Abstract
Objective
To compare the ease of use and accuracy of 5 feline AB blood-typing methods: card agglutination (CARD), immunochromatographic cartridge (CHROM), gel-based (GEL), and conventional slide (SLIDE) and tube (TUBE) agglutination assays.
Sample Population
490 anticoagulated blood samples from sick and healthy cats submitted to the Transfusion or Clinical Laboratory at the Veterinary Hospital of the University of Pennsylvania.
Procedures
Sample selection was purposely biased toward those from anemic, type B, or type AB cats or those with autoagglutination. All blood samples were tested by use of GEL, SLIDE, and TUBE methods. Fifty-eight samples were also tested by use of CARD and CHROM methods. The presence of alloantibodies in all cats expressing the B antigen as detected by use of any method was also assessed.
Results
Compared with the historical gold-standard TUBE method, good to excellent agreement was achieved with the other typing tests: CARD, 53 of 58 (91% agreement); CHROM, 55 of 58 (95%); GEL, 487 of 490 (99%); and SLIDE, 482 of 487 (99%; 3 samples were excluded because of autoagglutination). Four of the samples with discordant test results originated from cats with FeLV-related anemia.
Conclusions and Clinical Relevance
Current laboratory and in-clinic methods provide simple and accurate typing for the feline AB blood group system with few discrepancies. Retyping after in-clinic typing with the GEL or TUBE laboratory methods is recommended to confirm any type B or AB cats.
The feline AB blood group system is clinically important because of the varied prevalence of type A, type B, and type AB cats within DSH, DLH, and purebred feline populations and the presence of naturally occurring alloantibodies that may cause acute hemolytic transfusion reactions and neonatal isoerythrolysis.1–15 The biochemistry and molecular basis of blood types A and B are known,16,17 and a DNA test is available to identify the b allele,a allowing identification of type B cats or cats carrying the allele. However, serologic blood typing must be used in clinics and clinical pathology laboratories to differentiate type A, B, and AB cats.15,18
Serologic blood typing relies on identification of the presence of the A and B RBC surface antigens by agglutination or immunochromatographic reactions with antibody or lectins. Serum from type B cats is often used as an anti-A reagent owing to the presence of strong alloantibodies in all type B cats older than 3 months. Because the anti-B antibodies in serum of type A cats are generally weak, this reagent had been replaced by the lectin from Triticum vulgaris as an anti-B reagent.1,10,15,16,18,19 More recently, monoclonal antibodies against the type A and type B antigens have been developed by 2 laboratoriesb,c and are now commonly used in commercial assays.
Several commercially available serologic test kits are available for typing of the feline blood according to the AB system. A point-of-care test (CARD) has been available since 1995, consisting of a card with wells that now contain lyophilized monoclonal anti-A or anti-B antibody.18,19,b Also available is a newer point-of-care test (CHROM) based on immunochromatographic diffusion of RBCs passing through monoclonal antibody-containing strips.d For laboratory use, there is a standardized technique (GEL) that involves examination of agglutination in matrix gel columns containing serum or lectin.20,c The initial validation study18 of this GEL assay led to adding anti-A serum to the lectins for optimal recognition of the A antigen. In addition to these commercially available methods, the Transfusion Laboratory at the School of Veterinary Medicine, University of Pennsylvania has been performing serologic testing with lectin and anti-A sera in SLIDE and TUBE assays.18 An additional tube-typing kit is available in Japan, but not Europe or North America.18 The purpose of the study reported here was to assess the ease of use of available feline blood-typing methods and to compare their accuracy by use of blood samples from healthy and diseased cats.
Materials and Methods
Blood samples
The study included small (1- to 2-mL) EDTA-anticoagulated blood samples from healthy and sick cats and from blood donors at the Penn Animal Blood Bank submitted to the Transfusion Laboratory or the Clinical Laboratory at Veterinary Hospital of the University of Pennsylvania from 2005 through 2007. Although blood samples from DSH and DLH cats were included in the survey, samples were preferentially obtained from type AB or B cats, Ragdoll cats, anemic cats, and those with apparent autoagglutination in order to assist in recognizing any unique potential typing problems.
For each blood sample tested, the cat’s age, breed, and sex were recorded. When available, the cat’s health status or underlying disease was also recorded. All samples were analyzed with approval of the institutional animal care and use committee.
Typing methods
All samples were tested in accordance with the manufacturer’s instructionsb–d and published methods18 and as follows.
CARD method
One drop (approx 50 μL) of PBS solutione was placed in the 3 wells marked as autocontrol, A, or B on the feline blood-typing card.b One drop of anticoagulated blood was added to each well and then spread over the well area. A second drop of PBS solution was added to the anti-A well to overcome any potential prozone effect and thereby enhance agglutination. The card was agitated and agglutination interpreted after 1 minute as follows: 0, no agglutination; 1+, many small agglutinates with RBCs in suspension; 2+, some larger agglutinates and many small agglutinates; 3+, a few large agglutinates in clear suspension; and 4+, 1 large agglutinate in clear suspension. Agglutination reactions of 2+ or more were considered a positive test result. When autoagglutination was present in the autocontrol well, RBCs were washed 3 times in PBS solution by use of standard methods as described for the TUBE method before being resuspended at a 25% to 35% RBC suspension. When autoagglutination diminished to ≤1+, typing was repeated.
CHROM method
For the CHROM test kit,c 3 drops of diluent were placed into the supplied plastic well. The supplied absorbent paper strip was briefly dipped into anticoagulated blood and then in the diluent-containing well to suspend RBCs. An immunochromatographic strip, linearly impregnated at 3 distinct levels with monoclonal anti-A and anti-B antibodies and a control lectin, was then placed in the RBC suspension for approximately 2 minutes until the RBC suspension had diffused to the top of the strip. The cartridge was then placed in a holder and immediately read as follows: the presence of a red band at the position marked C (control) had to be present for result interpretation, the existence of a visible red band at the position marked A indicated the expression of the A antigen, and the presence of a red band at the B position indicated expression of the B antigen on RBCs.
GEL method
For the GEL laboratory method, 50 μL of anticoagulated blood (or 25 μL of washed, packed RBCs if the cat was markedly anemic or had strong autoagglutination) was added to 500 μL of low–ionic strength solution.f Then, 10 μL of this suspension was loaded on top of 3 gel columns containing anti-A antibodies, T vulgaris lectin, or no reagent. Gel columns were centrifuged for 10 minutes at 80 × g in the manufacturer’s centrifuge. The RBC retention in the gel was graded as follows: 0, all RBCs at the bottom of gel; 1+, few RBC agglutinates in the lower half of gel but most RBCs at the bottom of gel; 2+, RBC agglutinates dispersed throughout gel; 3+, RBC agglutinates throughout gel and RBCs on upper surface; and 4+, all RBCs on top of gel. An RBC retention of ≥ 2+ was considered a positive test result. Samples were washed, and the assay was repeated if the blank control was positive; results were disregarded if the control was still positive.
SLIDE method
For the SLIDE test, 25 μL of anticoagulated blood was mixed on a glass slide with 50 μL of serum from a type B cat (anti-A reagent), 64 μg of T vulgaris lectin/mL (anti-B reagent), or PBS solution (autoagglutination control). Slides were then agitated for 2 minutes, and the degree of agglutination was scored as described for the CARD method.
TUBE method
To prepare a RBC suspension for the TUBE method, 1 mL of anticoagulated blood was centrifuged for 2 minutes in a serologic centrifuge (1,000 × g at room temperature [approx 20°C]). Plasma was collected into a separate tube, and the RBC pellet was resuspended in 5 mL of PBS solution. The suspension was then recentrifuged and resuspended 3 times and finally reconstituted to a 2% to 5% RBC suspension. In 3 tubes, 25 μL of this suspension was then mixed with 50 μL of 1:4 type B serum diluted in PBS solution (anti-A reagent), 8 μg of T vulgaris lectin/mL in PBS solution (anti-B reagent), or PBS solution. These mixtures were incubated at room temperature for 15 minutes before centrifugation for 15 seconds at 1,000 × g. Tubes were then gently agitated, and the degree of agglutination was scored as for the CARD method.
Alloantibody testing
Washed 2% to 5% RBC suspensions from the test sample, a known type A cat, and a known type B cat were incubated with the sample serum or plasma as described for the TUBE method to detect the presence or absence of alloantibodies. This was performed in all cats that expressed B antigen on their RBC surface (type B or AB cats) by any typing method.
Statistical analysis
The strengths of all test reactions (anti-A, anti-B, and control) were recorded as well as the interpreted test result for each method. Sensitivity, specificity, and overall accuracy of test methods were then calculated by comparison with the TUBE method as the criterion reference (gold) standard.
Results
Blood samples
Blood samples from 490 cats were included in the study. Cat breeds represented were as follows: DSH (n = 174), Abyssinian (116), Birman (36), Ragdoll (30), Norwegian Forest Cat (26), Somali (25), DLH (21), Persian (20), Turkish Van (10), Bengal (5), Maine Coon (4), Devon Rex (4), Siamese (4), Himalayan (4), Sphynx (3), British Shorthair (2), Exotic Short-hair (2), Scottish Fold (2), Chartreux (1), and Russian Blue (1). Prior to development of the CHROM method, blood samples from 432 cats were typed by use of GEL, SLIDE, and TUBE methods only. Complete medical records were not available for this population of healthy and sick cats, but records were reviewed when available. After development of the CHROM method, an additional 58 samples from cats with complete medical records available were blood typed with CARD, CHROM, GEL, SLIDE, and TUBE methods. Of these additional 58 samples, 32 were from healthy blood donor or breeding cats and 26 were from diseased cats from which blood had been collected as a part of their clinical care.
Within the subpopulation of 26 diseased cats the diagnoses were as follows: lymphosarcoma (n = 5 cats), FeLV-related anemia (3), hepatic lipidosis (3), idiopathic immune-mediated hemolytic anemia (2), anemia of unknown origin (2), hyperthyroidism (2), dental disease (2), and 1 each of systemic mastocytosis, feline infectious peritonitis, inflammatory bowel disease, dog bite, septic peritonitis, chronic renal failure, and jejunal mass. Although type A was the predominant blood type detected, a high proportion of type B and type AB cats were included (Table 1).
Table 1.
Number (%) of cats with positive test results for various blood-typing methods.
| Blood type | Any method (n = 490) | GEL, SLIDE, and TUBE only* (n = 432) | CARD, CHROM, GEL, SLIDE, and TUBE |
|
|---|---|---|---|---|
| Healthy cats (n = 32) | Sick cats (n = 26) | |||
| A | 407 (83) | 372 (86) | 16 (50) | 19 (73) |
| B | 56 (11) | 42 (10) | 10 (31) | 4 (15) |
| AB | 27 (6) | 18 (4) | 6 (19) | 3 (12) |
Medical records were not available for all blood samples typed with GEL, SLIDE, and TUBE methods only, precluding classification of these samples by cat health status.
All cats identified as type B with the TUBE method had strong anti-A alloantibodies in their plasma (3+ or 4+ agglutination), whereas none of the cats identified as type AB by use of the TUBE method had detectable alloantibody against either the A or B antigen.
Test comparison
Overall agreement between blood-typing methods was good to excellent (Table 2), with identical results obtained in 52 of 58 (90%) cats tested with all 5 methods. Details of the 6 discrepancies identified among these cats were summarized (Table 3). Moreover, of the 490 samples tested with the GEL method, 2 type A DSH cats were misidentified as type AB according to results of the gold-standard TUBE method. Both of the cats mistyped by the GEL method had positive results of FeLV antigen testing (FeLV positive) and were anemic. The SLIDE method misidentified the same 2 cats and 1 additional type A cat as type AB and 1 type AB cat as type A. An additional 3 cats could not be typed by use of the SLIDE method because of the presence of strong autoagglutination.
Table 2.
Accuracy of CARD, CHROM, GEL, and SLIDE methods of feline blood typing as compared with the TUBE gold-standard method.
| Typing method (%) | No. of cats tested | A antigen detection | B antigen detection | Overall accuracy (%) | ||
|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |||
| CARD | 58 | 93.2 | 100.0 | 95.7 | 97.1 | 91.4 |
| CHROM | 58 | 97.7 | 100.0 | 95.7 | 97.1 | 94.8 |
| GEL | 490 | 100.0 | 100.0 | 100.0 | 99.3 | 99.4 |
| SLIDE | 487* | 100.0 | 100.0 | 98.8 | 99.0 | 99.0 |
Data for the SLIDE test were calculated after exclusion of 3 of the 490 blood samples that could not be determined through this method because of persistent autoagglutination.
Table 3.
Discordant results for 5 methods of blood typing in 6 of 58 cats as compared with results of the TUBE gold-standard method.
| Cat No. | TUBE | CARD | CHROM | GEL | SLIDE | Alloantibody detected | Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | A | AB | A | A | A | None | FeLV infection |
| 2 | A | A | AB | A ± B* | AB | Anti-B | FeLV infection |
| 3 | AB | B | B | AB | AB | None | Dog bite |
| 4 | AB | A | A | AB | AB | None | Healthy |
| 5 | AB | B† | AB | AB | AB | None | Healthy |
| 6 | AB | B | AB | AB | AB | None | Healthy |
| No. of discordant results | — | 5 | 3 | 1 | 1 | — | — |
For the indicated cat, B antigen positivity as indicated by the GEL method was equivocal.
The indicated blood sample had 1+ agglutination in the anti-A antibody well, which was lower than the cutoff for a positive test result (ie, ≥ 2+).
— = Not applicable.
Among the methods that involved interpretation of RBC agglutination in a suspension, the distinction between positive and negative anti-A and anti-B antibody results was greater with SLIDE and TUBE methods than with the CARD method (Table 4). With the exception of the 2 FeLV-positive cats that were misidentified as type AB, all positive GEL reactions were 3+ or 4+. Results acquired with CHROM and GEL methods were considered the simplest to interpret and could readily be archived by making a photocopy, which was not possible with the other methods. Digital photography could have been used to capture the results from all methods, although this was difficult to accomplish with the TUBE method.
Table 4.
Strength of agglutination in anti-A or anti-B antibody reactions with CARD, SLIDE, and TUBE methods used to type feline blood.
| Typing method | Negative test results |
Positive test results |
|||||
|---|---|---|---|---|---|---|---|
| No. of cats tested | 0 | 1+ | No. of cats tested | 2+ | 3+ | 4+ | |
| TUBE | 463 | 455 (98) | 8 (2) | 517 | 12 (2) | 159 (31) | 346 (67) |
| SLIDE | 458 | 439 (96) | 19 (4) | 516 | 22 (4) | 232 (45) | 262 (51) |
| CARD | 52 | 50 (96) | 2 (4) | 64 | 23 (36) | 25 (39) | 16 (25) |
Data are reported as number (%).
For these methods, agglutination of RBCs was scored in a suspension from 0 to 4+, with 0 or 1+ considered a negative result and ≥ 2+ considered a positive result. There were relatively fewer 1+ and 2+ results for TUBE and SLIDE methods than for the CARD method, making the distinction between positive and negative results greater for the TUBE and SLIDE methods.
Discordant results
Among the 490 samples examined in this study, there were 13 samples in which blood-typing problems or discrepancies arose. Four of these samples were from DSH cats that had a recorded diagnosis of an FeLV-related anemia on the basis of an Hct < 20% and FeLV antigen detection via ELISA or immunofluorescent antibody test by use of whole blood or bone marrow aspirates. While the TUBE assay identified these samples to be blood type A and all other methods detected the A antigen, they concurrently, to variable degrees, also reacted with the anti-B reagent (Figure 1), falsely identifying these cats as blood type AB. In addition, weak auto agglutination, which was eliminated by washing of RBCs, was seen with 3 of these samples and weak anti-B alloantibodies were also detected in 2 of those 3 samples.
Figure 1.
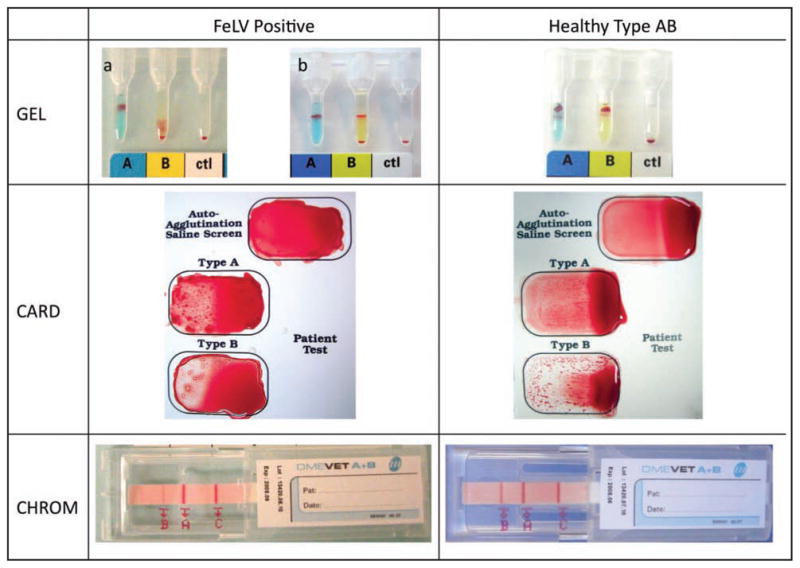
Photographs of results of GEL, CARD, and CHROM blood-typing methods for a type A cat with FeLV-related anemia and from a healthy type AB cat. Notice that the B antigen reaction is positive in the FeLV-positive cat but is weaker than in the healthy type AB cat. In 1 sample from a cat with FeLV-related anemia, the GEL test reaction shows a split population of RBCs in the anti-B column, making the results inconclusive. Also notice that for the CARD test, the anti-B reaction is stronger than the anti-A reaction for the healthy type AB cat.
Discussion
The AB blood group system is the most clinically important blood group system in cats because A-B mismatched breeding and transfusions can cause life-threatening hemolytic reactions without prior sensitization via pregnancy or transfusion. Therefore, it is crucial that veterinary clinicians accurately and swiftly identify a cat’s blood type.1–5,7,21 In the present study, 5 feline AB typing methods were compared for ease of use and accuracy. The GEL, SLIDE, and TUBE tests are generally restricted to the laboratory and performed by specifically trained personnel, whereas the CARD and CHROM assays are simple point-of-care kits commonly used by various veterinary staff in practice.
Finding the optimal reagent or reagent concentration for the recognition of different feline RBC antigens is an area of ongoing research and development. Over the past 15 to 20 years, the reagents in blood-typing kits or tests have occasionally changed, and it is important to remember this when interpreting and comparing our results with those from previous studies. The reagents used in the GEL method in the present study to recognize the feline A and B antigens were anti-A serum and T vulgaris lectin, respectively. These reagents were used on the basis of results of a previous study.18 However, since the completion of that and our study, the reagents used in the gel columns have again been changed by the manufacturer and are now monoclonal anti-A and anti-B antibodies. Additional studies will be needed to determine the sensitivity, specificity, and accuracy of the new method, which was introduced in 2009. Since the previous study,18 the reagents of the CARD assay have also been changed from T vulgaris lectin and anti-A serum to monoclonal antibodies.b Moreover, the CHROM test only recently became available and had not previously been compared with standard methods. In addition to the aforementioned differences in reagents, the present study involved a larger group of cats than the previous study18 and was not limited to healthy animals.
Compared with the TUBE method, which is considered the original gold-standard method,1,16,18 the previous study18 revealed excellent concordance of typing results by use of various techniques, and our larger survey confirmed those results. However, our survey also detected a few discrepancies, with the laboratory SLIDE and GEL techniques reaching ≥ 99% agreement and the 2 point-of-care kits (CHROM and CARD) achieving 95% and 91% agreement, respectively. Although AB typing with any of the evaluated methods was fairly easy to perform and, except for the TUBE test, can be performed with whole blood or unwashed dilute RBC suspensions, the GEL method appears to be the most standardized for the commercial laboratory, whereas the CHROM kit would appear to be the simplest to use, with the least subjective result interpretation in a clinic environment.
Because of very strong naturally occurring anti-A alloantibodies in the plasma of type B cats older than 3 months,10 and because A–B mismatches can cause fatal hemolytic reactions,3 it is particularly critical to differentiate type B cats from type A and AB cats, which both express the A antigen and can safely receive type A blood products. Similarly, because only type B queens mated to type A or AB toms can produce type A and AB kittens at risk for neonatal isoerythrolysis,4,5,12 it is important to recognize the queens lacking the A antigen. For these reasons, it is crucial that a point-of-care assay be both sensitive and specific for detection of the A antigen. The GEL, SLIDE, and TUBE methods were all 100% sensitive and specific for detection of the A antigen. The CARD and CHROM methods were both 100% specific for detection of the A antigen but were less sensitive (93.2% and 97.7%, respectively). Therefore, CARD and CHROM methods should be suitable for point-of-care testing in in-clinic settings when typing results are immediately needed, but as in human medicine, laboratory methods are preferred and should be used for definitive blood typing of breeding queens, blood donors, and, when possible, cats in need of transfusions. One should also consider that the prevalence of type B cats ranges from 0% in Siamese cats to approximately 50% in some breeds such as Turkish Van and Turkish Angora and similarly differs among DSH and DLH cats in different geographic regions.6,8,9,13 For this reason, the prevalence of type B cats in various breeds should be considered when evaluating the predictive value of CARD or CHROM assays in a specific setting.
Results of blood typing can be affected by disease states in humans22 and animals.15,g For example, FeLV infection and other diseases may cause anemia and auto agglutination and may thereby contribute to test inaccuracies as detected in the present study. In our study, blood samples from several FeLV-positive, anemic DSH cats had strongly positive results for the A antigen but only weakly positive results for the B antigen. Complete medical records were not available for all study samples, and FeLV antigen testing was not performed on all cats for which medical records were available. Consequently, no comment can be made regarding the commonness of such results within the FeLV-positive cat population. Some form of antigenic mimicry with the B antigen on the surface of RBCs or a reduction in CMAH activity converting N-acetylneuraminic acid (B-antigen) to N-glycolylneuraminic acid (A-antigen) in type A cats17 may explain our results. The presence of autoagglutination and anti-B alloantibodies may also suggest a possible immune-mediated mechanism for the anemia in the 2 study cats, although the precise pathogenesis would require additional investigation. A study in dogsg identified that anemia may cause spurious blood-typing results obtained by some methods; this factor was not specifically investigated but may have also played a role in our findings. Preparation of fixed-concentration RBC suspensions alleviates the effect of Hct on GEL and TUBE assays, and for the point-of-care assays, adding more blood to test reactions when dealing with anemic cats may overcome such problems; however, these suppositions require investigation.
In the study reported here, the cutoff of 2+ agglutination was used to differentiate between positive and negative test results. This cutoff was in keeping with the published guidelines and manufacturers’ recommendations for GEL, SLIDE, and TUBE assays18,c but differs from the manufacturer’s recommendations for the CARD method,b which suggest that any agglutination should be interpreted as a positive result. Had the manufacturer’s interpretation been applied to the data set, then one of the healthy type AB cats that was misidentified as type B by use of the CARD method would have been correctly identified as AB and 1 cat correctly identified as type B would have been misidentified as AB (Tables 3 and 4). Considering that most positive reactions are 3+ or 4+ and that any 1+ reaction obtained by use of the CARD method is difficult to accurately recognize,23 a 2+ cutoff appeared safer to use and did not noticeably change the typing accuracy.
Subjective test interpretation is a potential problem with any of the methods used in our study but is of particular concern when agglutination is scored in an RBC suspension because test interpretation is dependent on the time of reading and degree of agitation applied by the operator.23,24 When the SLIDE and TUBE methods were used, the distinction between positive and negative results was clearer than those for the CARD method. This was because there were smaller numbers of 1+ and 2+ results with the SLIDE and TUBE methods, and such results may be confused, altering test interpretation. The CHROM and GEL methods were advantageous in this respect because the test results are fixed, more objectively scored, and relatively stable over time, allowing results to be photocopied or viewed by multiple people and archived in laboratory or medical records.
Because the present study preferentially included samples from cats with autoagglutination and many more AB cats than expected for the target population (27/490 [5.5%] cats vs the expected 2/490 [≤ 0.4%] cats8,16), the typing accuracy being reported may have been underestimated. Indeed, most test errors identified involved blood samples from type AB cats or samples with autoagglutination. Concerns about the accuracy of blood typing of such samples have been reported.18,25 With the CHROM assay, only dissolved nonagglutinating RBCs migrate on the test strip and bind at the site of the control lectin and at one or both of the A and B bands. If enough RBCs migrate through the strip to cause a positive control band at the top, then theoretically, autoagglutination should not interfere with CHROM assay results.
In situations of autoagglutination and type AB or B results, it is particularly important to confirm these results at a reference laboratory in which staff apply appropriate laboratory typing techniques, wash RBCs, and screen for the presence of anti-A (or anti-B) allo-antibodies. A case report25 describes a kitten that was initially typed as AB with an in-clinic method and was given type B blood, but that, following the clearance of the transfused RBCs, was found to be type A a few weeks later. As has been reported for immunologically mature animals,10 the present study identified strong anti-A alloantibody in all type B cats, indicating the usefulness of this additional confirmatory (also known as back-typing) test for type B cats.
In human transfusion medicine, blood-typing errors remain a considerable problem, with ABO-mismatched transfusions accounting for 22% of transfusion-related deaths reported in the United States in 2008.26 Bedside ABO typing kits, which are used only as a final check prior to blood administration, are recognized to have an error rate of approximately 30%, and the experience of the nurse performing the test has a significant effect on test accuracy.24 By contrast, when blood typing is performed at a centralized laboratory that specializes in compatibility testing, the error rate is reduced to approximately 1 in 3,400, with most of these errors being clerical errors in patient and sample identification and recording rather than related to methods used.27 Therefore, human patients are blood typed and crossmatched prior to each transfusion event. As in humans, it may be prudent to retype cats prior to each transfusion in the future.
Results of the study reported here suggested that CARD, CHROM, GEL, SLIDE, and TUBE assays can be used for AB blood typing of cats with a high degree of agreement; however, a few discrepancies can arise among results of these typing methods. The commercially available GEL and original TUBE laboratory-based methods had a higher degree of agreement than do commercially produced point-of-care (CARD and CHROM) assays, and discrepancies should be considered potential contributing factors when results indicate cats are type B or AB but this condition is rare in similar cats. As such, blood types that are considered rare should be confirmed by use of laboratory techniques and alloantibody screening to avoid potentially fatal hemolytic reactions.
Acknowledgments
Supported in part by a grant from the National Institutes of Health (RR02512). Dr. Giger has been a scientific advisor for Alvedia, Lyon, France; DMS Laboratories, Flemington, NJ; and DiaMed AG, Cressier sur Morat, Switzerland.
The authors thank the Penn Animal Blood Bank for providing samples for this study.
Abbreviations
- CARD
Card agglutination
- CHROM
Immunochromatographic cartridge
- CMAH
Cytidine monophospho-N-acetylneur-aminic acid hydroxylase
- DLH
Domestic longhair
- DSH
Domestic shorthair
- GEL
Gel-based agglutination
- SLIDE
Slide agglutination
- TUBE
Tube agglutination
Footnotes
UC Davis InnovationAccess, Davis, Calif.
RapidVet-H Feline, provided by DMS Laboratories, Flemington, NJ.
ID Card Anti A+B (Cat), provided by DiaMed, Cressier sur Morat, Switzerland.
DME VET A+B, provided by Alvedia, Lyon, France.
Dulbecco’s Phosphate Buffered Saline without Calcium, Magnesium, or Phenol Red, Thermo Scientific, Logan, Utah.
ID-Diluent 2, DiaMed, Cressier sur Morat, Switzerland.
Seth M, Winzelberg S, Jackson KV, et al. Comparison of gel column, card and cartridge techniques for DEA 1.1 blood typing of dogs (abstr). J Vet Intern Med 2008;3:775.
Presented in abstract form at the American College of Veterinary Internal Medicine Forum, San Antonio, Tex, June 2008.
References
- 1.Auer L, Bell K. The AB blood group system of cats. Anim Blood Groups Biochem Genet. 1981;12:287–297. doi: 10.1111/j.1365-2052.1981.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 2.Auer L, Bell K, Coates S. Blood transfusion reactions in the cat. J Am Vet Med Assoc. 1982;180:729–730. [PubMed] [Google Scholar]
- 3.Auer L, Bell K. Transfusion reactions in cats due to AB blood group incompatibility. Res Vet Sci. 1983;35:145–152. [PubMed] [Google Scholar]
- 4.Cain GR, Suzuki Y. Presumptive neonatal isoerythrolysis in cats. J Am Vet Med Assoc. 1985;187:46–48. [PubMed] [Google Scholar]
- 5.Hubler M, Kaelin S, Hagen A, et al. Feline neonatal isoerythrolysis in two litters. J Small Anim Pract. 1987;28:833–838. [Google Scholar]
- 6.Giger U, Kilrain CG, Filippich LJ, et al. Frequencies of feline blood groups in the United States. J Am Vet Med Assoc. 1989;195:1230–1232. [PubMed] [Google Scholar]
- 7.Giger U, Bucheler J. Transfusion of type-A and type-B blood to cats. J Am Vet Med Assoc. 1991;198:411–418. [PubMed] [Google Scholar]
- 8.Giger U, Bucheler J, Patterson DF. Frequency and inheritance of A and B blood types in feline breeds of the United States. J Hered. 1991;82:15–20. doi: 10.1093/jhered/82.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Giger U, Griot-Wenk M, Bucheler J, et al. Geographical variation of the feline blood type frequencies in the United States. Feline Pract. 1991;19(6):21–27. [Google Scholar]
- 10.Bucheler J, Giger U. Alloantibodies against A and B blood types in cats. Vet Immunol Immunopathol. 1993;38:283–295. doi: 10.1016/0165-2427(93)90088-l. [DOI] [PubMed] [Google Scholar]
- 11.Griot-Wenk ME, Giger U. Feline transfusion medicine. Blood types and their clinical importance. Vet Clin North Am Small Anim Pract. 1995;25:1305–1322. doi: 10.1016/s0195-5616(95)50156-1. [DOI] [PubMed] [Google Scholar]
- 12.Bucheler J. Fading kitten syndrome and neonatal isoerythrolysis. Vet Clin North Am Small Anim Pract. 1999;29:853–870. [PubMed] [Google Scholar]
- 13.Arikan S, Duru SY, Gurkan M, et al. Blood type A and B frequencies in Turkish Van and Angora cats in Turkey. J Vet Med A Physiol Pathol Clin Med. 2003;50:303–306. doi: 10.1046/j.1439-0442.2003.00536.x. [DOI] [PubMed] [Google Scholar]
- 14.Hohenhaus AE. Importance of blood groups and blood group antibodies in companion animals. Transfus Med Rev. 2004;18:117–126. doi: 10.1016/j.tmrv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Giger U. Blood-typing and crossmatching. In: Bonagura JD, Twedt DC, editors. Kirk’s current veterinary therapy. St Louis: Elsevier; 2009. pp. 260–266. [Google Scholar]
- 16.Griot-Wenk M, Pahlsson P, Chisholm-Chait A, et al. Biochemical characterization of the feline AB blood group system. Anim Genet. 1993;24:401–407. doi: 10.1111/j.1365-2052.1993.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 17.Bighignoli B, Niini T, Grahn RA, et al. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genet. 2007;8:27–38. doi: 10.1186/1471-2156-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stieger K, Palos H, Giger U. Comparison of various blood-typing methods for the feline AB blood group system. Am J Vet Res. 2005;66:1393–1399. doi: 10.2460/ajvr.2005.66.1393. [DOI] [PubMed] [Google Scholar]
- 19.Kohn B, Niggemeier A, Reitemeyer S, et al. Blood typing in cats with a new test card method. Kleintierpraxis. 1997;42:941–950. [Google Scholar]
- 20.Lapierre Y, Rigal D, Adam J, et al. The gel test: a new way to detect red cell antigen-antibody reactions. Transfusion. 1990;30:109–113. doi: 10.1046/j.1537-2995.1990.30290162894.x. [DOI] [PubMed] [Google Scholar]
- 21.Giger U, Akol KG. Acute hemolytic transfusion reaction in an Abyssinian cat with blood type B. J Vet Intern Med. 1990;4:315–316. doi: 10.1111/j.1939-1676.1990.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 22.Garratty G, Arndt P, Co A, et al. Fatal hemolytic transfusion reaction resulting from ABO mistyping of a patient with acquired B antigen detectable only by some monoclonal anti-B reagents. Transfusion. 1996;36:351–357. doi: 10.1046/j.1537-2995.1996.36496226152.x. [DOI] [PubMed] [Google Scholar]
- 23.Greendyke RM, Wormer JL, Banzhaf JC. Quality assurance in the blood bank. Studies of technologist performance. Am J Clin Pathol. 1979;71:287–290. [PubMed] [Google Scholar]
- 24.Migeot V, Ingrand I, Salmi LR, et al. Reliability of bedside ABO testing before transfusion. Transfusion. 2002;42:1348–1355. doi: 10.1046/j.1537-2995.2002.00219.x. [DOI] [PubMed] [Google Scholar]
- 25.Niggemeier A, Haberstroh HF, Nelson VE, et al. An accidental transfusion of a type A kitten with type B blood causes a transient switch from blood type A to B. J Vet Intern Med. 2000;14:214–216. [PubMed] [Google Scholar]
- 26.FDA. Fatalities reported to FDA following blood collection and transfusion: annual summary for fiscal year 2008. [Accessed Oct 5, 2009]; Available at: www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Repor-taProblem/TransfusionDonationFatalities/ucm113649.htm.
- 27.Chiaroni J, Legrand D, Dettori I, et al. Analysis of ABO discrepancies occurring in 35 French hospitals. Transfusion. 2004;44:860–864. doi: 10.1111/j.1537-2995.2004.03337.x. [DOI] [PubMed] [Google Scholar]


