 50.Jayaraman MV, Do HM, Marks MP. Treatment of traumatic cervical arteriovenous fistulas with N-butyl-2-cyanoacrylate. AJNR Am J Neuroradiol. 2007;28:352–4. [PMC free article] [PubMed] [Google Scholar]
- 51.Joo JY, Ahn JY, Chung YS, Chung SS, Kim SH, Yoon PH, et al. Therapeutic endovascular treatments for traumatic carotid artery injuries. J Trauma. 2005;58:1159–66. doi: 10.1097/01.ta.0000171550.01402.ed. [DOI] [PubMed] [Google Scholar]
- 52.Katsaridis V, Papagiannaki C, Violaris C. Treatment of an iatrogenic vertebral artery laceration with the Symbiot self expandable covered stent. Clin Neurol Neurosurg. 2007;109:512–5. doi: 10.1016/j.clineuro.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Kessel D, Robertson I, Scott J, Phipp L. Re: successful exclusion of subclavian aneurysm with covered nitinol stents. Cardiovasc Intervent Radiol. 1999;22:86–7. doi: 10.1007/s002709900339. [DOI] [PubMed] [Google Scholar]
- 54.Koenigsberg RA, Urrutia V, McCormick D, Alani F, Ryu D, Nair B, Thomas C. Endovascular treatment of a left carotid artery ′bowtie‘ pseudoaneurysm with a covered Wallgraft stent. J Neuroimaging. 2003;13:362–6. [PubMed] [Google Scholar]
- 55.Krajcer Z, Sioco G, Reynolds T. Comparison of Wallgraft and Wallstent for treatment of complex iliac artery stenosis and occlusion.Preliminary results of a prospective randomized study. Tex Heart Inst J. 1997;24:193–9. [PMC free article] [PubMed] [Google Scholar]
- 56.Kropman RH, de Vries JP, Segers MJ. Surgical repair of a gunshot injury to the left carotid artery: Case report and review of literature. Vasc Endovascular Surg. 2008;42:180–3. doi: 10.1177/1538574407308366. [DOI] [PubMed] [Google Scholar]
- 57.Kubaska SM, 3rd, Greenberg RK, Clair D, Barber G, Srivastava SD, Green RM, et al. Internal carotid artery pseudoaneurysms: treatment with the Wallgraft endoprosthesis. J Endovasc Ther. 2003;10:182–9. doi: 10.1177/152660280301000204. [DOI] [PubMed] [Google Scholar]
- 58.Kudelko PE, 2nd, Alfaro-Franco C, Diethrich EB, Krajcer Z. Successful endoluminal repair of a popliteal artery aneurysm using the Wallgraft endoprosthesis. J Endovasc Surg. 1998;5:373–7. doi: 10.1583/1074-6218(1998)005<0373:SEROAP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Kwok PC, Cheung JY, Tang KW, Wong WK. Re: Endovascular treatment of acute carotid blow-out syndrome. J Vasc Interv Radiol. 2001;12:895–6. doi: 10.1016/s1051-0443(07)61520-6. [DOI] [PubMed] [Google Scholar]
- 60.Layton KF, Kim YW, Hise JH. Use of covered stent grafts in the extracranial carotid artery: Report of three patients with follow-up between 8 and 42 months. AJNR Am J Neuroradiol. 2004;25:1760–3. [PMC free article] [PubMed] [Google Scholar]
- 61.Lesley WS, Chaloupka JC, Weigele JB, Mangla S, Dogar MA. Preliminary experience with endovascular reconstruction for the management of carotid blowout syndrome. AJNR Am J Neuroradiol. 2003;24:975–81. [PMC free article] [PubMed] [Google Scholar]
- 62.Lim SP, Duddy MJ. Endovascular treatment of a carotid dissecting pseudoaneurysm in a patient with Ehlers-Danlos syndrome type IV with fatal outcome. Cardiovasc Intervent Radiol. 2008;31:201–4. doi: 10.1007/s00270-007-9135-1. [DOI] [PubMed] [Google Scholar]
- 63.Lin PH, Bush RL, Lumsden AB. Successful stent-graft exclusion of a bovine patch-related carotid artery pseudoaneurysm. J Vasc Surg. 2003;38:396. doi: 10.1016/s0741-5214(03)00428-2. [DOI] [PubMed] [Google Scholar]
- 64.Link J, Feyerabend B, Grabener M, Linstedt U, Brossmann J, Thomsen H, et al. Dacron-covered stent-grafts for the percutaneous treatment of carotid aneurysms: Effectiveness and biocompatibility--experimental study in swine. Radiology. 1996;200:397–4010. doi: 10.1148/radiology.200.2.8685332. [DOI] [PubMed] [Google Scholar]
- 65.Lupattelli T, Garaci FG, Hopkins CE, Simonetti G. Covered stent deployment and follow-up of a case of internal carotid artery pseudoaneurysm. Cerebrovasc Dis. 2003;16:98–101. doi: 10.1159/000070125. [DOI] [PubMed] [Google Scholar]
- 66.Macdonald S, Gan J, McKay AJ, Edwards RD. Endovascular treatment of acute carotid blow-out syndrome. J Vasc Interv Radiol. 2000;11:1184–8. doi: 10.1016/s1051-0443(07)61361-x. [DOI] [PubMed] [Google Scholar]
- 67.Maran AG, Amin M, Wilson JA. Radical neck dissection: A 19-year experience. J Laryngol Otol. 1989;103:760–4. doi: 10.1017/s002221510011000x. [DOI] [PubMed] [Google Scholar]
- 68.Maras D, Lioupis C, Magoufis G, Tsamopoulos N, Moulakakis K, Andrikopoulos V. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: A review. Cardiovasc Intervent Radiol. 2006;29:958–68. doi: 10.1007/s00270-005-0367-7. [DOI] [PubMed] [Google Scholar]
- 69.Marotta TR, Buller C, Taylor D, Morris C, Zwimpfer T. Autologous vein-covered stent repair of a cervical internal carotid artery pseudoaneurysm: Technical case report. Neurosurgery. 1998;42:408–12. doi: 10.1097/00006123-199802000-00138. [DOI] [PubMed] [Google Scholar]
- 70.Martin JB, Bednarkiewicz M, Christenson JT, Rufenacht DA. Endovascular repair using vein-covered stents in the carotid artery bifurcation. Cardiovasc Surg. 2002;8:499–502. doi: 10.1016/s0967-2109(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 71.May J, White GH, Waugh R, Brennan J. Endoluminal repair of internal carotid artery aneurysm: A feasible but hazardous procedure. J Vasc Surg. 1997;26:1055–60. doi: 10.1016/s0741-5214(97)70020-x. [DOI] [PubMed] [Google Scholar]
- 72.McCready RA, Divelbiss JL, Bryant MA, Denardo AJ, Scott JA. Endoluminal repair of carotid artery pseudoaneurysms: A word of caution. J Vasc Surg. 2004;40:1020–3. doi: 10.1016/j.jvs.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 73.McNeil JD, Chiou AC, Gunlock MG, Grayson DE, Soares G, Hagino RT. Successful endovascular therapy of a penetrating zone III internal carotid injury. J Vasc Surg. 2002;36:187–90. doi: 10.1067/mva.2002.125020. [DOI] [PubMed] [Google Scholar]
- 74.Mokri B, Piepgras DG, Sundt TM, Jr, Pearson BW. Extracranial internal carotid artery aneurysms. Mayo Clin Proc. 1982;57:310–21. [PubMed] [Google Scholar]
- 75.Mousa A, Bernheim J, Lyon R, Dayal R, Hollenbeck S, Henderson P, et al. Postcarotid endarterectomy pseudoaneurysm treated with combined stent graft and coil embolization--a case report. Vasc Endovascular Surg. 2005;39:191–4. doi: 10.1177/153857440503900209. [DOI] [PubMed] [Google Scholar]
- 76.Mukherjee D, Roffi M, Yadav JS. Endovascular treatment of carotid artery aneurysms with stent grafts. J Invasive Cardiol. 2002;14:269–72. [PubMed] [Google Scholar]
- 77.Nicholson A, Cook AM, Dyet JF, Galloway JM. Case report: Treatment of a carotid artery pseudoaneurism with a polyester covered nitinol stent. Clin Radiol. 1995;50:872–3. doi: 10.1016/s0009-9260(05)83113-7. [DOI] [PubMed] [Google Scholar]
- 78.Oruckaptan HH, Ozcan OE. Giant extracranial internal carotid artery aneurysm: A rare presentation with an oropharyngeal mass. Otolaryngol Head Neck Surg. 2001;125:571–3. doi: 10.1067/mhn.2001.118071. [DOI] [PubMed] [Google Scholar]
- 79.Parodi JC, Schonholz C, Ferreira LM, Bergan J. Endovascular stent-graft treatment of traumatic arterial lesions. Ann Vasc Surg. 1999;13:121–9. doi: 10.1007/s100169900230. [DOI] [PubMed] [Google Scholar]
- 80.Patel JV, Rossbach MM, Cleveland TJ, Gaines PA, Beard JD. Endovascular stent-graft repair of traumatic carotid artery pseudoaneurysm. Clin Radiol. 2002;57:308–11. doi: 10.1053/crad.2001.0808. [DOI] [PubMed] [Google Scholar]
- 81.Phatouros CC, Higashida RT, Malek AM, Meyers PM, Lempert TE, Dowd CF, et al. Carotid artery stent placement for atherosclerotic disease: Rationale, technique, and current status. Radiology. 2002;217:26–41. doi: 10.1148/radiology.217.1.r00oc2526. [DOI] [PubMed] [Google Scholar]
- 82.Powell RJ, Rzucidlo EM, Schermerhorn ML. Stent-graft treatment of a large internal carotid artery vein graft aneurysm. J Vasc Surg. 37:1310–3. doi: 10.1016/s0741-5214(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 83.Priestley R, Bray P, Bray A, Hunter J. Iatrogenic vertebral arteriovenous fistula treated with a hemobahn stent-graft. J Endovasc Ther. 2003;10:657–63. doi: 10.1177/152660280301000337. [DOI] [PubMed] [Google Scholar]
- 84.Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95:412–9. doi: 10.3171/jns.2001.95.3.0412. [DOI] [PubMed] [Google Scholar]
- 85.Reiter BP, Marin ML, Teodorescu VJ, Mitty HA. Endoluminal repair of an internal carotid artery pseudoaneurysm. J Vasc Interv Radiol. 1998;9:245–8. doi: 10.1016/s1051-0443(98)70264-7. [DOI] [PubMed] [Google Scholar]
- 86.Rich NM, Baugh JH, Hughes CW. Popliteal artery injuries in Vietnam. Am J Surg. 1969;118:531–4. doi: 10.1016/0002-9610(69)90176-7. [DOI] [PubMed] [Google Scholar]
- 87.Riesenman PJ, Mendes RR, Mauro MA, Farber MA. Endovascular exclusion of an external carotid artery pseudoaneurysm using a covered stent. Cardiovasc Intervent Radiol. 2007;30:1025–8. doi: 10.1007/s00270-007-9039-0. [DOI] [PubMed] [Google Scholar]
- 88.Robbs JV, Carrim AA, Kadwa AM, Mars M. Traumatic arteriovenous fistula: experience with 202 patients. Br J Surg. 1994;81:1296–9. doi: 10.1002/bjs.1800810912. [DOI] [PubMed] [Google Scholar]
- 89.Ruckert RI, Rutsch W, Filimonow S, Lehmann R. Successful stent-graft repair of a vertebrojugular arteriovenous fistula. J Endovasc Ther. 2001;8:495–500. doi: 10.1177/152660280100800511. [DOI] [PubMed] [Google Scholar]
- 90.Sadato A, Satow T, Ishii A, Takayama M, Hashimoto N. Large vertebral arteriovenous fistula treated with stent-grafts--case report. Neurol Med Chir (Tokyo) 2003;43:250–4. doi: 10.2176/nmc.43.250. [DOI] [PubMed] [Google Scholar]
- 91.Saket RR, Razavi MK, Sze DY, Frisoli JK, Kee ST, Dake MD. Stent-graft treatment of extracranial carotid and vertebral arterial lesions. J Vasc Interv Radiol. 2004;15:1151–6. doi: 10.1097/01.rvi.0000134496.71252. [DOI] [PubMed] [Google Scholar]
- 92.Sancak T, Bilgic S, Ustuner E. Endovascular stent-graft treatment of a traumatic vertebral artery pseudoaneurysm and vertebrojugular fistula. Korean J Radiol. 2008;9 Suppl:S68–72. doi: 10.3348/kjr.2008.9.s.s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanders EM, Davis KR, Whelan CS, Deckers PJ. Threatened carotid artery rupture: A complication of radical neck surgery. J Surg Oncol. 1986;33:190–3. doi: 10.1002/jso.2930330309. [DOI] [PubMed] [Google Scholar]
- 94.Scavee V, De Wispelaere JF, Mormont E, Coulier B, Trigaux JP, Schoevaerdts JC. Pseudoaneurysm of the internal carotid artery: Treatment with a covered stent. Cardiovasc Intervent Radiol. 2001;24:283–5. doi: 10.1007/s00270-001-0012-z. [DOI] [PubMed] [Google Scholar]
- 95.Self ML, Mangram A, Jefferson H, Slonim S, Dunn E, Kollmeyer K. Percutaneous stent-graft repair of a traumatic common carotid-internal jugular fistula and pseudoaneurysm in a patient with cervical spine fractures. J Trauma. 2004;57:1331–4. doi: 10.1097/01.ta.0000151256.20476.7e. [DOI] [PubMed] [Google Scholar]
- 96.Simionato F, Righi C, Scotti G. Post-traumatic dissecting aneurysm of extracranial internal carotid artery: Endovascular treatment with stenting. Neuroradiology. 1999;41:543–7. doi: 10.1007/s002340050801. [DOI] [PubMed] [Google Scholar]
- 97.Singer RJ, Dake MD, Norbash A, Abe T, Marcellus ML, Marks MP. Covered stent placement for neurovascular disease. AJNR Am J Neuroradiol. 1997;18:507–9. [PMC free article] [PubMed] [Google Scholar]
- 98.Smith BL, Munschauer CE, Diamond N, Rivera F. Ruptured internal carotid aneurysm resulting from neurofibromatosis: Treatment with intraluminal stent graft. J Vasc Surg. 2000;32:824–8. doi: 10.1067/mva.2000.107769. [DOI] [PubMed] [Google Scholar]
- 99.Smith TP, Alexander MJ, Enterline DS. Delayed stenosis following placement of a polyethylene terephthalate endograft in the cervical carotid artery. Report of three cases. J Neurosurg. 2003;98:421–5. doi: 10.3171/jns.2003.98.2.0421. [DOI] [PubMed] [Google Scholar]
- 100.Surber R, Werner GS, Cohnert TU, Wahlers T, Figulla HR. Recurrent vertebral arteriovenous fistula after surgical repair: Treatment with a self-expanding stent-graft. J Endovasc Ther. 2003;10:49–53. doi: 10.1177/152660280301000111. [DOI] [PubMed] [Google Scholar]
- 101.Szopinski P, Ciostek P, Kielar M, Myrcha P, Pleban E, Noszczyk W. A series of 15 patients with extracranial carotid artery aneurysms: Surgical and endovascular treatment. Eur J Vasc Endovasc Surg. 2005;29:256–61. doi: 10.1016/j.ejvs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 102.Thalhammer C, Kirchherr AS, Uhlich F, Waigand J, Gross CM. Postcatheterization pseudoaneurysms and arteriovenous fistulas: Repair with percutaneous implantation of endovascular covered stents. Radiology. 2000;214:127–31. doi: 10.1148/radiology.214.1.r00ja04127. [DOI] [PubMed] [Google Scholar]
- 103.Tseng A, Ramaiah V, Rodriguez-Lopez JA, Perkowshi PE, Del Santo PB, Gowda RG, et al. Emergent endovascular treatment of a spontaneous internal carotid artery dissection with pseudoaneurysm. J Endovasc Ther. 2003;10:643–6. doi: 10.1177/152660280301000334. [DOI] [PubMed] [Google Scholar]
- 104.ul Haq T, Yaqoob J, Munir K, Usman MU. Endovascular-covered stent treatment of posttraumatic cervical carotid artery pseudoaneurysms. Australas Radiol. 2004;48:220–3. doi: 10.1111/j.1440-1673.2004.01302.x. [DOI] [PubMed] [Google Scholar]
- 105.Usinskiene J, Mazighi M, Bisdorff A, Houdart E. Fatal peritoneal bleeding following embolization of a carotid-cavernous fistula in Ehlers-Danlos syndrome type IV. Cardiovasc Intervent Radiol. 2006;29:1104–6. doi: 10.1007/s00270-005-0331-6. [DOI] [PubMed] [Google Scholar]
- 106.Van Nieuwenhove Y, Van den Brande P, van Tussenbroek F, Debing E, von Kemp K. Iatrogenic carotid artery pseudoaneurysm treated by an autologous vein-covered stent. Eur J Vasc Endovasc Surg. 1998;16:262–5. doi: 10.1016/s1078-5884(98)80230-x. [DOI] [PubMed] [Google Scholar]
- 107.Vinchon M, Laurian C, George B, D’Arrigo G, Reizine D, Aymard A, et al. Vertebral arteriovenous fistulas: A study of 49 cases and review of the literature. Cardiovasc Surg. 1994;2:359–69. [PubMed] [Google Scholar]
- 108.Waigand J, Uhlich F, Gross CM, Thalhammer C, Dietz R. Percutaneous treatment of pseudoaneurysms and arteriovenous fistulas after invasive vascular procedures. Catheter Cardiovasc Interv. 1999;47:157–64. doi: 10.1002/(SICI)1522-726X(199906)47:2<157::AID-CCD5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 109.Waldman DL, Barquist E, Poynton FG, Numaguchi Y. Stent graft of a traumatic vertebral artery injury: Case report. J Trauma. 1998;44:1094–7. doi: 10.1097/00005373-199806000-00027. [DOI] [PubMed] [Google Scholar]
- 110.Wang C, Xie X, You C, Zhang C, Cheng M, He M, Sun H, Mao B. Placement of covered stents for the treatment of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. 2009;30:1342–6. doi: 10.3174/ajnr.A1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Willfort-Ehringer A, Ahmadi R, Gschwandtner ME, Haumer M, Lang W, Minar E. Single-center experience with carotid stent restenosis. J Endovasc Ther. 2002;9:299–307. doi: 10.1177/152660280200900308. [DOI] [PubMed] [Google Scholar]
- 112.Witz M, Gepstein R, Paran H, Shnaker A, Lehmann J, Gryton I, et al. Endovascular treatment of an open cervical fracture with carotid artery tear. Eur Spine J. 2006;15(Suppl 5):650–2. doi: 10.1007/s00586-006-0182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yi AC, Palmer E, Luh GY, Jacobson JP, Smith DC. Endovascular treatment of carotid and vertebral pseudoaneurysms with covered stents. AJNR Am J Neuroradiol. 2008;29:983–7. doi: 10.3174/ajnr.A0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zingler VC, Strupp M, Brandt T, Herrmann K, Mayer TE. Stent grafting resolved brachial plexus neuropathy due to cervical arteriovenous fistula. Eur Neurol. 2004;52:250–1. doi: 10.1159/000082370. [DOI] [PubMed] [Google Scholar]


